Black Plastic Film Mulching Increases Soil Nitrous Oxide Emissions in Arid Potato Fields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experimental Design
2.2. Soil Temperature and Moisture Measurement
2.3. N2O Flux Sampling
2.4. Soil Gas (CO2 and N2O) Sampling
2.5. DNA Extraction and Quantitative PCR
2.6. Statistical Analysis
3. Results
3.1. N2O Flux and Soil Temperature
3.2. Soil N2O Flux Variation between Two Irrigation Events
3.3. Soil Gas Variation
3.4. Soil N2O-Related Microbial Genes
3.5. Seasonal N2O Fluxes
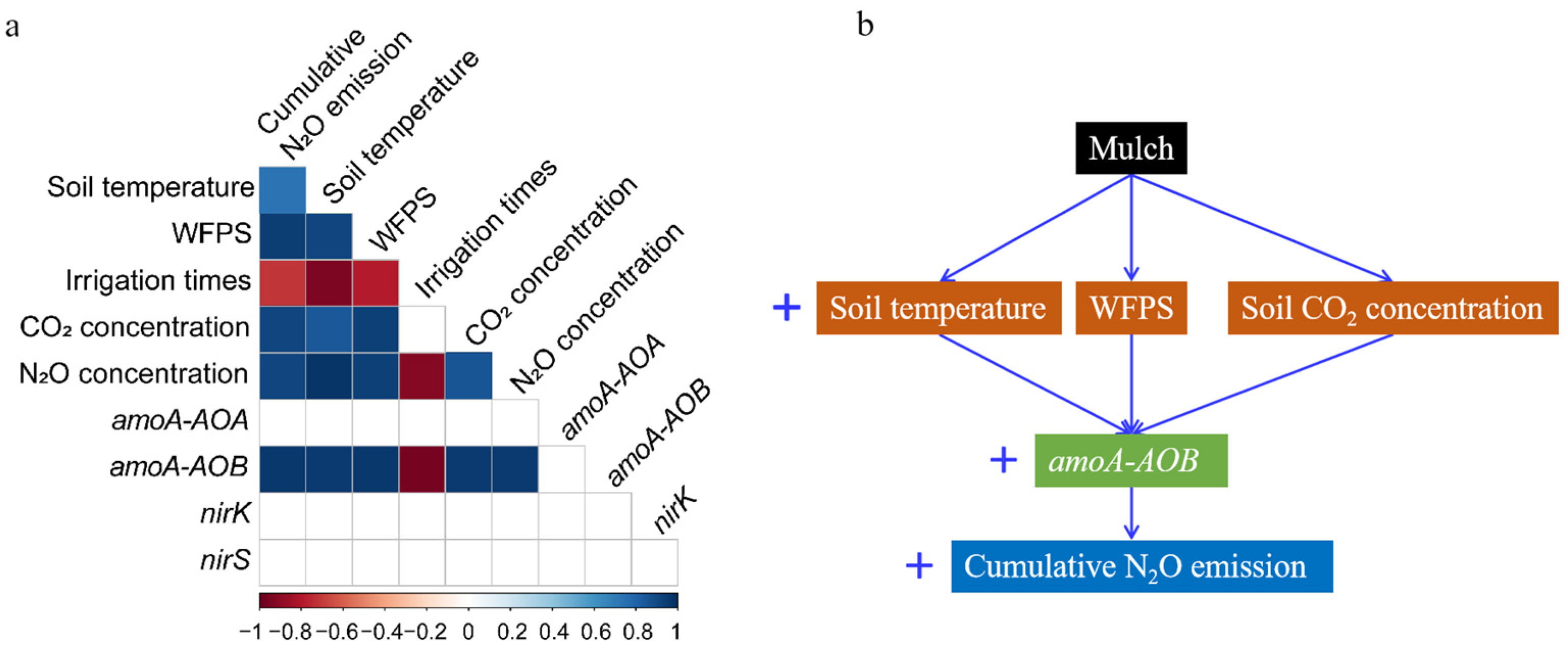
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change 2007: The Physical Science Basis; Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; 996p. [Google Scholar]
- WMO. WMO Greenhouse Gas Bulletin (GHG Bulletin)—No. 14: The State of Greenhouse Gases in the Atmosphere Based on Global Observations through 2017; WMO: Geneva, Switzerland, 2018. [Google Scholar]
- Reay, D.S.; Davidson, E.A.; Smith, K.A.; Smith, P.; Melillo, J.M.; Dentener, F.; Crutzen, P.J. Global agriculture and nitrous oxide emissions. Nat. Clim. Chang. 2012, 2, 410–416. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2007: Mitigation; Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Metz, B., Davidson, O.R., Bosch, P.R., Dave, R., Meyer, L.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- FAO. FAOSTAT Online Database. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data/RA (accessed on 3 November 2022).
- Li, Q.; Li, H.; Zhang, L.; Zhang, S.; Chen, Y. Mulching improves yield and water-use efficiency of potato cropping in China: A meta-analysis. Field Crop Res. 2018, 221, 50–60. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Tam, H.M.; Wani, S.P.; Long, T.D. Effect of mulch on soil temperature, moisture, weed infestation and yield of groundnut in northern Vietnam. Field Crop Res. 2006, 95, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Meng, C.; Wang, F.; Engel, B.A.; Yang, K.; Zhang, Y. Is Cattle Manure Application with Plastic-Film Mulch a Good Choice for Potato Production? Agronomy 2019, 9, 534. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Feng, S.; Hou, X.; Kang, S.; Han, J. Potato growth with and without plastic mulch in two typical regions of Northern China. Field Crop Res. 2009, 110, 123–129. [Google Scholar] [CrossRef]
- Zhao, M.; Jiang, C.; Li, X.; He, X.; Hao, Q. Variations in nitrous oxide emissions as manipulated by plastic film mulching and fertilization over three successive years in a hot pepper-radish rotated vegetable production system. Agric. Ecosyst. Environ. 2020, 304, 107127. [Google Scholar] [CrossRef]
- Duan, P.; Wu, Z.; Zhang, Q.; Fan, C.; Xiong, Z. Thermodynamic responses of ammonia-oxidizing archaea and bacteria explain N2O production from greenhouse vegetable soils. Soil Biol. Biochem. 2018, 120, 37–47. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Shock, C.C.; Yang, K.; Kang, S.; Qin, J.; Li, S. Effects of plastic mulch on the radiative and thermal conditions and potato growth under drip irrigation in arid Northwest China. Soil Tillage Res. 2017, 172, 1–11. [Google Scholar] [CrossRef]
- Yu, Y.; Jia, H.; Zhao, C. Evaluation of the effects of plastic mulching and nitrapyrin on nitrous oxide emissions and economic parameters in an arid agricultural field. Geoderma 2018, 324, 98–108. [Google Scholar] [CrossRef]
- Ding, D.; Zhao, Y.; Feng, H.; Hill, R.L.; Chu, X.; Zhang, T.; He, J. Soil water utilization with plastic mulching for a winter wheat-summer maize rotation system on the Loess Plateau of China. Agric. Water Manag. 2018, 201, 246–257. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhai, X.; Wang, Z.; Li, H.; Jiang, R.; Hill, R.L.; Si, B.; Hao, F. Simulation of soil water and heat flow in ridge cultivation with plastic film mulching system on the Chinese Loess Plateau. Agric. Water Manag. 2018, 202, 99–112. [Google Scholar] [CrossRef]
- Ma, D.; Chen, L.; Qu, H.; Wang, Y.; Misselbrook, T.; Jiang, R. Impacts of plastic film mulching on crop yields, soil water, nitrate, and organic carbon in Northwestern China: A meta-analysis. Agric. Water Manag. 2018, 202, 166–173. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Xing, Y. Effects of mulching and nitrogen on soil temperature, water content, nitrate-N content and maize yield in the Loess Plateau of China. Agric. Water Manag. 2015, 161, 53–64. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Y.; Zhang, Q.; Liu, X.; Xu, J.; Li, Y.; Di, H. Heterotrophic nitrification and denitrification are the main sources of nitrous oxide in two paddy soils. Plant Soil 2019, 445, 39–53. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C.; Podolyan, A.; Robinson, A. Effect of soil moisture status and a nitrification inhibitor, dicyandiamide, on ammonia oxidizer and denitrifier growth and nitrous oxide emissions in a grassland soil. Soil Biol. Biochem. 2014, 73, 59–68. [Google Scholar] [CrossRef]
- Shi, X.; Hu, H.; Zhu-Barker, X.; Hayden, H.; Wang, J.; Suter, H.; Chen, D.; He, J. Nitrifier-induced denitrification is an important source of soil nitrous oxide and can be inhibited by a nitrification inhibitor 3,4-dimethylpyrazole phosphate. Environ. Microbiol. 2017, 19, 4851–4865. [Google Scholar] [CrossRef]
- Meng, C.; Wang, F.; Yang, K.; Shock, C.C.; Engel, B.A.; Zhang, Y.; Tao, L.; Gu, X. Small wetted proportion of drip irrigation and non-mulched treatment with manure application enhanced methane uptake in upland field. Agric. For. Meteorol. 2020, 281, 107821. [Google Scholar] [CrossRef]
- Das, S.; Adhya, T.K. Dynamics of methanogenesis and methanotrophy in tropical paddy soils as influenced by elevated CO2 and temperature interaction. Soil Biol. Biochem. 2012, 47, 36–45. [Google Scholar] [CrossRef]
- Kim, G.W.; Das, S.; Hwang, H.Y.; Kim, P.J. Nitrous oxide emissions from soils amended by cover-crops and under plastic film mulching: Fluxes, emission factors and yield-scaled emissions. Atmos. Environ. 2017, 152, 377–388. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, L.; Luo, S.; Bu, L.; Chen, X.; Yue, S.; Li, S. Response of nitrous oxide emission to soil mulching and nitrogen fertilization in semi-arid farmland. Agric. Ecosyst. Environ. 2014, 188, 20–28. [Google Scholar] [CrossRef]
- Li, Y.; Guan, K.; Peng, B.; Franz, T.E.; Wardlow, B.; Pan, M. Quantifying irrigation cooling benefits to maize yield in the US Midwest. Global Chang. Biol. 2020, 26, 3065–3078. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; Zhao, J.; Shi, Y.; Yu, Z. Nitrogen use by winter wheat and changes in soil nitrate nitrogen levels with supplemental irrigation based on measurement of moisture content in various soil layers. Field Crop Res. 2014, 164, 117–125. [Google Scholar] [CrossRef]
- Luo, G.; Kiese, R.; Wolf, B.; Butterbach-Bahl, K. Effects of soil temperature and moisture on methane uptakes and nitrous oxide emissions across three different ecosystem types. Biogeosci. Discuss. 2013, 10, 927–965. [Google Scholar] [CrossRef] [Green Version]
- Lark, R.M.; Milne, A.E. Boundary line analysis of the effect of water-filled pore space on nitrous oxide emission from cores of arable soil. Eur. J. Soil Sci. 2016, 67, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.M.; Hu, H.W.; Shen, J.P.; He, J.Z. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2012, 6, 1032–1045. [Google Scholar] [CrossRef] [Green Version]
- Baker, B.J.; De Anda, V.; Seitz, K.W.; Dombrowski, N.; Santoro, A.E.; Lloyd, K.G. Diversity, ecology and evolution of Archaea. Nat. Microbiol. 2020, 5, 887–900. [Google Scholar] [CrossRef]
- Hink, L.; Nicol, G.W.; Prosser, J.I. Archaea produce lower yields of N2O than bacteria during aerobic ammonia oxidation in soil. Environ. Microbiol. 2017, 19, 4829–4837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hink, L.; Gubry-Rangin, C.; Nicol, G.W.; Prosser, J.I. The consequences of niche and physiological differentiation of archaeal and bacterial ammonia oxidisers for nitrous oxide emissions. ISME J. 2018, 12, 1084–1093. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Xiao, C.; Guenet, B.; Li, M.; Xu, L.; He, N. Short-term effects of labile organic C addition on soil microbial response to temperature in a temperate steppe. Soil Biol. Biochem. 2022, 167, 108589. [Google Scholar] [CrossRef]
- Hursh, A.; Ballantyne, A.; Cooper, L.; Maneta, M.; Kimball, J.; Watts, J. The sensitivity of soil respiration to soil temperature, moisture, and carbon supply at the global scale. Glob. Chang. Biol. 2017, 23, 2090–2103. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Broughton, K.; Osanai, Y.; Anderson, I.C.; Bange, M.P.; Tissue, D.T.; Singh, B.K. Effects of elevated temperature and elevated CO2 on soil nitrification and ammonia-oxidizing microbial communities in field-grown crop. Sci. Total Environ. 2019, 675, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, K.; Guo, Z.; Liu, X.; Bian, R.; Sun, B.; Li, J.; Chen, J. An antagonistic effect of elevated CO2 and warming on soil N2O emissions related to nitrifier and denitrifier communities in a Chinese wheat field. Plant Soil 2022, 470, 97–110. [Google Scholar] [CrossRef]
- Shen, L.; Yang, Y.; Liu, J.; Hu, Z.; Liu, X.; Tian, M.; Yang, W.; Jin, J.; Wang, H.; Wang, Y.; et al. Different responses of ammonia-oxidizing archaea and bacteria in paddy soils to elevated CO2 concentration. Environ. Pollut. 2021, 286, 117558. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Ju, X.; Topp, C.F.E.; Rees, R.M. Oxygen Regulates Nitrous Oxide Production Directly in Agricultural Soils. Environ. Sci. Technol. 2019, 53, 12539–12547. [Google Scholar] [CrossRef]
- Shudan, B. Soil Agro-Chemistry Analysi; China Agriculture Press: Beijing, China, 2005. [Google Scholar]
- Francis, C.A.; Roberts, K.J.; Beman, J.M.; Santoro, A.E.; Oakley, B.B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. USA 2005, 102, 14683–14688. [Google Scholar] [CrossRef] [Green Version]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microb. 1997, 63, 4704–4712. [Google Scholar] [CrossRef] [Green Version]
- Throbäck, I.N.; Enwall, K.; Jarvis, Ã.S.; Hallin, S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. Fems. Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef]
- Hallin, S.; Lindgren, P. PCR Detection of Genes Encoding Nitrite Reductase in Denitrifying Bacteria. Appl. Environ. Microb. 1999, 65, 1652–1657. [Google Scholar] [CrossRef]
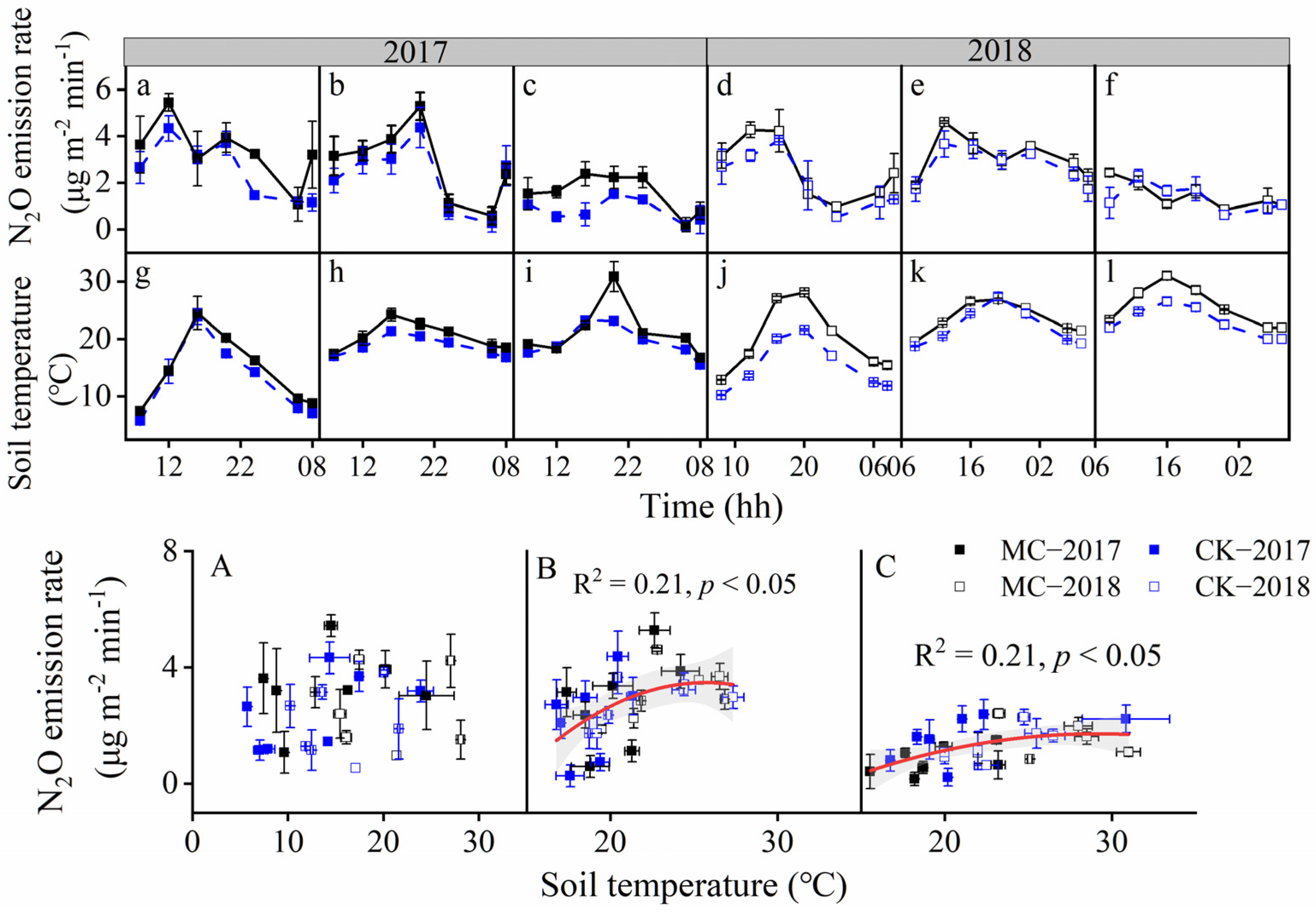
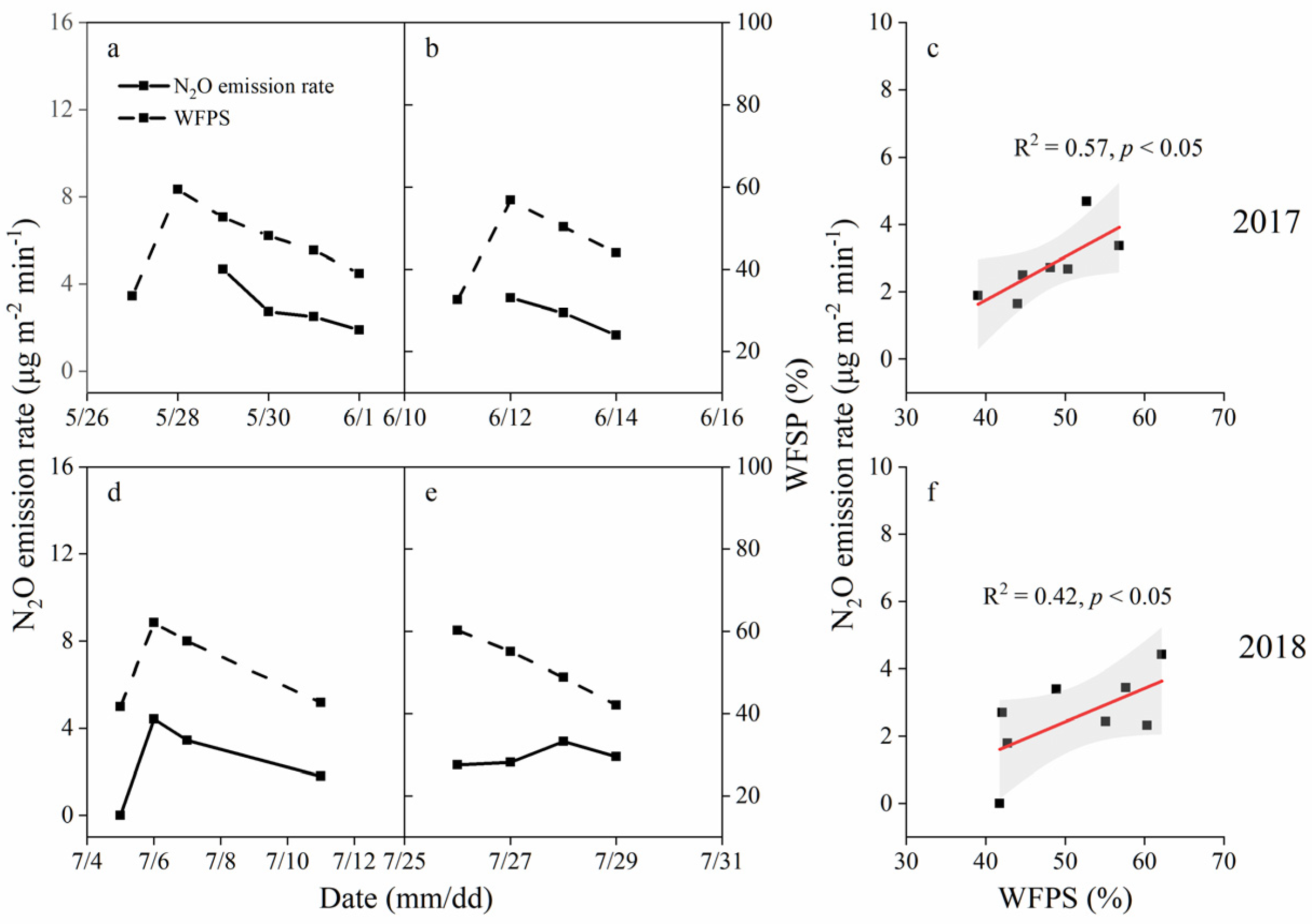
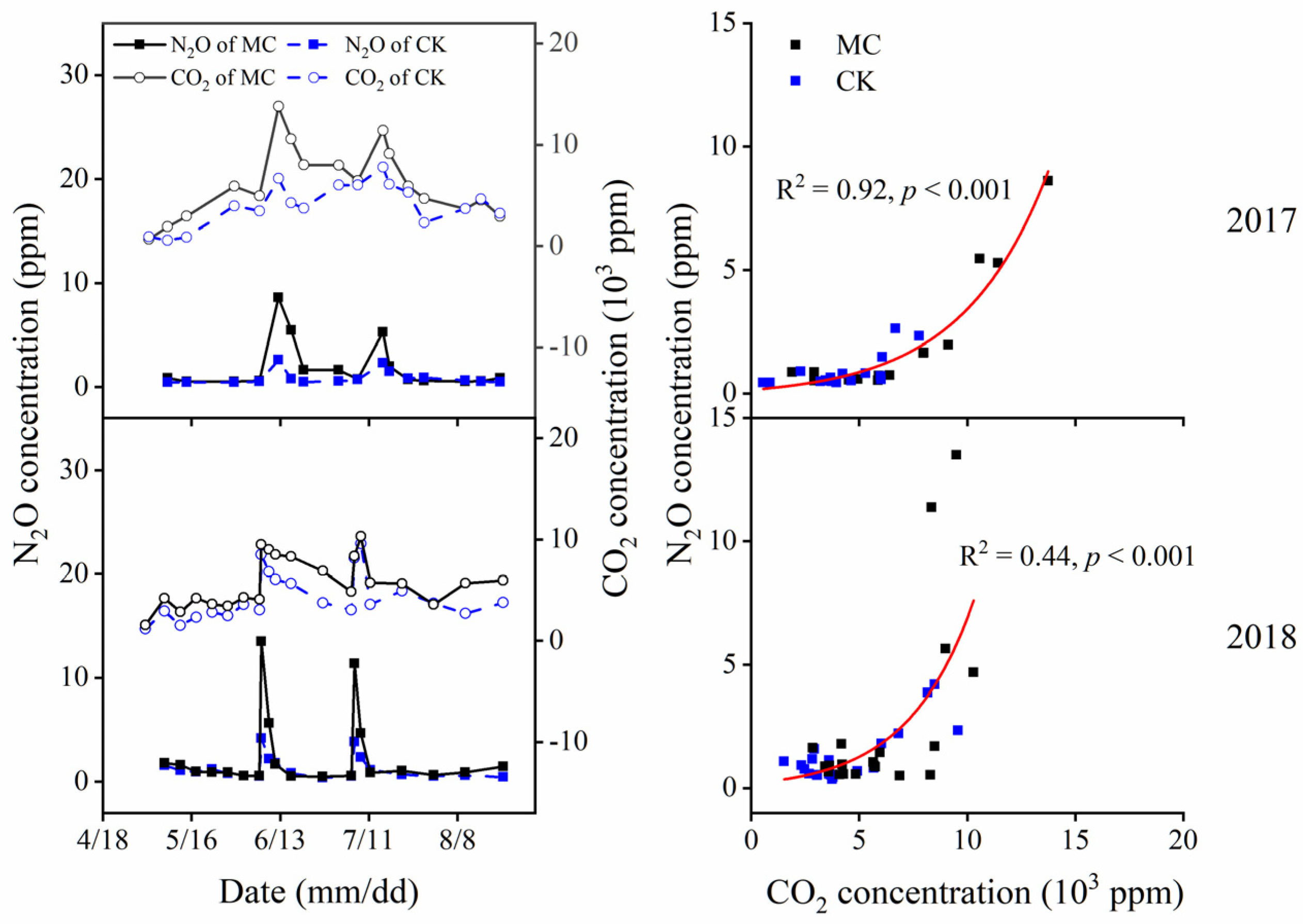
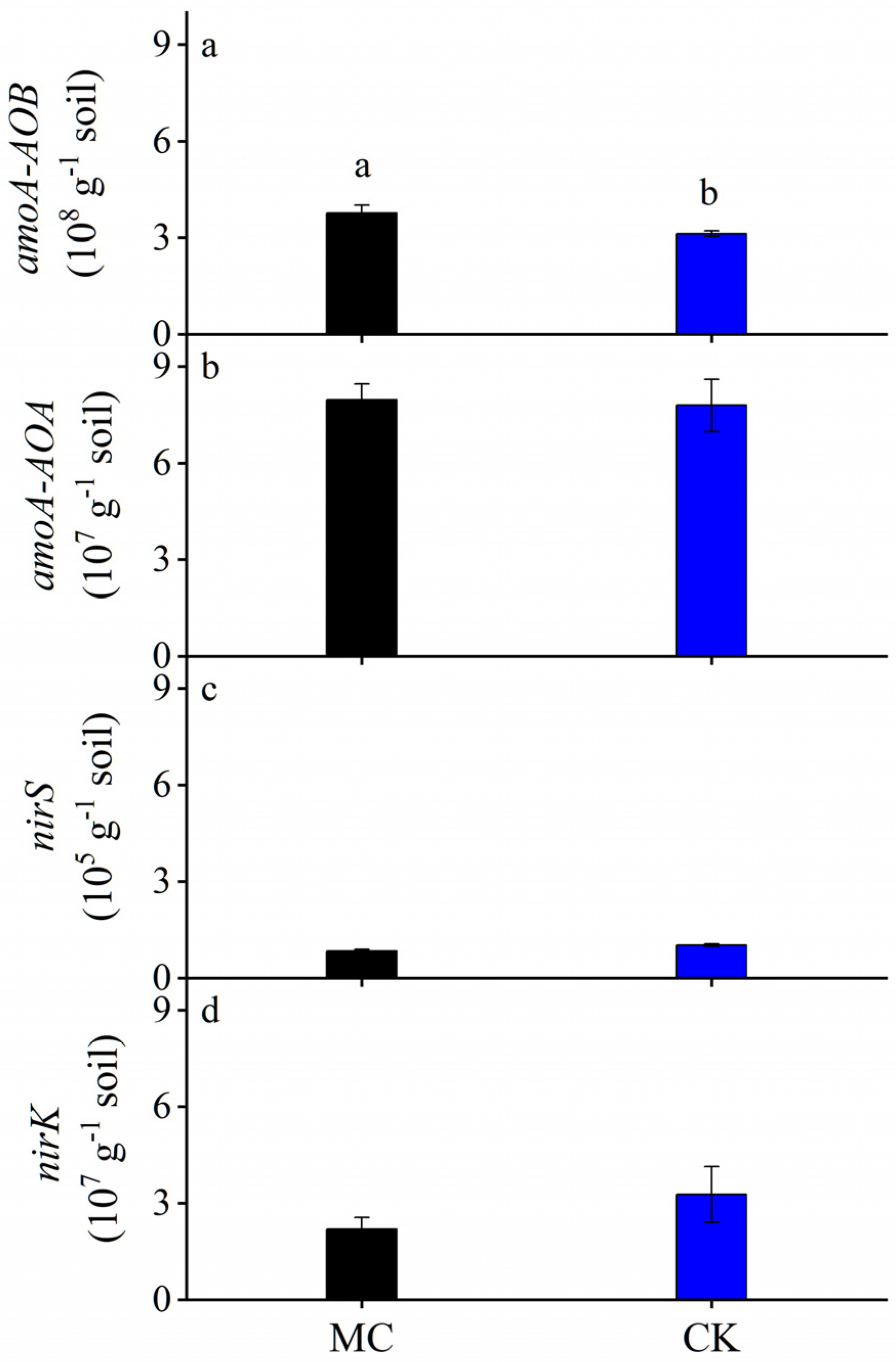

| Year | Parameter | MC 1 | CK | ANOVA |
|---|---|---|---|---|
| 2017 | Cumulative N2O emission (kg hm−2) | 5.56 ± 0.15 a | 4.58 ± 0.25 b | * |
| Soil temperature (°C) | 20.7 ± 0.5 a | 18.2 ± 0.3 b | * | |
| WFPS (%) | 48.4 ± 0.4 | 46.1 ± 0.2 | * | |
| 2018 | Cumulative N2O emission (kg hm−2) | 5.77 ± 0.13 a | 4.57 ± 0.28 b | * |
| Soil temperature (°C) | 21.6 ± 0.0 a | 19.6 ± 0.3 b | * | |
| WFPS (%) | 48.4 ± 0.1 | 46.7 ± 0.1 | * | |
| amoA-AOA (107 g−1 soil) | 7.96 ± 0.50 | 7.80 ± 0.85 | ns | |
| amoA-AOB (108 g−1 soil) | 3.76 ± 0.20 a | 3.12 ± 0.04 b | * | |
| nirK (105 g−1 soil) | 7.34 ± 0.07 | 7.51 ± 0.12 | ns | |
| nirS (107 g−1 soil) | 4.91 ± 0.03 | 5.00 ± 0.02 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, C.; Zhao, J.; Wang, N.; Yang, K.; Wang, F. Black Plastic Film Mulching Increases Soil Nitrous Oxide Emissions in Arid Potato Fields. Int. J. Environ. Res. Public Health 2022, 19, 16030. https://doi.org/10.3390/ijerph192316030
Meng C, Zhao J, Wang N, Yang K, Wang F. Black Plastic Film Mulching Increases Soil Nitrous Oxide Emissions in Arid Potato Fields. International Journal of Environmental Research and Public Health. 2022; 19(23):16030. https://doi.org/10.3390/ijerph192316030
Chicago/Turabian StyleMeng, Chaobiao, Jianyu Zhao, Ning Wang, Kaijing Yang, and Fengxin Wang. 2022. "Black Plastic Film Mulching Increases Soil Nitrous Oxide Emissions in Arid Potato Fields" International Journal of Environmental Research and Public Health 19, no. 23: 16030. https://doi.org/10.3390/ijerph192316030
APA StyleMeng, C., Zhao, J., Wang, N., Yang, K., & Wang, F. (2022). Black Plastic Film Mulching Increases Soil Nitrous Oxide Emissions in Arid Potato Fields. International Journal of Environmental Research and Public Health, 19(23), 16030. https://doi.org/10.3390/ijerph192316030





