Evaluation of an Air Cleaning Device Equipped with Filtration and UV: Comparison of Removal Efficiency on Particulate Matter and Viable Airborne Bacteria in the Inlet and Treated Air
Abstract
:1. Introduction
2. Materials and Methods
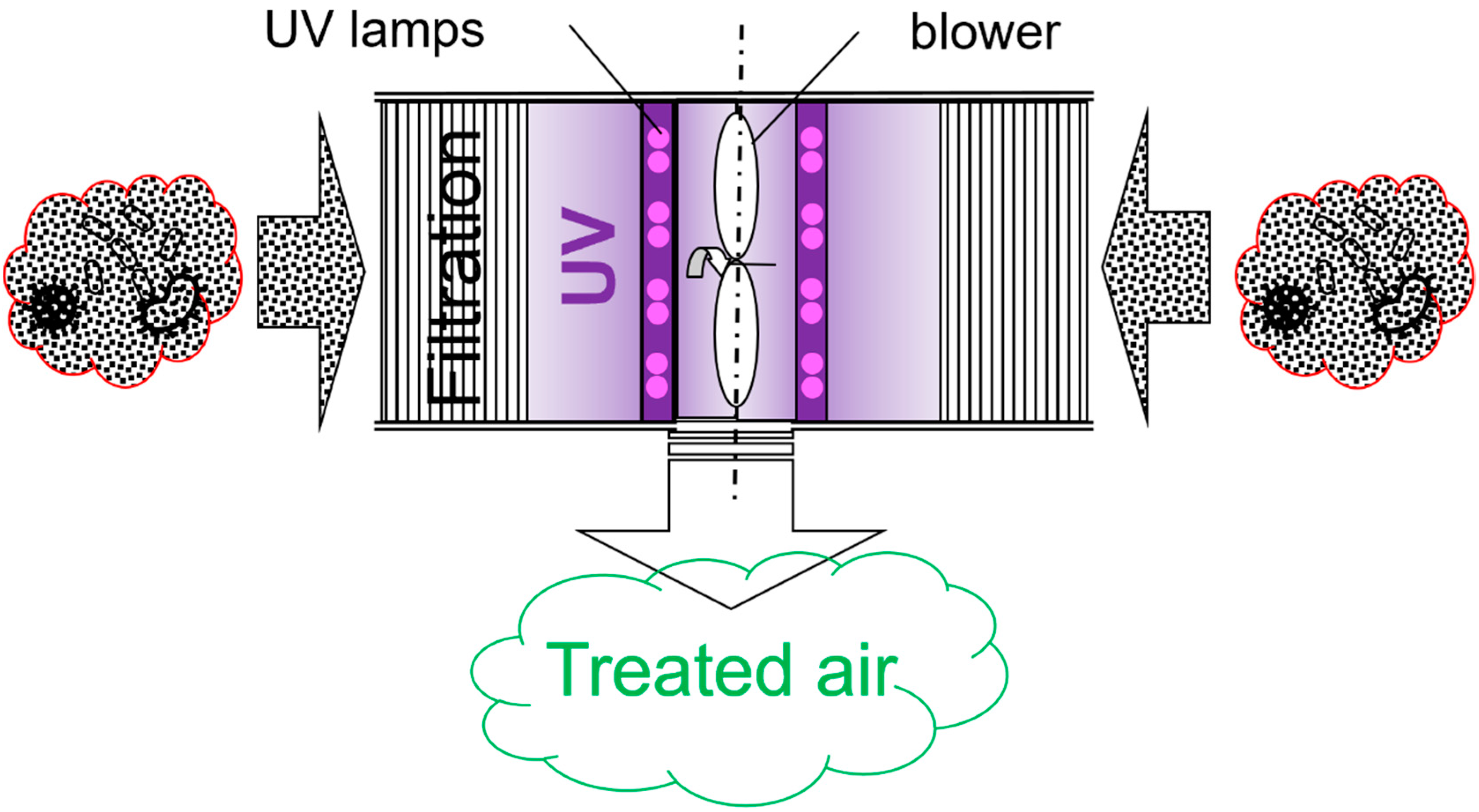
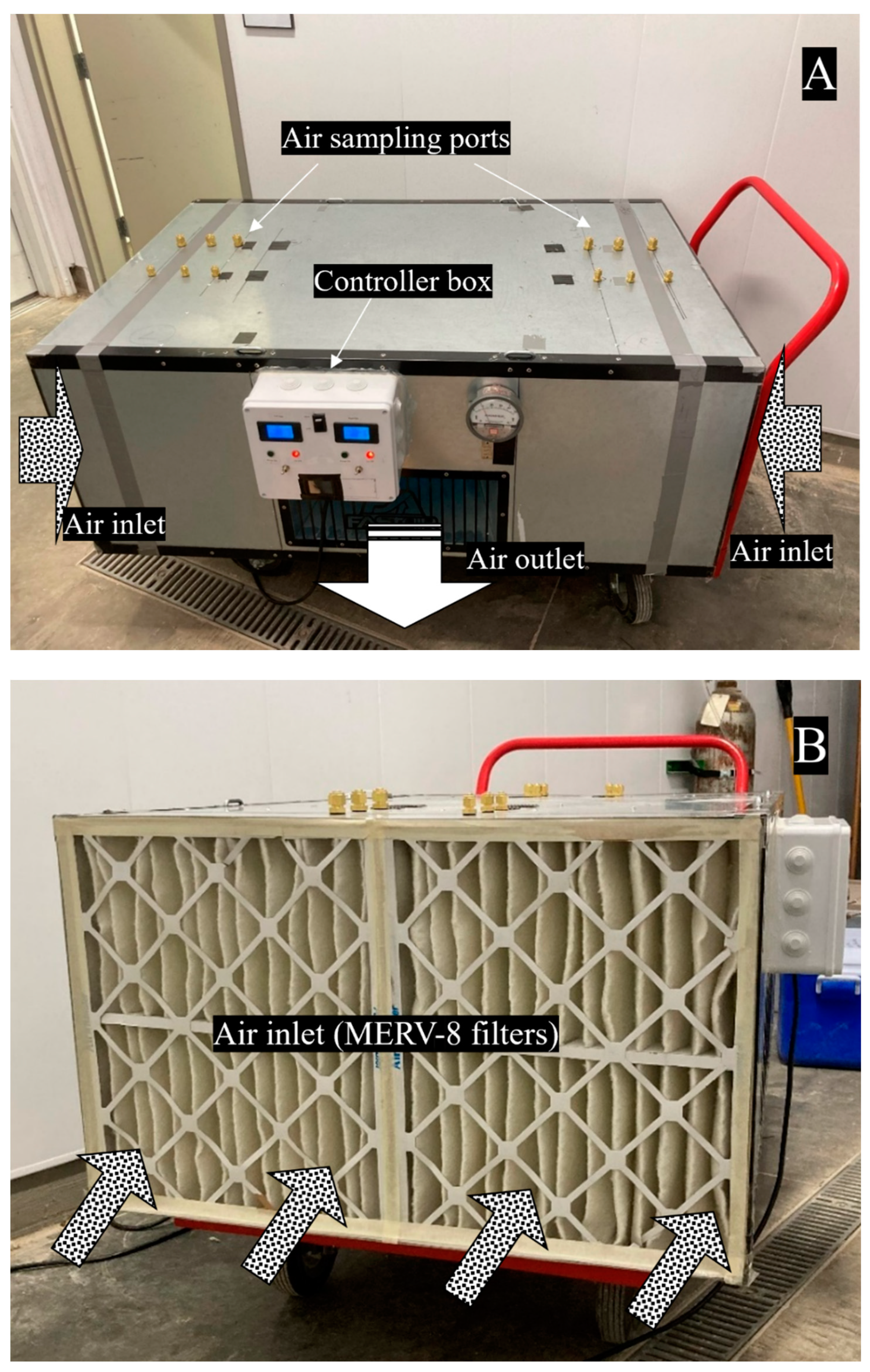
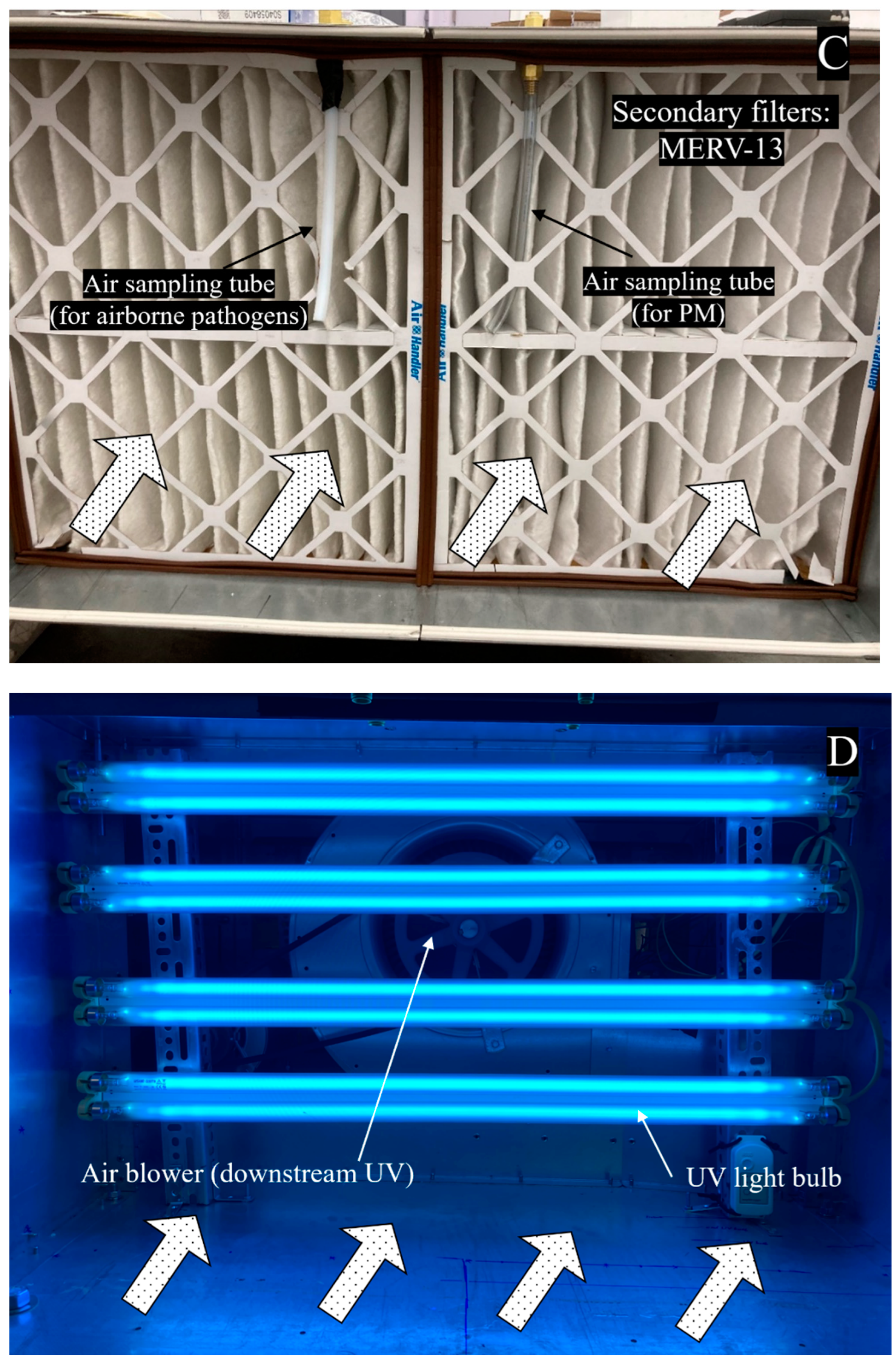
2.1. Upgrade of an Air-Cleaning Prototype
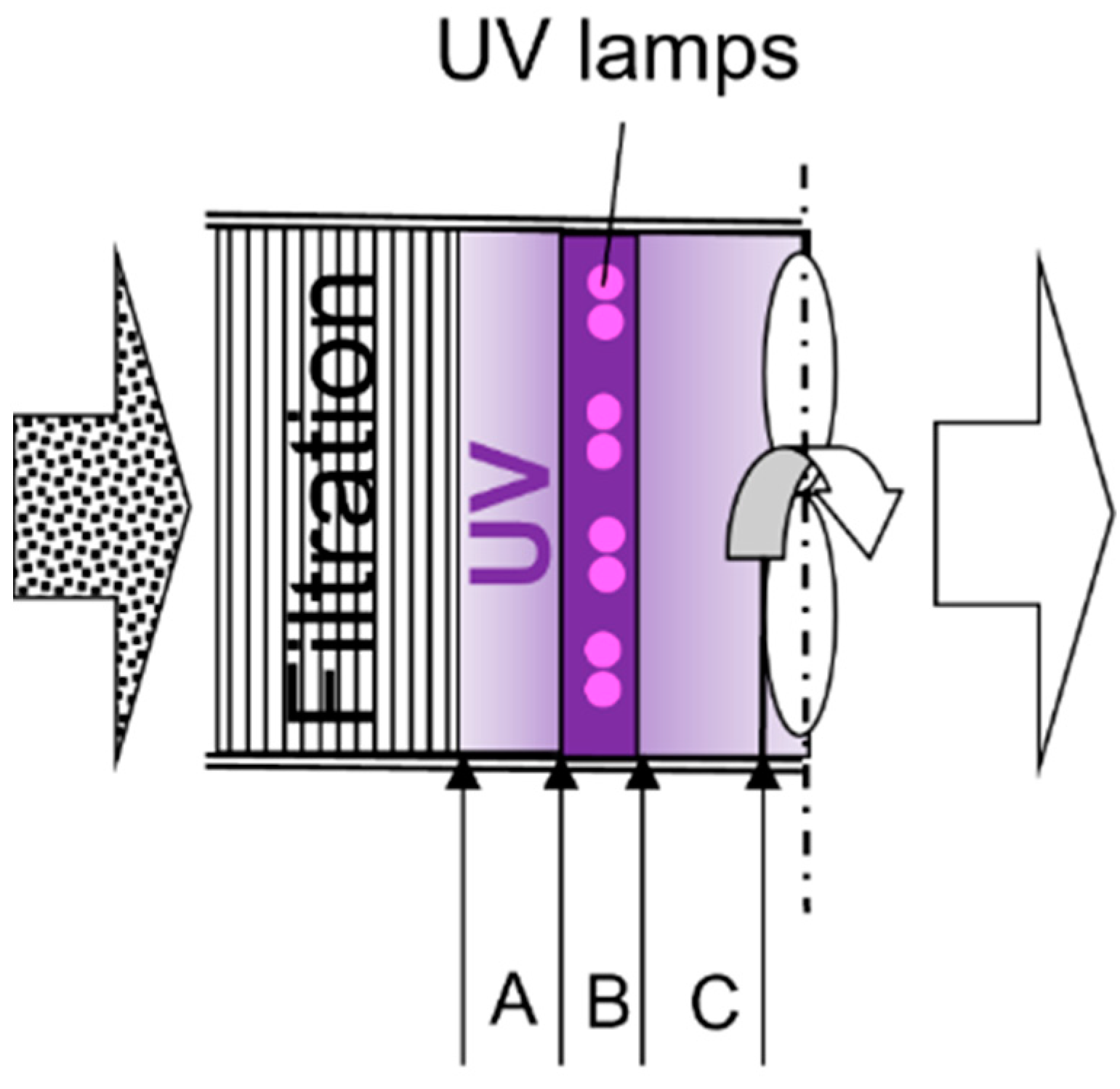
2.2. UV Irradiance and UV Dose Estimation
2.2.1. UV Light Measurement
2.2.2. UV Irradiance and Dose Estimation
Method 1: Estimation by UV Energy Output
- 2-D area-based estimation
- 3-D volume-based estimation
Method 2: Estimation by Measured Irradiance of a Single Plane
- 2-D area-based estimation
- 3-D volume-based estimation
Method 3: Estimation by Integration of Measured Irradiance across Multiple Planes
- 2-D area-based estimation
- 3-D volume-based estimation
2.2.3. Air Flow Rate Measurement and Conversion
2.3. Experimental Design
2.3.1. Testing Sites
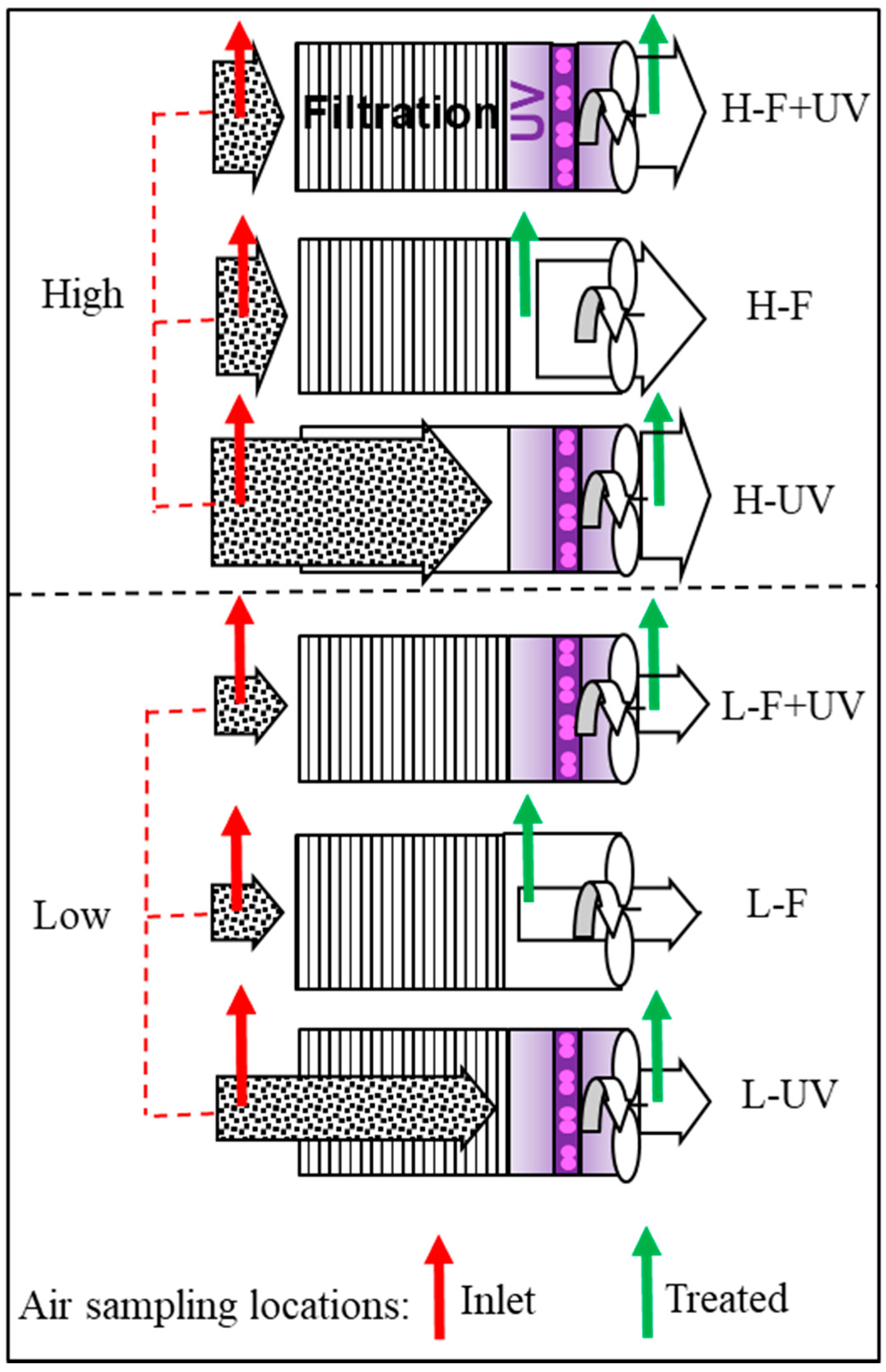
2.3.2. Air Cleaning Configuration Options
2.4. Air Quality Measurements
2.4.1. Sampling and Recovery of Viable Airborne Bacteria
2.4.2. Particulate Matter Concentration Measurement
2.5. Data Analysis
Statistical Analysis
3. Results
3.1. Evaluation of the Actual Air Flow of the FastAir Prototype
3.2. Mitigation of Airborne Pathogens
3.3. Mitigation of Particulate Matter
3.4. Normalization of Airborne Pathogen Concentrations
3.5. UV Dose Estimation
4. Discussion
4.1. Testing Environment
4.2. Airborne Pathogen and PM Mitigation
4.3. Data Analysis
4.4. Potential Improvement
4.5. Safety Concern on Ozone
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tellier, R. COVID-19: The case for aerosol transmission. Interface Focus 2022, 12, 20210072. [Google Scholar] [CrossRef]
- Cowling, B.; Ip, D.; Fang, V.; Suntarattiwong, P.; Olsen, S.; Levy, J.; Uyeki, T.; Leung, G.; Peiris, J.M.; Chotpitayasunondh, T.; et al. Aerosol transmission is an important mode of influenza A virus spread. Nat. Commun. 2013, 4, 1935. [Google Scholar] [CrossRef] [Green Version]
- Lerner, A.M.; Folkers, G.K.; Fauci, A.S. Preventing the spread of SARS-CoV-2 with masks and other “low-tech” interventions. JAMA 2020, 324, 1935. [Google Scholar] [CrossRef]
- Guidelines for Environmental Infection Control in Health-Care Facilities. Available online: https://www.cdc.gov/infectioncontrol/pdf/guidelines/environmental-guidelines-P.pdf (accessed on 10 August 2022).
- Kim, H.-J.; Han, B.; Kim, Y.-J.; Jeong, C.-S.; Lee, S.-H. A simple and efficient method for evaluating air-cleaning performance against airborne allergen particles. Build. Environ. 2013, 60, 272–279. [Google Scholar] [CrossRef]
- Allen, J.G.; Ibrahim, A.M. Indoor air changes and potential implications for SARS-CoV-2 transmission. JAMA 2021, 325, 2112. [Google Scholar] [CrossRef]
- Li, Y.; Leung, G.M.; Tang, J.W.; Yang, X.; Chao, C.Y.; Lin, J.Z.; Lu, J.W.; Nielsen, P.V.; Niu, J.; Qian, H.; et al. Role of ventilation in airborne transmission of infectious agents in the built environment? A multidisciplinary systematic review. Indoor Air 2007, 17, 2–18. [Google Scholar] [CrossRef]
- ANSI/ASHRAE Standard 52.2-2017; Method of Testing General Ventilation Air-Cleaning Devices for Removal Efficiency by Particle Size. ASHRAE Standards Committee: Peachtree Corners, GA, USA, 2017. Available online: https://www.ashrae.org/File%20Library/Technical%20Resources/COVID-19/52_2_2017_COVID-19_20200401.pdf (accessed on 30 November 2022).
- Bergey, D.H.; Brenner, D.J.; Krieg, N.R.; Staley, J.T. Bergey’s Manual of Systematic Bacteriology. In The Proteobacteria. Part B. The Gammaproteobacteria; Springer: New York, NY, USA, 2005; Volume 2. [Google Scholar]
- Louten, J. Virus structure and classification. Essent. Hum. Virol. 2016, 2016, 19–29. [Google Scholar]
- Qian, J.; Hospodsky, D.; Yamamoto, N.; Nazaroff, W.W.; Peccia, J. Size-resolved emission rates of airborne bacteria and fungi in an occupied classroom. Indoor Air 2012, 22, 339–351. [Google Scholar] [CrossRef] [Green Version]
- Cutler, T.D.; Zimmerman, J.J. Ultraviolet irradiation and the mechanisms underlying its inactivation of infectious agents. Anim. Health Res. Rev. 2011, 12, 15–23. [Google Scholar] [CrossRef]
- Eadie, E.; Hiwar, W.; Fletcher, L.; Tidswell, E.; O’Mahoney, P.; Buonanno, M.; Welch, D.; Adamson, C.S.; Brenner, D.J.; Noakes, C.; et al. Far-UVC (222 nm) efficiently inactivates an airborne pathogen in a room-sized chamber. Sci. Rep. 2022, 12, 4373. [Google Scholar] [CrossRef]
- Biasin, M.; Bianco, A.; Pareschi, G.; Cavalleri, A.; Cavatorta, C.; Fenizia, C.; Galli, P.; Lessio, L.; Lualdi, M.; Tombetti, E.; et al. UV-C irradiation is highly effective in inactivating SARS-CoV-2 replication. Sci. Rep. 2021, 11, 6260. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, U.; Tai, H.; Sheng, W. Effectiveness of an ultraviolet-C disinfection system for reduction of healthcare-associated pathogens. J. Microbiol. Immunol. Infect. 2019, 52, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Buonanno, M.; Ponnaiya, B.; Welch, D.; Stanislauskas, M.; Randers-Pehrson, G.; Smilenov, L.; Lowy, F.D.; Owens, D.M.; Brenner, D.J. Germicidal efficacy and mammalian skin safety of 222-nm UV light. Radiat. Res. 2017, 187, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Koziel, J.A.; Li, P.; Jenks, W.S. Mitigation of air pollutants by UV-a photocatalysis in livestock and poultry farming: A mini-review. Catalysts 2022, 12, 782. [Google Scholar] [CrossRef]
- Küpper, M.; Asbach, C.; Schneiderwind, U.; Finger, H.; Spiegelhoff, D.; Schumacher, S. Testing of an indoor air cleaner for particulate pollutants under realistic conditions in an office room. Aerosol Air Qual. Res. 2019, 19, 1655–1665. [Google Scholar] [CrossRef] [Green Version]
- Ultraviolet Low Pressure Mercury-Arc (UVC): Ushio America, Inc. Available online: https://www.ushio.com/product/ultraviolet-low-pressure-mercury-arc-uvc/ (accessed on 24 November 2022).
- Heßling, M.; Hönes, K.; Vatter, P.; Lingenfelder, C. Ultraviolet irradiation doses for coronavirus inactivation—Review and analysis of coronavirus photoinactivation studies. GMS Hyg. Infect. Control 2020, 15, Doc08. [Google Scholar] [CrossRef]
- Holtkamp, D.J.; Johnson, C.; Koziel, J.A.; Li, P.; Murray, D.; Ruston, C.R.; Stephan, A.; Torremorell, M.; Wedel, K. Ultraviolet C (UVC) Standards and Best Practices for the Swine Industry. Swine Health Information Center. 2020. Available online: https://www.swinehealth.org/wp-content/uploads/2020/10/SHIC-UVC-white-paper-10-19-2020.pdf (accessed on 25 October 2022).
- Bolton, J.R.; Cotton, C.A. Ultraviolet Disinfection Handbook; American Water Works Association: Denver, CO, Canada, 2008. [Google Scholar]
- Lawal, O.; Dussert, B.; Howarth, C.; Platzer, K.; Sasges, M.; Muller, J.; Whitby, E.; Stowe, R.; Adam, V.; Witham, D.; et al. Method for the Measurement of the Output of Monochromatic (254 nm) Low-Pressure UV Lamps. IUVA News 2017, 19, 9–16. Available online: https://iuva.org/resources/Resource%20Documents/Lawal-Method%20for%20the%20Measurement%20of%20the%20Output%20of%20Monochromatic%20Low%20Pressure%20UV%20Lamps.pdf (accessed on 30 November 2022).
- ISO Technical Committee ISO/TC 142. UV-C Devices—Measurement of the Output of a UV-C Lamp; ISO 15727:2020(E); ISO: Geneva, Switzerland, 2020. [Google Scholar]
- Gates, R.S.; Casey, K.D.; Xin, H.; Wheeler, E.F.; Simmons, J.D. Fan assessment numeration system (FANS) design and calibration specifications. Trans. ASAE 2004, 47, 1709–1715. [Google Scholar] [CrossRef] [Green Version]
- Hoff, S.J.; Bundy, D.S.; Nelson, M.A.; Zelle, B.C.; Jacobson, L.D.; Heber, A.J.; Ni, J.; Zhang, Y.; Koziel, J.A.; Beasley, D.B. Real-time air flow rate measurements from mechanically ventilated animal buildings. J. Air Waste Manag. Assoc. 2009, 59, 683–694. [Google Scholar] [CrossRef]
- Hermann, J.R.; Hoff, S.J.; Yoon, K.J.; Burkhardt, A.C.; Evans, R.B.; Zimmerman, J.J. Optimization of a sampling system for recovery and detection of airborne porcine reproductive and respiratory syndrome virus and swine influenza virus. Appl. Environ. Microbiol. 2006, 72, 4811–4818. [Google Scholar] [CrossRef] [Green Version]
- Laird, D.T.; Gambrel-Lenarz, S.A.; Scher, F.M.; Graham, T.E.; Reddy, R. Microbiological Count Methods. In Standard Methods for the Examination of Dairy Products; Wehr, H.W., Frank, J.F., Eds.; American Public Health Association: Washington, DC, USA, 2004; pp. 153–186. [Google Scholar]
- Nelson, P.R.; Sears, S.B.; Heavner, D.L. Application of methods for evaluating air cleaner performance. Indoor Environ. 1993, 2, 111–117. [Google Scholar]
- Lee, M.; Koziel, J.A.; Macedo, N.R.; Li, P.; Chen, B.; Jenks, W.S.; Zimmerman, J.; Paris, R.V. Mitigation of Particulate Matter and Airborne Pathogens in Swine Barn Emissions with Filtration and UV-A Photocatalysis. Catalysts 2021, 11, 1302. [Google Scholar] [CrossRef]
- Sabino, C.P.; Sellera, F.P.; Sales-Medina, D.F.; Machado, R.R.; Durigon, E.L.; Freitas-Junior, L.H.; Ribeiro, M.S. UV-C (254 nm) lethal doses for SARS-CoV-2. Photodiagnosis Photodyn. Ther. 2020, 32, 101995. [Google Scholar] [CrossRef] [PubMed]
- ASHRAE Epidemic Task Force ASHRAE Epidemic Task Force—Filtration & Disinfection. Available online: https://www.ashrae.org/file%20library/technical%20resources/covid-19/ashrae-filtration_disinfection-c19-guidance.pdf (accessed on 16 September 2022).
- Zhang, Y. Indoor Air Quality Engineering; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Claus, H. Ozone generation by ultraviolet lamps. Photochem. Photobiol. 2021, 97, 471–476. [Google Scholar] [CrossRef]


| Prototype | Flow Rate Mode | Conditions (Load) | Standard Air Flow Rate * | |
|---|---|---|---|---|
| m3/s | CFM ** | |||
| Original | High | No filters | 1.28 | 2704 |
| MERV 13 only | 1.08 | 2280 | ||
| MERV 8 & 13 | 1.01 | 2146 | ||
| Low | No filters | 0.86 | 1813 | |
| MERV 13 only | 0.61 | 1283 | ||
| MERV 8 & 13 | 0.57 | 1200 | ||
| Upgraded with UV | High | UV light only | 1.20 | 2537 |
| UV light + Al mesh | 1.16 | 2440 | ||
| UV light + Al mesh + MERV 13 | 1.02 | 2154 | ||
| UV light + Al mesh + MERV 8 & 13 | 1.00 | 2101 | ||
| Low | UV light only | 0.53 | 1115 | |
| UV light + Al mesh | 0.51 | 1076 | ||
| UV light + Al mesh + MERV 13 | 0.49 | 1037 | ||
| UV light + Al mesh + MERV 8 & 13 | 0.49 | 1027 | ||
| Configuration Option | Mean Percentage Mitigation of Airborne Bacteria Concentrations ± SD * |
|---|---|
| H-F + UV | 100% |
| H-F | 100% |
| H-UV | 79% ± 5% |
| L-F + UV | 99% ± 2% |
| L-F | 98% ± 3% |
| L-UV | 95% ± 5% |
| Configuration Option | Mean Percentage of PM Removed (%R) ± SD * | ||||
|---|---|---|---|---|---|
| TSP | PM10 | PM4 | PM2.5 | PM1 | |
| H-F + UV | 95% ± 2% | 85% ± 5% | 78% ± 7% | 77% ± 8% | 76% ± 8% |
| H-F | 97% ± **% | 91% ± 1% | 87% ± 3% | 87% ± 3% | 86% ± 3% |
| H-UV | 50% ± 25% | 27% ± 24% | 27% ± 17% | 30% ± 19% | 30% ± 19% |
| L-F + UV | 97% ± 3% | 91% ± 6% | 87% ± 8% | 87% ± 9% | 88% ± 9% |
| L-F | 100% ± **% | 100% ± **% | 100% ± **% | 100% ± **% | 100% ± **% |
| L-UV | 46% ± 5% | 11% ± 2% | 6% ± 2% | 7% ± 2% | 7% ± 1% |
| Configuration Options | Normalized Percentage of Airborne Bacteria Mitigation (%R) | ||||
|---|---|---|---|---|---|
| by TSP | by PM10 | by PM4 | by PM2.5 | by PM1 | |
| H-F + UV | 100% | 100% | 100% | 100% | 100% |
| H-F | 100% | 100% | 100% | 100% | 100% |
| H-UV | 54% ± 9% | 71% ± 2% | 71% ± 1% | 70% ± 1% | 70% ± 1% |
| L-F + UV | 68% ± 56% | 90% ± 17% | 93% ± 12% | 94% ± 11% | 94% ± 11% |
| L-F | N/A * | N/A | N/A | N/A | N/A |
| L-UV | 91% ± 7% | 94% ± 4% | 95% ± 4% | 95% ± 4% | 94% ± 4% |
| p-Values | 0.0171 | 0.0005 | 0.0003 | 0.0003 | 0.0006 |
| UV Dose | Method 1 | Method 2 | Method 3 | ||||
|---|---|---|---|---|---|---|---|
| 2-D (mJ/cm2) | 3-D (mJ/cm3) | 2-D (mJ/cm2) | 3-D (mJ/cm3) | 2-D (mJ/cm2) | 3-D (mJ/cm3) | ||
| Flow Rate Mode | High | 6.3 | 0.45 | 1.5 | 0.22 | 1.4 | 0.22 |
| Low | 12.8 | 0.92 | 3.1 | 0.11 | 2.9 | 0.11 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Koziel, J.A.; Macedo, N.; Zimmerman, J.J.; Wrzesinski, D.; Sobotka, E.; Balderas, M.; Walz, W.B.; Paris, R.V.; Lee, M.; et al. Evaluation of an Air Cleaning Device Equipped with Filtration and UV: Comparison of Removal Efficiency on Particulate Matter and Viable Airborne Bacteria in the Inlet and Treated Air. Int. J. Environ. Res. Public Health 2022, 19, 16135. https://doi.org/10.3390/ijerph192316135
Li P, Koziel JA, Macedo N, Zimmerman JJ, Wrzesinski D, Sobotka E, Balderas M, Walz WB, Paris RV, Lee M, et al. Evaluation of an Air Cleaning Device Equipped with Filtration and UV: Comparison of Removal Efficiency on Particulate Matter and Viable Airborne Bacteria in the Inlet and Treated Air. International Journal of Environmental Research and Public Health. 2022; 19(23):16135. https://doi.org/10.3390/ijerph192316135
Chicago/Turabian StyleLi, Peiyang, Jacek A. Koziel, Nubia Macedo, Jeffrey J. Zimmerman, Danielle Wrzesinski, Erin Sobotka, Mateo Balderas, William B. Walz, Reid Vincent Paris, Myeongseong Lee, and et al. 2022. "Evaluation of an Air Cleaning Device Equipped with Filtration and UV: Comparison of Removal Efficiency on Particulate Matter and Viable Airborne Bacteria in the Inlet and Treated Air" International Journal of Environmental Research and Public Health 19, no. 23: 16135. https://doi.org/10.3390/ijerph192316135







