Clinical Characterization of the Three Waves of COVID-19 Occurring in Southern Italy: Results of a Multicenter Cohort Study
Abstract
1. Introduction
2. Methods
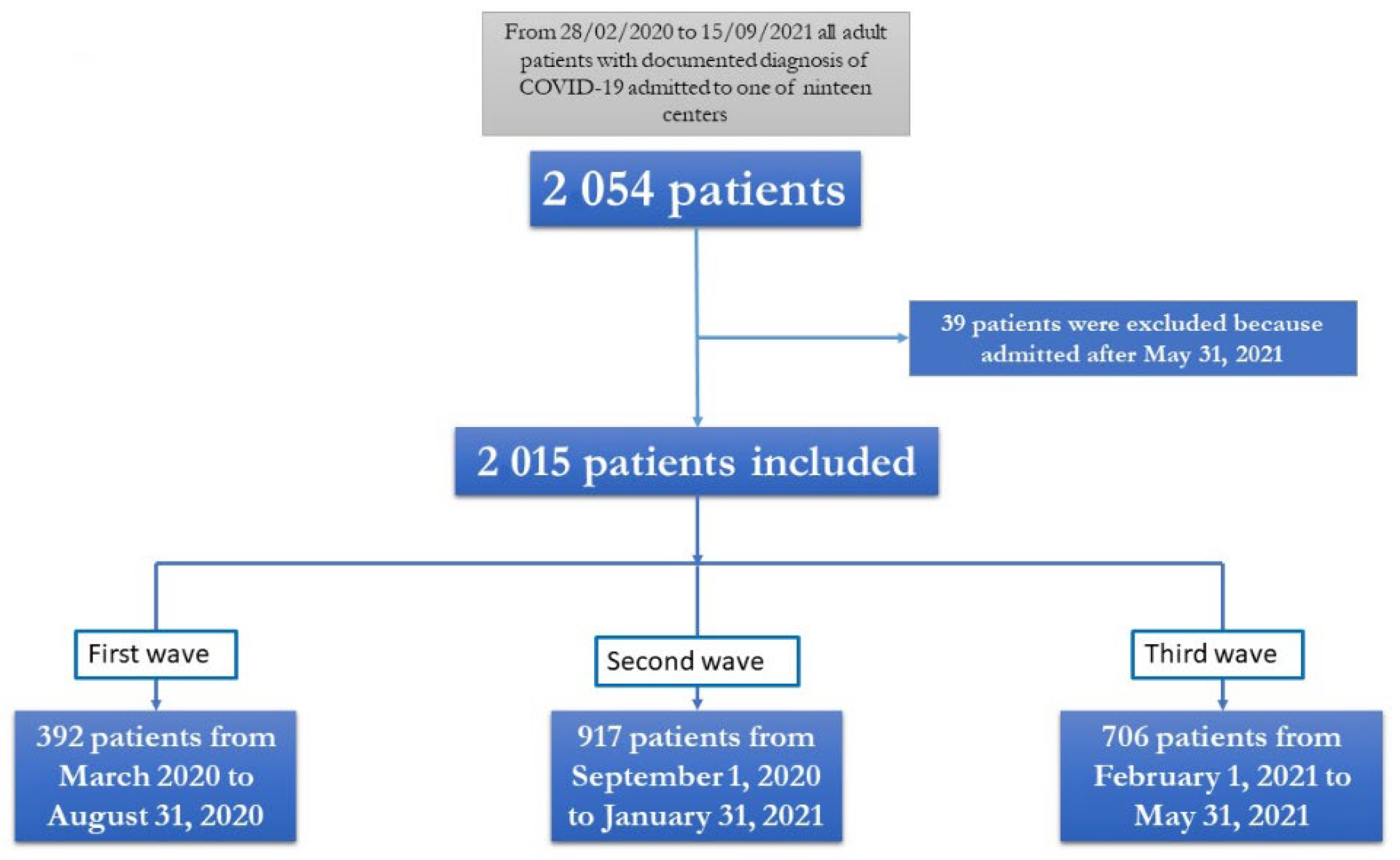
2.1. Study Population
2.2. Data Collection
2.3. Definition
2.4. Ethical issue
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Geneve. Listings of WHO’s Response to COVID-19. Available online: https://www.who.int/news/item/29-06-2020-covidtimeline (accessed on 13 September 2022).
- Macera, M.; De Angelis, G.; Sagnelli, C.; Coppola, N.; Vanvitelli COVID-Group. Clinical Presentation of COVID-19: Case Series and Review of the Literature. Int. J. Environ. Res. Public Health 2020, 17, 5062. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Kim, G.-U.; Kim, M.-J.; Ra, S.; Lee, J.; Bae, S.; Jung, J.; Kim, S.-H. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin. Microbiol. Infect. 2020, 26, 948.e1–948.e3. [Google Scholar] [CrossRef]
- Monari, C.; Sagnelli, C.; Maggi, P.; Sangiovanni, V.; Numis, F.G.; Gentile, I.; Masullo, A.; Rescigno, C.; Calabria, G.; Megna, A.S.; et al. More Severe COVID-19 in Patients with Active Cancer: Results of a Multicenter Cohort Study. Front Oncol. 2021, 11, 662746. [Google Scholar] [CrossRef]
- Pisaturo, M.; Calò, F.; Russo, A.; Camaioni, C.; Giaccone, A.; Pinchera, B.; Gentile, I.; Simeone, F.; Iodice, A.; Maggi, P.; et al. Dementia as Risk Factor for Severe Coronavirus Disease 2019: A Case-Control Study. Front. Aging Neurosci. 2021, 13, 698184. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Guan, W.J.; Liang, W.H.; Zhao, Y.; Liang, H.R.; Chen, Z.S.; Li, Y.M.; Liu, X.Q.; Chen, R.C.; Tang, C.L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef]
- Ssentongo, P.; Ssentongo, A.E.; Heilbrunn, E.S.; Ba, D.M.; Chinchilli, V.M. Association of car-diovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0238215. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Lv, Y.; Zha, W.; Zhou, N.; Hong, X. Association of body mass index (BMI) with critical COVID-19 and in-hospital mortality: A dose-response meta-analysis. Metabolism 2021, 117, 154373. [Google Scholar] [CrossRef] [PubMed]
- Mahamat-Saleh, Y.; Fiolet, T.; Rebeaud, M.E.; Mulot, M.; Guihur, A.; El Fatouhi, D.; Laouali, N.; Peiffer-Smadja, N.; Aune, D.; Severi, G. Diabetes, hypertension, body mass index, smoking and COVID-19-related mortality: A systematic review and meta-analysis of observational studies. BMJ Open. 2021, 11, e052777. [Google Scholar] [CrossRef] [PubMed]
- Italy: COVID-19 Patients Hospitalized since the Outbreak 2021. Statista. Available online: https://www.statista.com/statistics/1125030/covid-19-patients-hospitalized-since-the-outbreak-italy (accessed on 13 September 2022).
- Meschiari, M.; Cozzi-Lepri, A.; Tonelli R Modena COVID-19 Working Group. First and second waves among hospitalised patients with COVID-19 with severe pneumonia: A comparison of 28-day mortality over the 1-year pandemic in a tertiary university hospital in Italy. BMJ Open 2022, 12, e054069. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, L.; Palmer, K.; Lo Noce, C.; Meli, P.; Giuliano, M.; Floridia, M.; Tamburo de Bella, M.; Piccioli, A.; Brusaferro, S.; Onder, G. Differences in the clinical characteristics of COVID-19 patients who died in hospital during different phases of the pandemic: National data from Italy. Aging Clin. Exp. Res. 2021, 33, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Diebold, M.; Martinez, A.E.; Adam, K.M.; Bassetti, S.; Osthoff, M.; Kassi, E.; Steiger, J.; Pargger, H.; Siegemund, M.; Battegay, M.; et al. Temporal trends of COVID-19 related in-hospital mortality and demographics in Switzerland—A retrospective single centre cohort study. Swiss Med. Wkly. 2021, 151, w20572. [Google Scholar] [CrossRef] [PubMed]
- Bociąga-Jasik, M.; Wojciechowska, W.; Terlecki, M.; Wizner, B.; Rajzer, M.; Garlicki, A.; Sładek, K.; Krzanowska, K.; Wordliczek, J.; Krzanowski, M.; et al. Comparison between COVID 19 outcomes in the first 3 waves of the pandemic: A reference hospital report. Pol. Arch. Intern. Med. 2022, 132, 16286. [Google Scholar] [CrossRef]
- Dell’Antonio, L.S.; Leite, F.M.C.; Dell’Antonio, C.S.D.S.; Souza, C.B.; Garbin, J.R.T.; Santos, A.P.B.D.; Medeiros Junior, N.F.; Lopes-Júnior, L.C. COVID-19 Mortality in Public Hospitals in a Brazilian State: An Analysis of the Three Waves of the Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 14077. [Google Scholar] [CrossRef]
- Leidi, F.; Boari, G.E.M.; Scarano, O.; Mangili, B.; Gorla, G.; Corbani, A.; Accordini, B.; Napoli, F.; Ghidelli, C.; Archenti, G.; et al. Comparison of the characteristics, morbidity and mortality of COVID-19 between first and second/third wave in a hospital setting in Lombardy: A retrospective cohort study. Intern. Emerg. Med. 2022, 17, 1941–1949. [Google Scholar] [CrossRef]
- Marietta, M.; Vandelli, P.; Mighali, P.; Vicini, R.; Coluccio, V.; D’Amico, R.; COVID-19 HD Study Group. Randomised controlled trial comparing efficacy and safety of high versus low low-molecular weight heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): A structured summary of a study protocol. Trials 2020, 21, 574. [Google Scholar]
- Farmaci Utilizzabili per IL Trattamento DELLA Malattia COVID-19. Agenzia Italiana del Farmaco. Available online: https://aifa.gov.it/aggiornamento-sui-farmaci-utilizzabili-per-il-trattamento-dellamalattia-covid19 (accessed on 1 September 2022).
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A. Dexamethasone in hospitalized patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [PubMed]
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Haymet, A.; Bassi, G.L.; Fraser, J.F. Airborne spread of SARS-CoV-2 while using high-flow nasal cannula oxygen therapy: Myth or reality? Intensive Care. Med. 2020, 46, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Sartini, C.; Tresoldi, M.; Scarpellini, P.; Tettamanti, A.; Carcò, F.; Landoni, G.; Zangrillo, A. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA 2020, 323, 2338–2340. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, R.; Pisani, L.; Tabbì, L.; Comellini, V.; Prediletto, I.; Fantini, R.; Marchioni, A.; Andrisani, D.; Gozzi, F.; Bruzzi, G.; et al. Early awake proning in critical and severe COVID-19 patients undergoing noninvasive respiratory support: A retrospective multicenter cohort study. Pulmonology 2021, 28, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Calò, F.; Di Fraia, A.; Russo, A.; Di Biase, A.; Misso, S.; Coppola, N. Blood donor serological screening for SARS-CoV-2 as a tool to estimate the prevalence of asymptomatic infection in a low-intermediate endemic area of southern Italy after the first wave of the pandemic. Blood Transfus. 2022, 20, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Rome, Italy. 2022. Available online: https://www.salute.gov.it/portale/nuovocoronavirus/homeNuovoCoronavirus.jsp (accessed on 13 September 2022).
- Kokudo, N.; Sugiyama, H. Hospital capacity during the COVID-19 pandemic. Glob. Health Med. 2021, 3, 56–59. [Google Scholar] [CrossRef]
- Chang, A.Y.; Cullen, M.R.; Harrington, R.A.; Barry, M. The impact of novel coronavirus COVID-19 on noncommunicable disease patients and health systems: A review. J. Intern. Med. 2021, 289, 450–462. [Google Scholar] [CrossRef]
| Numbers of Data Available | |
|---|---|
| DEMOGRAPHIC VARIABLES | |
| N° (%) of males | 1241 (61.3) |
| Age, years, Median(Q1–Q3) | 63 (51–74) |
| Days of enrolment after onset of symptoms, Median (Q1–Q3) | 7 (3–10) |
| N° (%) of healthcare workers | 23 (1.4) |
| CLINICAL AND HEMATOBIOCHEMICAL VARIABLES | |
| Charlson comorbidity index, Median (Q1–Q3) | 2 (1–4) |
| N° (%) of subjects with underlying chronic disease | |
| With hypertension | 935 (46.2) |
| With cardio-vascular disease | 561 (27.7) |
| With diabetes | 406 (20) |
| With malignancy | 143 (7.1) |
| With chronic kidney disease | 173 (8.5) |
| With chronic obstructive pulmonary disease | 210 (10.4) |
| With hepatopathy | 68 (3.4) |
| N° (%) of asymptomatic subjects | 239 (11.8) |
| N° (%) of symptomatic patients | 1752 (86.5) |
| N° (%) of symptomatic subjects with | |
| Fever | 1170 (57.8) |
| Cough | 681 (33.6) |
| Dyspnea | 1232 (60.8) |
| hypo ageusia | 108 (5.3) |
| hypo-anosmia | 92 (4.5) |
| diarrhea | 90 (4.4) |
| cutaneous lesions | 10 (0.5) |
| WBC, Median (Q1–Q3) | 7910 (5680–10780) |
| INR, Median (Q1–Q3) | 1.1 (1.02–1.19) |
| AST, Median (Q1–Q3) | 31 (21.7–47) |
| ALT, Median (Q1–Q3) | 31 (20–53) |
| LDH, Median (Q1–Q3) | 300 (234–414) |
| Creatinine, Median (Q1–Q3) | 0.9 (0.7–1.1) |
| Total bilirubin, Median (Q1–Q3) | 0.6 (0.41–0.83) |
| PO2/FiO2, Median (Q1–Q3) | 230 (140–319) |
| OUTCOME | |
| Clinical outcome of COVID-19, n° (%) | |
| non-severe COVID-19 | 1393 (68.8) |
| severe COVID-19 | 622 (30.7) |
| Length of hospitalization expressed in days, median (Q1–Q3) | 14 (9–21) |
| Number of patients who died during hospitalization, N° (%) | 215 (10.6) |
| DEMOGRAPHIC VARIABLES | First Wave | Second Wave | Third Wave | p Value |
|---|---|---|---|---|
| N° of subjects | 392 | 917 | 706 | |
| N° (%) of males | 249 (63.5) | 561 (61.2) | 431 (61.0) | 0.680 a |
| Age, years, Median (Q1–Q3) | 60 (47–71) | 64 (52–75) | 63 (52–74) | 0.001 b |
| Days of enrolment after onset of symptoms, Median (Q1–Q3) | 5 (3–8) | 6 (2–10) | 8 (4–10) | 0.001 b |
| CLINICAL AND HEMATOBIOCHEMICAL VARIABLES | ||||
| Charlson comorbidity index, Median (Q1–Q3) | 2 (0–4) | 3 (1–5) | 2 (1–4) | 0.001 c |
| N° (%) of subjects with underlying chronic disease | ||||
| With hypertension | 163 (42) | 420 (46.2) | 352 (49.9) | 0.039 a |
| With cardio-vascular disease | 89 (22.9) | 281 (30.9) | 191 (27.01) | 0.011 a |
| With diabetes | 63 (16.2) | 212 (23.3) | 131 (18.6) | 0.006 a |
| With malignancy | 36 (9.3) | 68 (7.5) | 39 (5.5) | 0.061 a |
| With chronic kidney disease | 32 (8.2) | 100 (11) | 41 (5.8) | 0.001 a |
| With chronic obstructive pulmonary disease | 56 (14.4) | 98 (10.8) | 56 (8) | 0.003 a |
| With hepatopathy | 8 (2.1) | 31 (3.4) | 29 (4.1) | 0.196 a |
| N° (%) of asymptomatic subjects | 31 (8.4) | 149 (16.2) | 59 (8.4) | 0.001 a |
| N° (%) of symptomatic patients | 337 (91.6) | 768 (83.8) | 647 (91.6) | |
| N° (%) OF SYMPTOMATIC SUBJECTS WITH | ||||
| fever | 237 (70.5) | 511 (56.2) | 422 (60) | 0.001 a |
| cough | 141 (41.8) | 307 (33.8) | 233 (33.2) | 0.015 a |
| dyspnea | 140 (41.4) | 558 (61.4) | 534 (76.1) | 0.001 a |
| hypo ageusia | 50 (20) | 37 (4.1) | 21 (3.0) | 0.001 a |
| hypo-anosmia | 43 (16.9) | 37 (4.1) | 12 (1.7) | 0.001 a |
| diarrhea | 24 (8.6) | 41 (4.5) | 25 (3.6) | 0.003 a |
| cutaneous lesions | 3 (1.5) | 3 (0.3) | 4 (0.6) | 0.144 a |
| WBC, Median (Q1–Q3) | 6660 (4010–9210) | 8285 (5900–11,090) | 7525 (5420–10,400) | 0.001 c |
| INR, Median (Q1–Q3) | 1.16 (1.06–1.29) | 1.09 (1.01–1.2) | 1.1 (1.03–1.18) | 0.113 c |
| AST, Median (Q1–Q3) | 19 (16–27) | 30 (20–44) | 34 (24–49) | 0.001 c |
| ALT, Median (Q1–Q3) | 23 (13–37.5) | 28 (20–52) | 34 (22–57) | 0.004 c |
| LDH, Median (Q1–Q3) | 198.5 (151–308) | 301 (232–419) | 301.5 (239–411) | 0.008 c |
| Creatinine, Median (Q1–Q3) | 0.8 (0.7–1.01) | 0.88 (0.7–1.14) | 0.9 (0.71–1.09) | 0.835 c |
| Total bilirubin, Median (Q1–Q3) | 0.59 (0.4–1.13) | 0.6 (0.4–0.85) | 0.58 (0.41–0.8) | 0.811 c |
| PO2/FiO2, Median (Q1–Q3) | 257.5 (119–363) | 240 (147–328) | 214.5 (133–305) | 0.027 c |
| OUTCOME | ||||
| Length of hospitalization expressed in days, median (Q1–Q3) | 17 (13–25) | 14 (9–21) | 14 (9–19) | 0.001 c |
| Clinical outcome of COVID-19, N° (%) | ||||
| non-severe COVID-19 | 287 (73.2) | 599 (65.3) | 503 (71.8) | 0.003 a |
| severe COVID-19 | 105 (26.8) | 318 (34.7) | 199 (28.2) | 0.003 a |
| Number of patients who died during hospitalization, N° (%) | 65 (16.6) | 104 (11.3) | 46 (6.5) | 0.001 a |
| Patients Death in First Wave | Patients Death in Second Wave | Patients Death in Third Wave | p Value | |
|---|---|---|---|---|
| N° of subjects | 65 | 104 | 46 | |
| N° (%) of males | 47 (72.3) | 58 (55.8) | 23 (50) | 0.034 a |
| Age, years, mean (SD) | 77 (66–82) | 81 (72–86) | 84 (75–88) | 0.001 b |
| Days of enrolment after onset of symptoms, median (Q1–Q3) | 5 (2.5–7) | 3 (0–7) | 3 (0–8) | 0.090 b |
| Charlson comorbidity index, median (Q1–Q3) | 3 (2–6) | 5 (4–7) | 6 (5–6) | 0.001 c |
| N° (%) of subjects with underlying chronic disease | ||||
| With hypertension | 34 (54.8) | 59 (57.3) | 26 (56.5) | 0.892 a |
| With cardio-vascular disease | 30 (48.4) | 53 (51.5) | 26 (56.5) | 0.704 a |
| With diabetes | 16 (25.8) | 37 (35.9) | 16 (34.8) | 0.384 a |
| With malignancy | 14 (22.6) | 13 (12.6) | 5 (10.9) | 0.148 a |
| With chronic kidney disease | 9 (14.5) | 25 (24.3) | 10 (21.7) | 0.323 a |
| With chronic obstructive pulmonary disease | 14 (22.6) | 23 (22.3) | 6 (13) | 0.377 a |
| With hepatopathy | 2 (3.2) | 8 (7.8) | 0 (0) | 0.093 a |
| N° (%) of asymptomatic subjects | 1 (1.6) | 16 (15.4) | 4 (8.7) | 0.014 a |
| N° (%) of symptomatic patients | 62 (98.4) | 88 (84.6) | 42 (91.3) | |
| N° (%) of symptomatic subjects with | ||||
| fever | 17 (70.8) | 42 (40.8) | 24 (53.3) | 0.022 a |
| cough | 10 (41.7) | 20 (19.6) | 9 (20) | 0.060 a |
| dyspnea | 11 (45.8) | 76 (74.5) | 36 (80) | 0.007 a |
| hypo ageusia | 0 (0) | 1 (1.0) | 0 (0) | 0.739 a |
| hypo-anosmia | 0 (0) | 1 (1) | 0 (0) | 0.739 a |
| diarrhea | 3 (14.3) | 1 (1.0) | 0 (0) | 0.001 a |
| cutaneous lesions | 0 (0) | 1 (1) | 0 (0) | 0.743 a |
| Length of hospitalization expressed in days, median (Q1–Q3) | 6 (2–22) | 10 (5–15) | 8 (4–12) | 0.150 b |
| OR | 95% Lower Confident Interval | 95% Lower Confident Interval | p Value | |
|---|---|---|---|---|
| Age, years * | 1.107 | 10.080 | 1.135 | 0.001 |
| Charlson comorbidity index * | 1.135 | 1.022 | 1.260 | 0.018 |
| Third wave reference value | ||||
| second wave | 1.078 | 0.634 | 1.834 | 0.781 |
| first wave | 1.917 | 1.054 | 3.485 | 0.033 |
| Days of enrolment after onset of symptoms * | 0.965 | 0.921 | 1.010 | 0.127 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisaturo, M.; Russo, A.; Pattapola, V.; Astorri, R.; Maggi, P.; Numis, F.G.; Gentile, I.; Sangiovanni, V.; Rossomando, A.; Gentile, V.; et al. Clinical Characterization of the Three Waves of COVID-19 Occurring in Southern Italy: Results of a Multicenter Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 16003. https://doi.org/10.3390/ijerph192316003
Pisaturo M, Russo A, Pattapola V, Astorri R, Maggi P, Numis FG, Gentile I, Sangiovanni V, Rossomando A, Gentile V, et al. Clinical Characterization of the Three Waves of COVID-19 Occurring in Southern Italy: Results of a Multicenter Cohort Study. International Journal of Environmental Research and Public Health. 2022; 19(23):16003. https://doi.org/10.3390/ijerph192316003
Chicago/Turabian StylePisaturo, Mariantonietta, Antonio Russo, Viraj Pattapola, Roberta Astorri, Paolo Maggi, Fabio Giuliano Numis, Ivan Gentile, Vincenzo Sangiovanni, Annamaria Rossomando, Valeria Gentile, and et al. 2022. "Clinical Characterization of the Three Waves of COVID-19 Occurring in Southern Italy: Results of a Multicenter Cohort Study" International Journal of Environmental Research and Public Health 19, no. 23: 16003. https://doi.org/10.3390/ijerph192316003
APA StylePisaturo, M., Russo, A., Pattapola, V., Astorri, R., Maggi, P., Numis, F. G., Gentile, I., Sangiovanni, V., Rossomando, A., Gentile, V., Calabria, G., Pisapia, R., Codella, A. V., Masullo, A., Iodice, V., Giolitto, G., Parrella, R., Dell’Aquila, G., Gambardella, M., ... Coppola, N., on behalf of CoviCam Group. (2022). Clinical Characterization of the Three Waves of COVID-19 Occurring in Southern Italy: Results of a Multicenter Cohort Study. International Journal of Environmental Research and Public Health, 19(23), 16003. https://doi.org/10.3390/ijerph192316003








