Mortality Trends Due to Skin Melanoma in Poland in the Years 2000–2020
Abstract
:1. Introduction
2. Materials and Methods
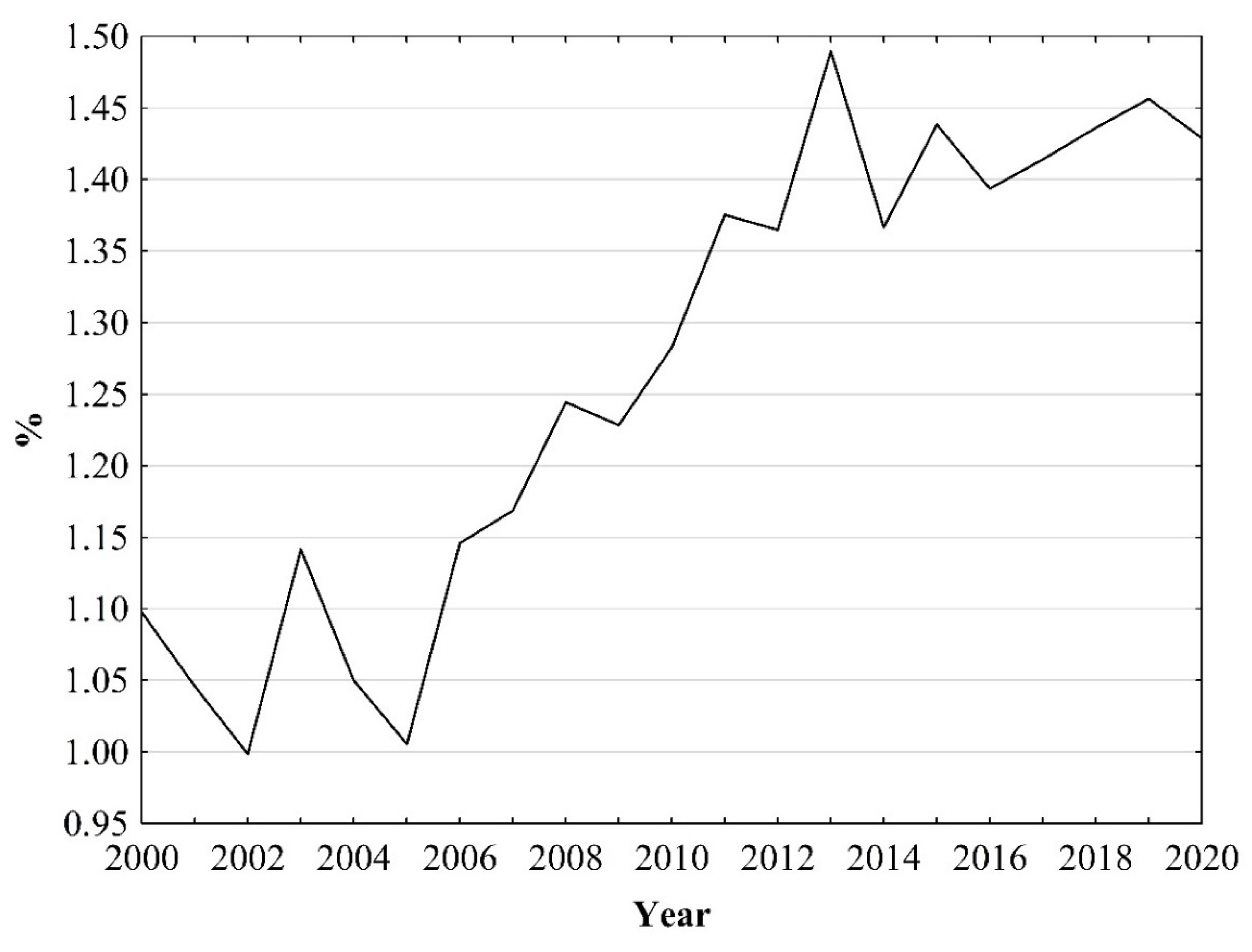
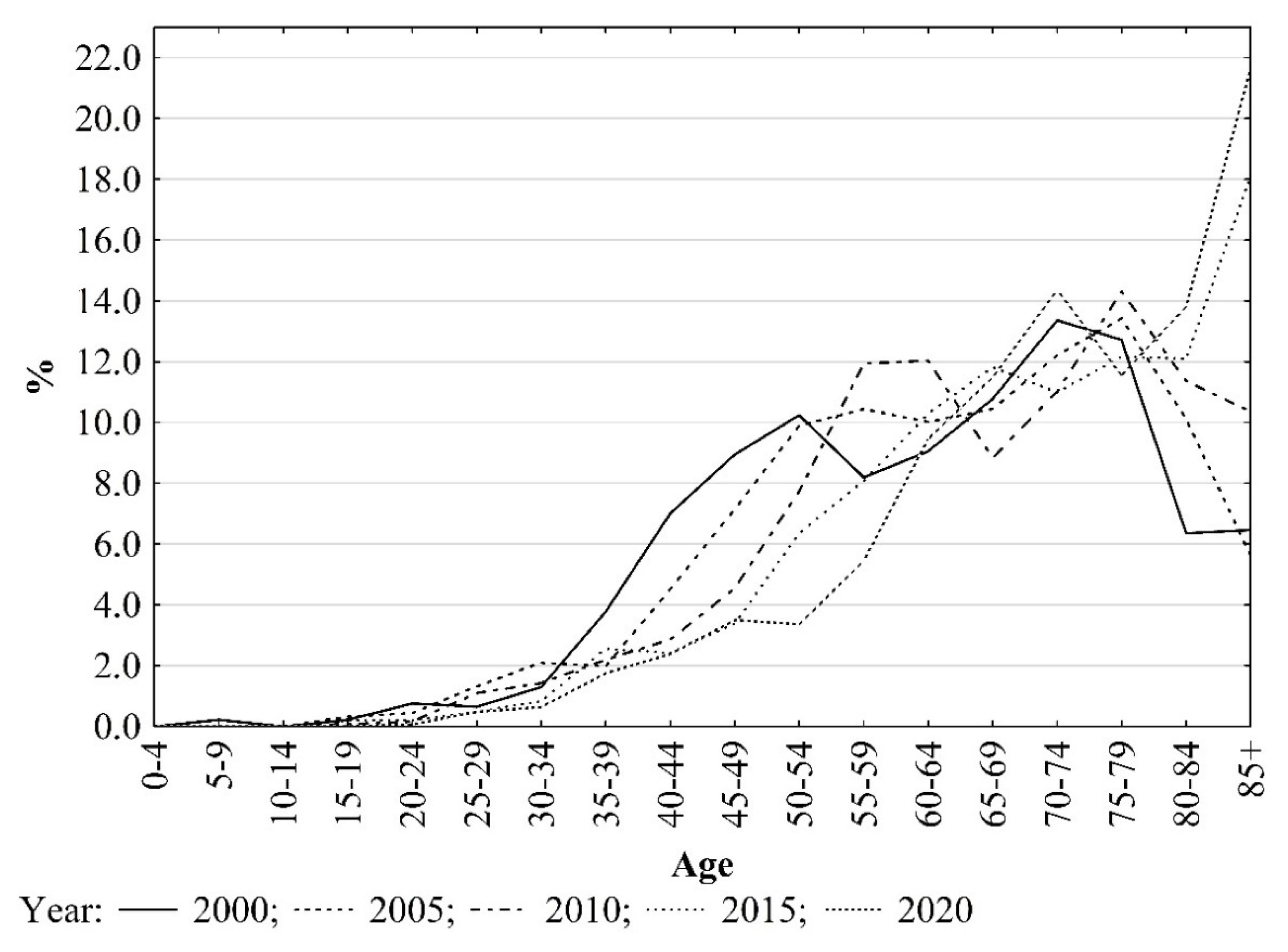
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, Z.; Yousaf, N.; Larkin, J. Melanoma epidemiology, biology and prognosis. EJC Suppl. 2013, 11, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- 2O2O Melanoma Skin Cancer Report. Stemming the Global epidemic. Global Coalition for Melanoma Patient Advocacy. Available online: https://spotthedot.org/wp2019/wp-content/uploads/2020/04/2020-campaign-report-GC-version-FINAL.pdf (accessed on 12 May 2022).
- Rastrelli, M.; Tropea, S.; Rossi, C.R.; Alaibac, M. Melanoma: Epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo 2014, 28, 1005–1011. [Google Scholar] [PubMed]
- Osterlind, A. Epidemiology on malignant melanoma in Europe. Acta Oncol. 1992, 31, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Markovic, S.N.; Erickson, L.A.; Rao, R.D.; Weenig, R.H.; Pockaj, B.A.; Bardia, A.; Vachon, C.M.; Schild, S.E.; McWilliams, R.R.; Hand, J.L.; et al. Malignant melanoma in the 21st century, part 1: Epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin. Proc. 2007, 82, 364–380. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- How does the Sun Cause Cancer? Worldwide Cancer Research. Available online: https://www.worldwidecancerresearch.org/news-opinion/2022/march/how-does-the-sun-cause-skin-cancer/ (accessed on 30 May 2022).
- Armstrong, B.K.; Kricker, A. How much melanoma is caused by sun exposure? Melanoma Res. 1993, 3, 395–401. [Google Scholar] [CrossRef]
- Autier, P. Cutaneous malignant melanoma: Facts about sunbeds and sunscreen. Expert Rev. Anticancer Ther. 2005, 5, 821–833. [Google Scholar] [CrossRef]
- Erdmann, F.; Lortet-Tieulent, J.; Schüz, J.; Zeeb, H.; Greinert, R.; Breitbart, E.W.; Bray, F. International trends in the incidence of malignant melanoma 1953–2008—Are recent generations at higher or lower risk? Int. J. Cancer 2013, 132, 385–400. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Didkowska, J.; Wojciechowska, U.; Olasek, P.; Caetano dos Santos, F.; Michałek, I. Nowotwory złośliwe w Polsce w 2019 roku. Cancer in Poland in 2019. Krajowy Rejestr Nowotworów. Narodowy Instytut Onkologii im. Marii Skłodowskiej-Curie. Państwowy Instytut Badawczy. Warszawa 2021. Available online: http://onkologia.org.pl/wp-content/uploads/Nowotwory_2019.pdf (accessed on 8 June 2022).
- Cancer Tooday. International Agency for Research on Cancer. WHO. Available online: https://gco.iarc.fr/today/online-analysis-sunburst?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=16&type=1&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=0&include_nmsc_other=1 (accessed on 29 April 2022).
- Cancer over Time. International Agency for Research on Cancer. WHO. Available online: https://gco.iarc.fr/overtime/en/dataviz/trends?populations=61600&sexes=1_2&types=1&multiple_populations=1&cancers=12&key=total&mode=population&multiple_cancers=0 (accessed on 29 April 2022).
- Arnold, M.; Holterhues, C.; Hollestein, L.M.; Coebergh, J.W.; Nijsten, T.; Pukkala, E.; Holleczek, B.; Tryggvadóttir, L.; Comber, H.; Bento, M.J.; et al. Trends in incidence and predictions of cutaneous melanoma across Europe up to 2015. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Briatico, G.; Mancuso, P.; Argenziano, G.; Longo, C.; Mangone, L.; Moscarella, E.; Brancaccio, G.; Pampena, R. Trends in cutaneous melanoma mortality in Italy from 1982 to 2016. Int. J. Derematol. 2022, 61, 1237–1244. [Google Scholar] [CrossRef]
- Forsea, A.M.; Del Marmol, V.; de Vries, E.; Bailey, E.E.; Geller, A.C. Melanoma incidence and mortality in Europe: New estimates, persistent disparities. Br. J. Dermatol. 2012, 167, 1124–1130. [Google Scholar] [CrossRef]
- Barbaric, J.; Sekerija, M.; Agius, D.; Coza, D.; Dimitrova, N.; Demetriou, A.; Safaei Diba, C.; Eser, S.; Gavric, Z.; Primic-Zakelj, M.; et al. Disparities in melanoma incidence and mortality in South-Eastern Europe: Increasing incidence and divergent mortality patterns. Is progress around the corner? Eur. J. Cancer 2016, 55, 47–55. [Google Scholar] [CrossRef] [PubMed]
- de Vries, E.; Bray, F.I.; Coebergh, J.W.; Parkin, D.M. Changing epidemiology of malignant cutaneous melanoma in Europe 1953–1997: Rising trends in incidence and mortality but recent stabilizations in western Europe and decreases in Scandinavia. Int. J. Cancer 2003, 107, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, U.; Didkowska, J.; Michałek, I.; Olasek, P.; Ciuba, A. Nowotwory Złośliwe w Polsce w 2018 Roku. Cancer in Poland in 2018. Krajowy Rejestr Nowotworów. Narodowy Instytut Onkologii im. Marii Skłodowskiej-Curie. Państwowy Instytut Badawczy. Warszawa 2020. Available online: http://onkologia.org.pl/wp-content/uploads/Nowotwory_2018.pdf (accessed on 10 June 2022).
- Czerniak Skóry (C43). KRN. Available online: http://onkologia.org.pl/czerniak-skory-c43/#e (accessed on 1 June 2022).
- Causes of Death-Standarized Death Rate by NUTS 2 Region of Residence. Eurostat. Available online: https://ec.europa.eu/eurostat/databrowser/view/HLTH_CD_ASDR2__custom_2977926/default/table?lang=en (accessed on 26 May 2022).
- Bank Danych Lokalnych. Główny Urząd Statystyczny. Available online: https://bdl.stat.gov.pl/bdl/dane/podgrup/wymiary/3/7/2137 (accessed on 10 April 2022).
- Revision of the European Standard Population. Available online: https://ec.europa.eu/eurostat/documents/3859598/5926869/KS-RA-13-028-EN.PDF/e713fa79-1add-44e8-b23d-5e8fa09b3f8f (accessed on 20 April 2022).
- Joinpoint Regression Program, Version 4.0.4, Statistical Methodology and Applications Branch, Surveillance Research Program; National Cancer Institute: Bethesda, MD, USA, 2013.
- Kim, H.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Yang, D.D.; Salciccioli, J.D.; Marshall, D.C.; Sheri, A.; Shalhoub, J. Trends in malignant melanoma mortality in 31 countries from 1985 to 2015. Br. J. Dermatol. 2020, 183, 1056–1064. [Google Scholar] [CrossRef]
- Leiter, U.; Keim, U.; Garbe, C. Epidemiology of Skin Cancer: Update 2019. Adv. Exp. Med. Biol. 2020, 1268, 123–139. [Google Scholar] [CrossRef]
- Karimkhani, C.; Green, A.C.; Nijsten, T.; Weinstock, M.A.; Dellavalle, R.P.; Naghavi, M.; Fitzmaurice, C. The global burden of melanoma: Results from the Global Burden of Disease Study 2015. Br. J. Dermatol. 2017, 177, 134–140. [Google Scholar] [CrossRef]
- Khazaei, Z.; Ghorat, F.; Jarrahi, A.M.; Adineh, H.A.; Sohrabivafa, M.; Goodarzi, E. Global Incidence and Mortality of Skin Cancer by Histological Subtype and Its Relationship with the Human Development Index (HDI); An Ecology Study in 2018. World Cancer Res. J. 2019, 6, e1265. Available online: https://www.wcrj.net/wp-content/uploads/sites/5/2019/04/e1265-Global-incidence-and-mortality-of-skin-cancer-by-histological-subtype-and-its-relationship-with-the-Human-Development-Index-HDI-an-ecology-study-in-2018.pdf (accessed on 18 April 2022).
- Barbarić, J.; Znaor, A. Incidence and mortality trends of melanoma in Croatia. Croat. Med. J. 2012, 53, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burzyńska, M.; Maniecka-Bryła, I.; Pikala, M. Trends of mortality due to breast cancer in Poland, 2000–2016. BMC Public Health 2020, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Pikala, M.; Burzyńska, M.; Maniecka-Bryła, I. Epidemiology of Mortality Due to Prostate Cancer in Poland, 2000–2015. Int. J. Environ. Res. Public Health 2019, 16, 2881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pikala, M.; Burzyńska, M.; Maniecka-Bryła, I. Changes in mortality and years of life lost due to lung cancer in Poland, 2000-2016. J. Transl. Med. 2020, 18, 188. [Google Scholar] [CrossRef]
- Pikala, M.; Burzyńska, M.; Maniecka-Bryła, I. Standard Expected Years of Life Lost Due to Malignant Neoplasms in Poland, 2000-2014. Int. J. Environ. Res. Public Health 2019, 16, 4898. [Google Scholar] [CrossRef] [Green Version]
- Pikala, M.; Burzyńska, M.; Maniecka-Bryła, I. Years of Life Lost Due to Cervical Cancer in Poland in 2000 to 2015. Int. J. Environ. Res. Public Health 2019, 16, 1545. [Google Scholar] [CrossRef] [Green Version]
- Paciej-Gołębiowska, P.; Pikala, M.; Maniecka-Bryła, I. Years of life lost due to malignant neoplasms of the digestive system in Poland in the years 2000–2014. United Eur. Gastroenterol. J. 2018, 6, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Jobczyk, M.; Pikala, M.; Różański, W.; Maniecka-Bryła, I. Years of life lost due to bladder cancer among the inhabitants of Poland in the years 2000 to 2014. Cent. European J. Urol. 2017, 70, 338–343. [Google Scholar] [CrossRef] [Green Version]
- Boniol, M.; Autier, P.; Gandini, S. Melanoma mortality following skin cancer screening in Germany. BMJ Open 2015, 5, e008158. [Google Scholar] [CrossRef] [Green Version]
- Ebisz, M.; Brokowska, M. Szkodliwe oddziaływanie promieniowania ultrafioletowego na skórę człowieka. Harmful impact of ultraviolet radiation on human skin. Hygeia Public Health 2015, 50, 467–473. [Google Scholar]
- Wojciechowska, U.; Didkowska, J. Poprawa przeżyć chorych na nowotwory złośliwe w Polsce.Analiza przeżyć pacjentów zdiagnozowanych w latach 2003–2005. Nowotw. J. Oncol. 2013, 63, 279–285. [Google Scholar] [CrossRef]
- Didkowska, J.; Wojciechowska, U.; Czaderny, K.; Olasek, P.; Ciuba, A. Nowotwory w Polsce 2017. Cancer in Poland in 2017. Krajowy Rejestr Nowotworów. Narodowy Instytut Onkologii im. Marii Skłodowskiej-Curie. Państwowy Instytut Badawczy. Warszawa 2019. Available online: http://onkologia.org.pl/wp-content/uploads/Nowotwory_2017.pdf (accessed on 9 June 2022).
- Dréno, B. Mélanome [Melanoma]. Rev. Prat. 1999, 49, 833–837. [Google Scholar] [PubMed]
- Dzień Walki z Czerniakiem 2021. Available online: https://www.gov.pl/web/wsse-lodz/dzien-walki-z-czerniakiem-2021 (accessed on 10 July 2022).
- Czerniak w Świadomości Internautów. Available online: https://www.raknroll.pl/wp-content/uploads/2016/11/czerniak-w-swiadomosci-internautow_raport.pdf (accessed on 12 July 2022).
- Mayer, J.E.; Swetter, S.M.; Fu, T.; Geller, A.C. Screening, early detection, education, and trends for melanoma: Current status (2007–2013) and future directions: Part I. Epidemiology, high-risk groups, clinical strategies, and diagnostic technology. J. Am. Acad. Dermatol. 2014, 71, 599.e1. [Google Scholar] [CrossRef]
- Cohn-Cedermark, G.; Månsson-Brahme, E.; Rutqvist, L.E.; Larsson, O.; Johansson, H.; Ringborg, U. Trends in mortality from malignant melanoma in Sweden, 1970–1996. Cancer 2000, 89, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, A.; Rodríguez-Domínguez, S.; Lapetra-Peralta, J.; Conejo-Mir, J.S. Has mortality from malignant melanoma stopped rising in Spain? Analysis of trends between 1975 and 2001. Br. J. Dermatol. 2005, 152, 997–1000. [Google Scholar] [CrossRef]
- Cristofolini, M.; Bianchi, R.; Boi, S.; DeCarli, A.; Micciolo, R.; Cristofolini, P.; Zumiani, G. Effectiveness of the health campaign for the early diagnosis of cutaneous melanoma in Trentino, Italy. J. Dermatol. Surg. Oncol. 1993, 19, 117–120. [Google Scholar] [CrossRef]
- Marks, R. Two decades of the public health approach to skin cancer control in Australia: Why, how and where are we now? Australas. J. Dermatol. 1999, 40, 1–5. [Google Scholar] [CrossRef]
- Iannacone, M.R.; Green, A.C. Towards skin cancer prevention and early detection: Evolution of skin cancer awareness campaigns in Australia. Melanoma Manag. 2014, 1, 75–84. [Google Scholar] [CrossRef]


| Year | Place of Residence | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Poland Total | Urban | Rural | |||||||
| Total | Men | Women | Total | Men | Women | Total | Men | Women | |
| 2000 | 62.4 | 61.7 | 63.0 | 62.4 | 61.7 | 63.0 | 63.0 | 61.0 | 64.9 |
| 2010 | 67.0 | 65.9 | 68.3 | 67.0 | 65.9 | 68.3 | 66.4 | 63.8 | 69.2 |
| 2020 | 72.4 | 70.8 | 74.2 | 72.4 | 70.8 | 74.2 | 71.7 | 67.6 | 76.3 |
| Year | Total | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | CDR | SDR | n | CDR | SDR | n | CDR | SDR | |
| 2000 | 928 | 2.43 | 3.60 | 463 | 2.50 | 4.45 | 465 | 2.36 | 3.12 |
| 2005 | 909 | 2.38 | 3.14 | 488 | 2.64 | 4.14 | 421 | 2.14 | 2.47 |
| 2010 | 1188 | 3.10 | 3.84 | 618 | 3.33 | 4.89 | 570 | 2.88 | 3.16 |
| 2015 | 1447 | 3.76 | 4.42 | 765 | 4.11 | 5.82 | 682 | 3.44 | 3.51 |
| 2020 | 1427 | 3.73 | 4.03 | 760 | 4.11 | 5.44 | 667 | 3.38 | 3.10 |
| Year | Total | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | CDR | SDR | n | CDR | SDR | n | CDR | SDR | |
| 2000 | 608 | 2.57 | 3.56 | 311 | 2.76 | 4.57 | 297 | 2.40 | 2.94 |
| 2005 | 575 | 2.45 | 3.22 | 315 | 2.83 | 4.59 | 260 | 2.11 | 2.40 |
| 2010 | 736 | 3.16 | 3.77 | 384 | 3.49 | 5.00 | 352 | 2.87 | 3.01 |
| 2015 | 926 | 4.00 | 4.44 | 497 | 4.52 | 6.08 | 429 | 3.52 | 3.44 |
| 2020 | 889 | 3.88 | 3.91 | 471 | 4.34 | 5.39 | 418 | 3.47 | 3.00 |
| Year | Total | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | CDR | SDR | n | CDR | SDR | n | CDR | SDR | |
| 2000 | 320 | 2.19 | 3.00 | 152 | 2.09 | 3.24 | 168 | 2.29 | 2.83 |
| 2005 | 334 | 2.27 | 3.03 | 173 | 2.36 | 3.55 | 161 | 2.18 | 2.59 |
| 2010 | 452 | 3.03 | 3.89 | 234 | 3.15 | 4.60 | 218 | 2.90 | 3.33 |
| 2015 | 521 | 3.41 | 4.29 | 268 | 3.52 | 5.23 | 253 | 3.30 | 3.55 |
| 2020 | 538 | 3.50 | 4.24 | 289 | 3.78 | 5.48 | 249 | 3.23 | 3.29 |
| Age Group | Year | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2010 | 2020 | |||||||
| n | CDR | SDR | n | CDR | SDR | n | CDR | SDR | |
| Total | |||||||||
| 0–25 | 11 | 0.08 | 0.08 | 3 | 0.03 | 0.02 | 1 | 0.01 | 0.01 |
| 25–49 | 201 | 1.45 | 1.42 | 144 | 1.04 | 1.13 | 125 | 0.88 | 0.89 |
| 50–69 | 355 | 4.78 | 4.84 | 482 | 5.11 | 5.38 | 425 | 4.34 | 4.11 |
| 70+ | 361 | 11.79 | 13.55 | 559 | 14.69 | 15.09 | 876 | 18.98 | 19.28 |
| Men | |||||||||
| 0–24 | 5 | 0.07 | 0.07 | 3 | 0.05 | 0.05 | 0 | 0.00 | 0.00 |
| 25–49 | 109 | 1.57 | 1.58 | 78 | 1.11 | 1.23 | 77 | 1.07 | 1.09 |
| 50–69 | 186 | 5.44 | 5.58 | 281 | 6.34 | 6.76 | 272 | 5.85 | 5.65 |
| 70+ | 163 | 15.18 | 17.96 | 256 | 18.85 | 19.87 | 411 | 23.86 | 26.13 |
| Women | |||||||||
| 0–24 | 6 | 0.09 | 0.09 | 0 | 0.00 | 0.00 | 1 | 0.02 | 0.02 |
| 25–49 | 92 | 1.33 | 1.30 | 66 | 0.96 | 1.05 | 48 | 0.68 | 0.69 |
| 50–69 | 169 | 4.22 | 4.24 | 201 | 4.02 | 4.21 | 153 | 2.97 | 2.73 |
| 70+ | 198 | 5.14 | 11.43 | 303 | 6.84 | 12.52 | 465 | 8.17 | 15.54 |
| Urban | |||||||||
| 0–24 | 8 | 0.10 | 0.09 | 1 | 0.02 | 0.01 | 1 | 0.02 | 0.02 |
| 25–49 | 133 | 1.51 | 1.45 | 81 | 0.95 | 1.07 | 75 | 0.89 | 0.91 |
| 50–69 | 233 | 4.77 | 4.87 | 307 | 4.98 | 5.2 | 251 | 4.18 | 3.88 |
| 70+ | 234 | 13.06 | 14.85 | 347 | 14.70 | 15.1 | 562 | 18.44 | 18.8 |
| Men | |||||||||
| 0–24 | 3 | 0.07 | 0.07 | 1 | 0.03 | 0.03 | 0 | 0.00 | 0.00 |
| 25–49 | 70 | 1.32 | 1.58 | 44 | 1.04 | 1.19 | 40 | 0.95 | 0.95 |
| 50–69 | 126 | 5.70 | 5.90 | 174 | 6.25 | 6.70 | 155 | 5.66 | 5.39 |
| 70+ | 112 | 18.19 | 20.75 | 165 | 19.77 | 20.93 | 276 | 24.51 | 26.60 |
| Women | |||||||||
| 0–24 | 5 | 0.12 | 0.12 | 0 | 0.00 | 0.00 | 1 | 0.04 | 0.04 |
| 25–49 | 63 | 1.39 | 1.32 | 37 | 0.86 | 0.95 | 35 | 0.83 | 0.86 |
| 50–69 | 107 | 4.00 | 4.05 | 133 | 3.93 | 4.02 | 96 | 2.94 | 2.61 |
| 70+ | 122 | 10.37 | 11.75 | 182 | 11.93 | 12.06 | 286 | 14.89 | 14.68 |
| Rural | |||||||||
| 0–24 | 3 | 0.05 | 0.06 | 2 | 0.04 | 0.04 | 0 | 0.00 | 0.00 |
| 25–49 | 68 | 1.35 | 1.39 | 63 | 1.18 | 1.29 | 50 | 0.87 | 0.90 |
| 50–69 | 122 | 4.67 | 4.71 | 175 | 5.33 | 5.65 | 174 | 4.60 | 4.50 |
| 70+ | 127 | 9.58 | 9.86 | 212 | 14.43 | 14.58 | 314 | 20.03 | 20.12 |
| Men | |||||||||
| 0–24 | 2 | 0.07 | 0.08 | 2 | 0.08 | 0.07 | 0 | 0.00 | 0.00 |
| 25–49 | 39 | 1.46 | 1.49 | 34 | 1.22 | 2.15 | 37 | 1.25 | 1.97 |
| 50–69 | 60 | 4.83 | 4.95 | 107 | 6.52 | 7.78 | 117 | 6.13 | 7.30 |
| 70+ | 51 | 10.61 | 11.02 | 91 | 17.09 | 14.00 | 135 | 22.64 | 21.78 |
| Women | |||||||||
| 0–24 | 1 | 0.04 | 0.04 | 0 | 0.00 | 0.00 | 0 | 0.00 | 0.00 |
| 25–49 | 29 | 1.21 | 1.27 | 29 | 1.12 | 1.77 | 13 | 0.47 | 0.80 |
| 50–69 | 62 | 4.53 | 4.55 | 68 | 4.14 | 5.09 | 57 | 3.04 | 4.24 |
| 70+ | 76 | 9.00 | 8.89 | 121 | 12.92 | 10.72 | 179 | 18.43 | 14.28 |
| Place of Residence/Gender | Number of Joinpoints | Years | APC | 95% CI | AAPC | 95% CI |
|---|---|---|---|---|---|---|
| Poland | ||||||
| Men | 0 | 2000–2020 | 3.2 * | (2.7; 3.7) | ||
| Women | 0 | 2000–2020 | 2.4 * | (2.0; 2.9) | ||
| Total | 0 | 2000–2020 | 2.8 * | (2.4; 3.2) | ||
| Urban | ||||||
| Men | 1 | 2000–2011 2011–2020 | 4.4 * 1.6 | (2.9; 5.9) (−0.4; 3.6) | 3.1 * | (2.0; 4.3) |
| Women | 0 | 2000–2020 | 2.4 * | (1.8; 3.0) | ||
| Total | 0 | 2000–2020 | 2.8 * | (2.3; 3.3) | ||
| Rural | ||||||
| Men | 0 | 2000–2020 | 3.2 * | (2.7; 3.7) | ||
| Women | 0 | 2000–2020 | 2.5 * | (1.9; 3.1) | ||
| Total | 0 | 2000–2020 | 2.9 * | (2.4; 3.3) |
| Place of Residence/Gender | Number of Joinpoints | Years | APC | 95% CI | AAPC | 95% CI |
|---|---|---|---|---|---|---|
| Poland | ||||||
| Men | 0 | 2000–2020 | 2.1 * | (1.6; 2.6) | ||
| Women | 2 | 2000–2005 2005–2013 2013–2020 | −2.4 2.8 * −1.3 | (−5.0; 0.2) (1.2; 4.5) (−2.8; 0.3) | 0.0 | (−0.9; 1.0) |
| Total | 1 | 2000–2015 2015–2020 | 1.9 * −1.0 | (1.2; 2.5) (−4.3; 2.4) | 1.1 * | (0.2; 2.1) |
| Urban | ||||||
| Men | 0 | 2000–2020 | 1.8 * | (1.2; 2.5) | ||
| Women | 0 | 2000–2020 | 0.6 * | (0.0; 1.3) | ||
| Total | 0 | 2000–2020 | 1.2 * | (0.7; 1.6) | ||
| Rural | ||||||
| Men | 0 | 2000–2020 | 3.0 * | (2.4; 3.5) | ||
| Women | 0 | 2000–2020 | 1.5 * | (0.9; 2.1) | ||
| Total | 0 | 2000–2020 | 2.2 * | (1.7; 2.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziankowska-Zaborszczyk, E.; Maniecka-Bryła, I.; Pikala, M. Mortality Trends Due to Skin Melanoma in Poland in the Years 2000–2020. Int. J. Environ. Res. Public Health 2022, 19, 16118. https://doi.org/10.3390/ijerph192316118
Dziankowska-Zaborszczyk E, Maniecka-Bryła I, Pikala M. Mortality Trends Due to Skin Melanoma in Poland in the Years 2000–2020. International Journal of Environmental Research and Public Health. 2022; 19(23):16118. https://doi.org/10.3390/ijerph192316118
Chicago/Turabian StyleDziankowska-Zaborszczyk, Elżbieta, Irena Maniecka-Bryła, and Małgorzata Pikala. 2022. "Mortality Trends Due to Skin Melanoma in Poland in the Years 2000–2020" International Journal of Environmental Research and Public Health 19, no. 23: 16118. https://doi.org/10.3390/ijerph192316118





