A Randomized Trial of a Swimming-Based Alternative Treatment for Children with Attention Deficit Hyperactivity Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Intervention Training Program
2.4. Measure of Outcomes
2.4.1. The Junior Hayling Test
2.4.2. The Child Behavior Checklist (CBCL)
2.4.3. Academic Performance
2.5. Statistical Analysis
3. Results
3.1. Maximum Oxygen Consumption
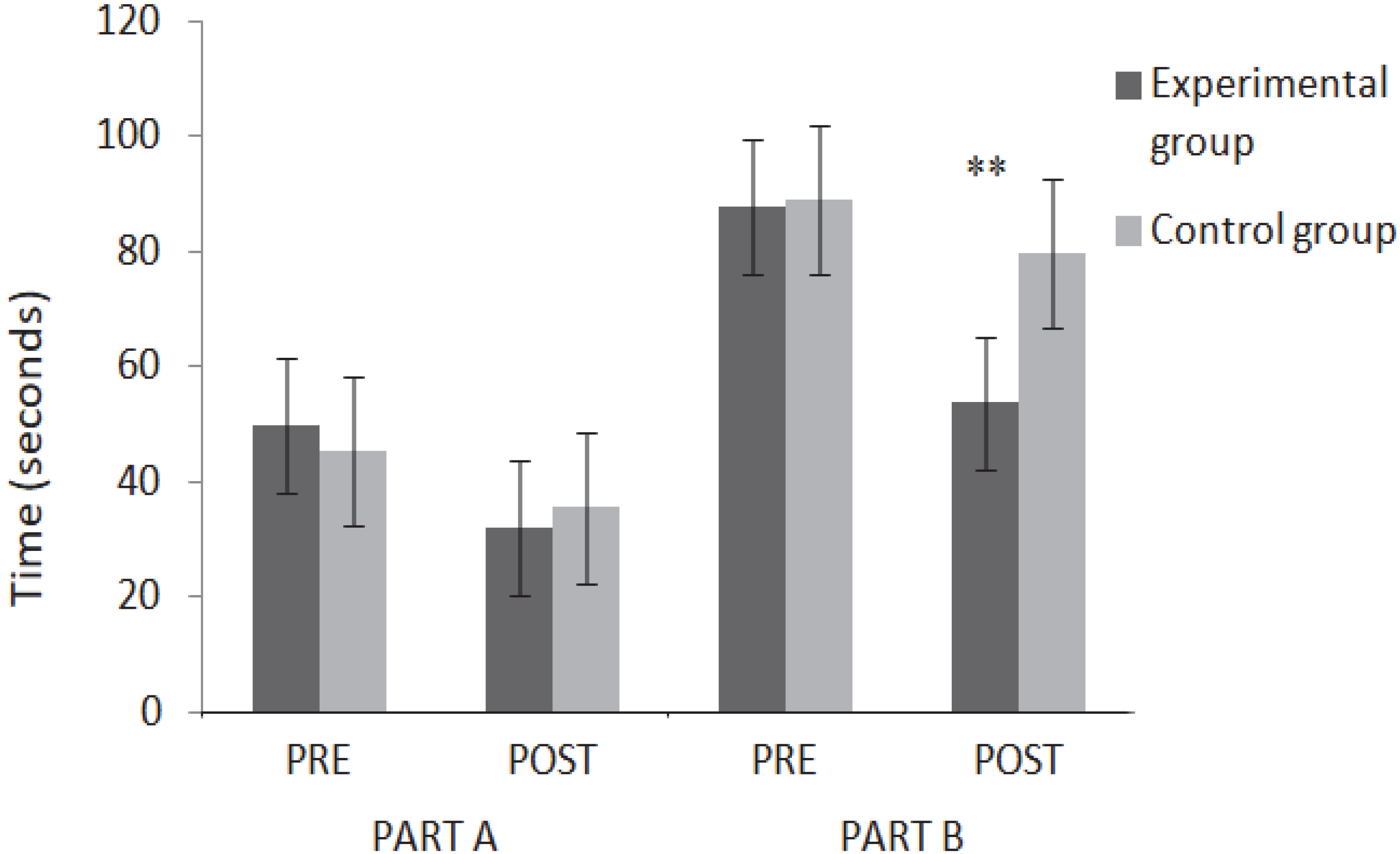
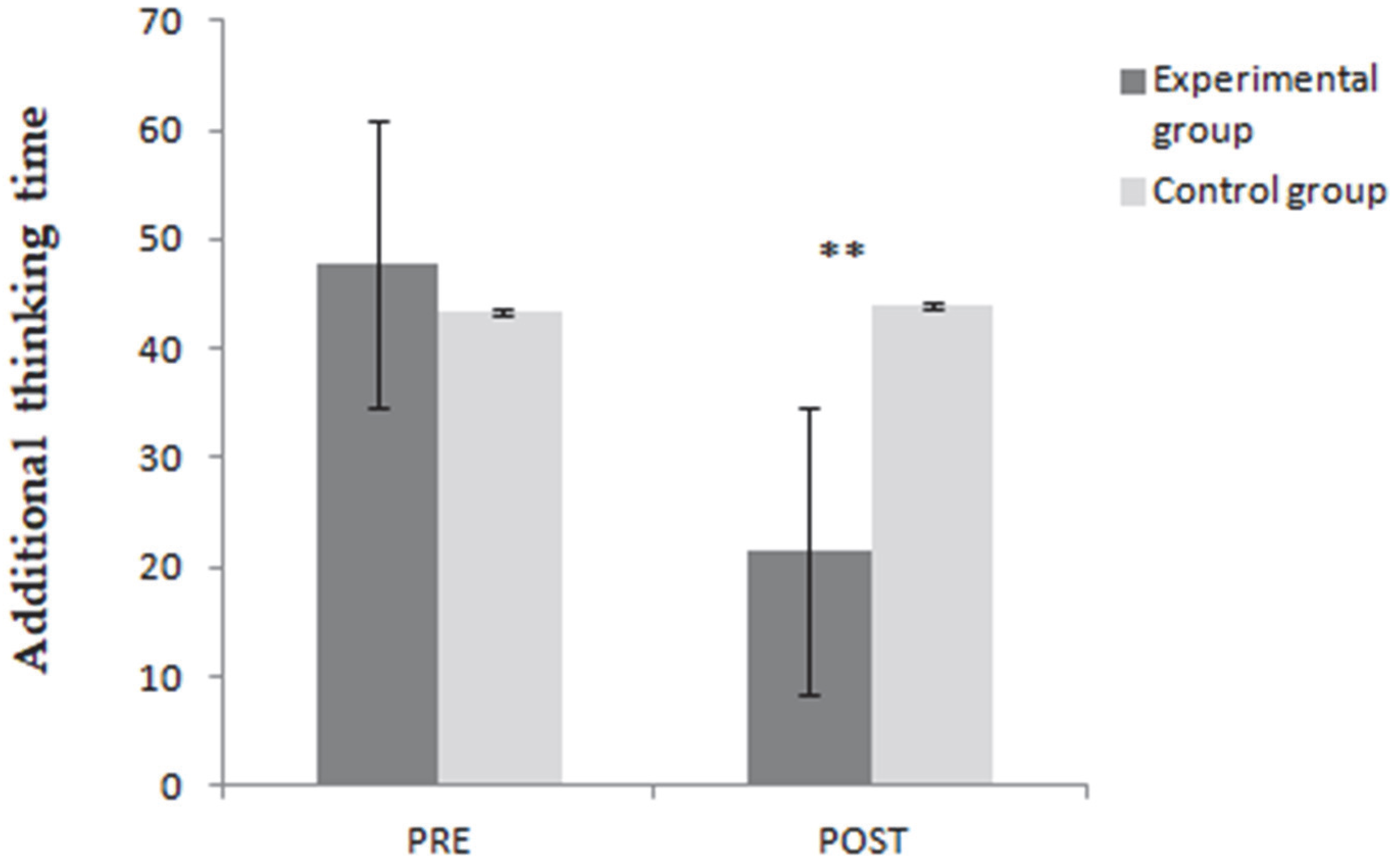
3.2. Hayling Test—Analysis of Temporal Data
3.3. CBCL Tasks
3.4. Academic Performance
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambez, B.; Harwood-Gross, A.; Golumbic, E.Z.; Rassovsky, Y. Non-pharmacological interventions for cognitive difficulties in ADHD: A systematic review and meta-analysis. J. Psychiatr. Res. 2019, 120, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-Y.; Chu, C.-H.; Tsai, C.-L.; Lo, S.-Y.; Cheng, Y.-W.; Liu, Y.-J. A racket-sport intervention improves behavioral and cognitive performance in children with attention-deficit/hyperactivity disorder. Res. Dev. Disabil. 2016, 57, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barkley, R.A. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol. Bull. 1997, 121, 65–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohlin, G.; Eninger, L.; Brocki, K.C.; Thorell, L.B. Disorganized Attachment and Inhibitory Capacity: Predicting Externalizing Problem Behaviors. J. Abnorm. Child Psychol. 2011, 40, 449–458. [Google Scholar] [CrossRef]
- Nejati, V. Cognitive rehabilitation in children with attention deficit- hyperactivity disorder: Transferability to untrained cognitive domains and behavior. Asian J. Psychiatry 2020, 49, 101949. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.-L.; Gau, S.S.-F. Executive function as a mediator in the link between attention-deficit/hyperactivity disorder and social problems. J. Child Psychol. Psychiatry 2013, 54, 996–1004. [Google Scholar] [CrossRef]
- Vlad, A.R.; Lungu, A.I. Can a Person with Attention Deficit Hyperactivity Disorder Be an Athlete? Acta Med. Marisiensis 2017, 63, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Liotti, M.; Pliszka, S.R.; Perez, R.; Kothmann, D.; Woldorff, M.G. Abnormal Brain Activity Related to Performance Monitoring and Error Detection in Children with ADHD. Cortex 2005, 41, 377–388. [Google Scholar] [CrossRef]
- Yang, B.; Chan, R.C.K.; Zou, X.; Jing, J.; Mai, J.; Li, J. Time perception deficit in children with ADHD. Brain Res. 2007, 1170, 90–96. [Google Scholar] [CrossRef]
- Martinussen, R.; Hayden, J.; Hogg-Johnson, S.; Tannock, R. A Meta-Analysis of Working Memory Impairments in Children with Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2005, 44, 377–384. [Google Scholar] [CrossRef]
- Carlson, C.L.; Pelham, J.W.E.; Swanson, J.M.; Wagner, J.L. A Divided Attention Analysis of the Effects of Methylphenidate on the Arithmetic Performance of Children with Attention-Deficit Hyperactivity Disorder. J. Child Psychol. Psychiatry 1991, 32, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Polanczyk, G.; De Lima, M.S.; Horta, B.L.; Biederman, J.; Rohde, L.A. The Worldwide Prevalence of ADHD: A Systematic Review and Metaregression Analysis. Am. J. Psychiatry 2007, 164, 942–948. [Google Scholar] [CrossRef]
- AlZaben, F.N.; Sehlo, M.G.; Alghamdi, W.A.; Tayeb, H.O.; Khalifa, D.A.; Mira, A.T.; Alshuaibi, A.M.; Alguthmi, M.A.; Derham, A.A.; Koenig, H.G. Prevalence of attention deficit hyperactivity disorder and comorbid psychiatric and behavioral problems among primary school students in western Saudi Arabia. Saudi. Med. J. 2018, 39, 52–58. [Google Scholar] [CrossRef]
- Skogli, E.W.; Teicher, M.H.; Andersen, P.N.; Hovik, K.T.; Øie, M. ADHD in girls and boys–gender differences in coexisting symptoms and executive function measures. BMC Psychiatry 2013, 13, 298. [Google Scholar] [CrossRef] [Green Version]
- Guevara, J.; Stein, M.T. Evidence based paediatrics: Evidence based management of attention deficit hyperactivity disorder. BMJ 2001, 323, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Clément, C. Hypothèses et modèles théoriques du TDA/H: Vers une approche holistique du trouble. J. Thérapie Comport. Cogn. 2010, 20, 79–86. [Google Scholar] [CrossRef]
- Sagvolden, T.; Johansen, E.B.; Aase, H.; Russell, V.A. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav. Brain Sci. 2005, 28, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokobane, M.; Pillay, B.J.; Meyer, A.M. Fine motor deficits and attention deficit hyperactivity disorder in primary school children. S. Afr. J. Psychiatry 2019, 25, 7. [Google Scholar] [CrossRef]
- Berger, I.; Nevo, Y. Early Developmental Cues for Diagnosis of Attention Deficit/Hyperactivity Disorder in Young Children. Dev. Disabil. Res. Rev. 2011, 17, 170–179. [Google Scholar] [CrossRef]
- Haenlein, M.; Caul, W.F. Attention Deficit Disorder with Hyperactivity: A Specific Hypothesis of Reward Dysfunction. J. Am. Acad. Child Adolesc. Psychiatry 1987, 26, 356–362. [Google Scholar] [CrossRef]
- Sergeant, J.A.; Oosterlaan, J.; van der Meere, J. Information Processing and Energetic Factors in Attention-Deficit/Hyperactivity Disorder. In Handbook of Disruptive Behavior Disorders; Quay, H.C., Hogan, A.E., Eds.; Springer: Boston, MA, USA, 1999; pp. 75–104. ISBN 978-1-4615-4881-2. [Google Scholar]
- Dovis, S.; Van Der Oord, S.; Wiers, R.; Prins, P.J.M. Can Motivation Normalize Working Memory and Task Persistence in Children with Attention-Deficit/Hyperactivity Disorder? The Effects of Money and Computer-Gaming. J. Abnorm. Child Psychol. 2011, 40, 669–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martella, D.; Aldunate, N.; Fuentes, L.J.; Sánchez-Pérez, N. Arousal and Executive Alterations in Attention Deficit Hyperactivity Disorder (ADHD). Front. Psychol. 2020, 11, 1991. [Google Scholar] [CrossRef] [PubMed]
- Grygiel, P.; Humenny, G.; Rębisz, S.; Bajcar, E.; Świtaj, P. Peer Rejection and Perceived Quality of Relations with Schoolmates Among Children with ADHD. J. Atten. Disord. 2014, 22, 738–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moudi Saud, S.A.; Nawda, S.A.; Hana, A.A. Developing the Social Skills of Attention Deficit Hyperactivity Disorder Children: A Systematic Reviewthe Social Skills of Attention Deficit Hyperactivity Disorder Children: A Systematic Review. J. Spec. Educ. Rehabil. 2021, 12, 65–101. [Google Scholar] [CrossRef]
- Johansen, E.B.; Aase, H.; Meyer, A.; Sagvolden, T. Attention-deficit/h–yperactivity disorder (ADHD) behaviour explained by dysfunctioning reinforcement and extinction processes. Behav. Brain Res. 2001, 130, 37–45. [Google Scholar] [CrossRef]
- Puyjarinet, F.; Franc, N.; Purper-Ouakil, D. Pédopsychiatrie et Psychomotricité: Apports Spécifiques, Complémentarité Thérapeutique et Réflexions Communes Autour de La Prise En Charge Des Enfants TDA/H et de Leur Famille. Entret. Psychomot. 2012, 2012, 13–25. [Google Scholar]
- McMorris, T.; Tomporowski, P.; Audiffren, M. Exercise and Cognitive Function; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 978-0-470-74067-5. [Google Scholar]
- World Health Organization. World Health Statistics 2010; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Sowa, M.; Meulenbroek, R. Effects of physical exercise on Autism Spectrum Disorders: A meta-analysis. Res. Autism Spectr. Disord. 2012, 6, 46–57. [Google Scholar] [CrossRef]
- Kramer, A.F.; Erickson, K.I. Capitalizing on cortical plasticity: Influence of physical activity on cognition and brain function. Trends Cogn. Sci. 2007, 11, 342–348. [Google Scholar] [CrossRef]
- Netz, Y.; Wu, M.-J.; Becker, B.J.; Tenenbaum, G. Physical Activity and Psychological Well-Being in Advanced Age: A Meta-Analysis of Intervention Studies. Psychol. Aging 2005, 20, 272–284. [Google Scholar] [CrossRef] [Green Version]
- Zang, Y. Impact of Physical Exercise on Children with Attention Deficit Hyperactivity Disorders: Evidence through a Meta-Analysis. Medicine 2019, 98, e17980. [Google Scholar] [CrossRef]
- Sun, W.; Yu, M.; Zhou, X. Effects of Physical Exercise on Attention Deficit and Other Major Symptoms in Children with ADHD: A Meta-analysis. Psychiatry Res. 2022, 311, 114509. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, A.M.; Campaniço, J.; Garrido, N.D.; Silva, A.J. Competência Aquática: Um valor acrescentado à Educação Básica. Motricidade 2019, 15, 1–16. [Google Scholar] [CrossRef]
- Langendorfer, S.J. Developmental Aquatic Kinesiology Self-agency and Swimming: Letting Babies Be Your Teachers. Int. J. Aquat. Res. Educ. 2019, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Moura, O.M.; Marinho, D.A.; Forte, P.; Faíl, L.B.; Neiva, H.P. School-Based Swimming Lessons Enhance Specific Skills and Motor Coordination in Children: The Comparison between Two Interventions. Motricidade 2021, 17, 367–374. [Google Scholar] [CrossRef]
- Sortwell, A.; Behringer, M.; Granacher, U.; Trimble, K.; Forte, P.; Neiva, H.P.; Clemente-Suárez, V.J.; Ramirez-Campillo, R.; Konukman, F.; Tufekcioglu, E.; et al. Advancing Sports Science and Physical Education Research Through a Shared Understanding of the Term Motor Performance Skills: A Scoping Review with Content Analysis. Int. J. Kinesiol. Sport. Sci. 2022, 10, 18–27. [Google Scholar] [CrossRef]
- Moura, O.; Neiva, H.; Faíl, L.B.; Morais, J.E.; Marinho, D.A. A influência da prática regular de natação no desenvolvimento motor global na infância. Retos 2021, 40, 296–304. [Google Scholar] [CrossRef]
- Hattabi, S.; Bouallegue, M.; Ben Yahya, H.; Bouden, A. Rehabilitation of ADHD children by sport intervention: A Tunisian experience. Tunis. Med. 2019, 97, 874–881. [Google Scholar]
- Chang, Y.-K.; Hung, C.-L.; Huang, C.-J.; Hatfield, B.D.; Hung, T.-M. Effects of an Aquatic Exercise Program on Inhibitory Control in Children with ADHD: A Preliminary Study. Arch. Clin. Neuropsychol. 2014, 29, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, L.A.; Doyenart, R.; Salvan, P.H.; Rodrigues, W.; Lopes, J.F.; Gomes, K.; Thirupathi, A.; Pinho, R.; Silveira, P.C. Swimming training improves mental health parameters, cognition and motor coordination in children with Attention Deficit Hyperactivity Disorder. Int. J. Environ. Health Res. 2019, 30, 584–592. [Google Scholar] [CrossRef]
- Raven, J.C.; Court, J.H.; Raven, J. Raven’s Coloured Progressive Matrices; Oxford Psychologists Press: Oxford, UK, 2004. [Google Scholar]
- Williams, J.R. The Declaration of Helsinki and public health. Bull. World Health Organ. 2008, 86, 650–651. [Google Scholar] [CrossRef]
- Olstad, B.; Bjørlykke, V.; Olstad, D. Maximal Heart Rate for Swimmers. Sports 2019, 7, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, P.W.; Shallice, T. The Hayling and Brixton Tests; Thames Valley Test Company: Bury St Edmunds, UK, 1997. [Google Scholar]
- Shallice, T. Fractionation of the Supervisory System; Oxford University Press: Oxford, UK, 2002; pp. 261–277. [Google Scholar] [CrossRef]
- Bellaj, T.; Salhi, I.; Le Gall, D.; Roy, A. Development of executive functioning in school-age Tunisian children. Child Neuropsychol. 2015, 22, 919–954. [Google Scholar] [CrossRef]
- Yunis, F.; Zoubeidi, T.; Eapen, V.; Yousef, S. Psychometric Properties of the Child Behavior Checklist/2-3 in an Arab Population. Psychol. Rep. 2007, 100, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Elkhayat, H.; Shehata, M.; Nada, A.; Deifalla, S.; Ammar, M. Impact of functional constipation on psychosocial functioning and quality of life of children: A cross sectional study. Egypt. Pediatr. Assoc. Gaz. 2016, 64, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Al-Hendawi, M.; Keller, C.; Cloninger, L. A Psychometric Analysis of the Child Behavior Checklist for Elementary School Children in Qatar. Assess. Eff. Interv. 2016, 41, 220–229. [Google Scholar] [CrossRef]
- Achenbach, T.M.; Ruffle, T.M. The Child Behavior Checklist and Related Forms for Assessing Behavioral/Emotional Problems and Competencies. Pediatr. Rev. 2000, 21, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.J. An Effect Size Primer: A Guide for Clinicians and Researchers. In Methodological Issues and Strategies in Clinical Research, 4th ed.; American Psychological Association: Washington, DC, USA, 2016; p. 310. ISBN 978-1-4338-2091-5. [Google Scholar]
- Tomkinson, G.R.; Lang, J.J.; Tremblay, M.S.; Dale, M.; LeBlanc, A.G.; Belanger, K.; Ortega, F.B.; Léger, L. International normative 20 m shuttle run values from 1 142 026 children and youth representing 50 countries. Br. J. Sport. Med. 2016, 51, 1545–1554. [Google Scholar] [CrossRef] [Green Version]
- da Costa, A.V.; Costa, M.D.C.; de Oliveira, S.F.M.; de Albuquerque, F.L.; Guimarães, F.J.D.S.P.; Barbosa, T.M. Validation of an equation for estimating maximal oxygen consumption of nonexpert adult swimmers. Open Access J. Sport. Med. 2013, 4, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.L.; Hoza, B.; Linnea, K.; McQuade, J.D.; Tomb, M.; Vaughn, A.J.; Shoulberg, E.K.; Hook, H. Pilot Physical Activity Intervention Reduces Severity of ADHD Symptoms in Young Children. J. Atten. Disord. 2011, 17, 70–82. [Google Scholar] [CrossRef]
- Hung, C.-L.; Chang, Y.-K.; Chan, Y.-S.; Shih, C.-H.; Huang, C.-J.; Hung, T.-M. Motor ability and inhibitory processes in children with ADHD: A neuroelectric study. J. Sport Exerc. Psychol. 2013, 35, 322–328. [Google Scholar] [CrossRef]
- Piek, J.P.; Dyck, M.J.; Nieman, A.; Anderson, M.; Hay, D.; Smith, L.M.; McCoy, M.; Hallmayer, J. The relationship between motor coordination, executive functioning and attention in school aged children. Arch. Clin. Neuropsychol. 2004, 19, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Dail, T.; Smith, C. Swimming and Children with Attention-Deficit Hyperactive Disorder: A Winning Combination. J. Phys. Educ. Recreat. Danc. 2016, 87, 16–20. [Google Scholar] [CrossRef]
- Dwyer, T.; Sallis, J.F.; Blizzard, L.; Lazarus, R.; Dean, K. Relation of Academic Performance to Physical Activity and Fitness in Children. Pediatr. Exerc. Sci. 2001, 13, 225–237. [Google Scholar] [CrossRef]
- Macdonald, K.; Milne, N.; Orr, R.; Pope, R. Relationships between Motor Proficiency and Academic Performance in Mathematics and Reading in School-Aged Children and Adolescents: A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Fels, I.M.J.; Te Wierike, S.C.M.; Hartman, E.; Elferink-Gemser, M.T.; Smith, J.; Visscher, C. The relationship between motor skills and cognitive skills in 4–16 year old typically developing children: A systematic review. J. Sci. Med. Sport 2015, 18, 697–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomporowski, P.D.; Davis, C.L.; Miller, P.H.; Naglieri, J.A. Exercise and Children’s Intelligence, Cognition, and Academic Achievement. Educ. Psychol. Rev. 2007, 20, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Verret, C.; Gardiner, P.; Béliveau, L. Fitness level and gross motor performance of children with attention-deficit hyperactivity disorder. Adapt. Phys. Act. Q. 2010, 27, 337–351. [Google Scholar] [CrossRef]
- Buck, S.M.; Hillman, C.; Castelli, D.M. The Relation of Aerobic Fitness to Stroop Task Performance in Preadolescent Children. Med. Sci. Sport. Exerc. 2008, 40, 166–172. [Google Scholar] [CrossRef]
- Robinson, A.M.; Hopkins, M.E.; Bucci, D.J. Effects of physical exercise on ADHD-like behavior in male and female adolescent spontaneously hypertensive rats. Dev. Psychobiol. 2011, 53, 383–390. [Google Scholar] [CrossRef]
- Sabzi, A.H.; Dana, A.; Salehian, M.H.; Yekta, H.S. The Effect of Water Treadmill Exercise on Children with Attention Deficit Hyperactivity Disorder. Int. J. Pediatr. 2021, 9, 13671–13681. [Google Scholar] [CrossRef]
- Hillman, C.H.; Snook, E.M.; Jerome, G.J. Acute cardiovascular exercise and executive control function. Int. J. Psychophysiol. 2003, 48, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Seidman, L.J.; Valera, E.M.; Makris, N. Structural Brain Imaging of Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2005, 57, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The functions of the orbitofrontal cortex. Brain Cogn. 2004, 55, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Tantillo, M.; Kesick, C.M.; Hynd, G.W.; Dishman, R.K. The effects of exercise on children with attention-deficit hyperactivity disorder. Med. Sci. Sport. Exerc. 2002, 34, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.T. Fundamentals of attention-deficit/hyperactivity disorder: Circuits and pathways. J. Clin. Psychiatry 2006, 67 (Suppl. 8), 7–12. [Google Scholar]
- Aktop, A.; Karahan, N. Physical Education Teacher’s Views of Effective Teaching Methods in Physical Education. Procedia-Soc. Behav. Sci. 2012, 46, 1910–1913. [Google Scholar] [CrossRef] [Green Version]
- Branquinho, L.; Forte, P.; Ferraz, R. Pedagogical Concerns in Sports and Physical Education for Child Growth and Health Promotion. Int. J. Environ. Res. Public Health 2022, 19, 8128. [Google Scholar] [CrossRef]
- Metzler, M. Instructional Models in Physical Education, 3rd ed.; Routledge: New York, NY, USA, 2017; ISBN 978-1-315-21352-1. [Google Scholar]
| Variables | Experimental Group (n = 20) | Control Group (n = 20) |
|---|---|---|
| Gender (Females/Males) | 3/17 | 2/18 |
| Age (years) | 9.95 ± 1.31 | 9.75 ± 1.33 |
| Weight (kg) | 34.95 ± 8.95 | 34.70 ± 6.6 |
| Height (m) | 1.4 ± 1 | 1.4 ± 0.73 |
| BMI (kg/m2) | 17.56 ± 2.91 | 17.54 ± 2.31 |
| VO2max (mL/L/min) | 35.35 ± 2.09 | 35.79 ± 2.16 |
| Resting HR (bpm) | 74.85 ± 4.09 | 74.85 ± 4.09 |
| ADHD-I | 4 (20%) | 5 (25%) |
| ADHD-HI | 6 (30%) | 4 (20%) |
| ADHD-C | 10 (50%) | 11 (55%) |
| Exercise Category | Activities |
|---|---|
| Warm-up period | Duration: 15 min. Intensity: (moderate intensity) <50% of maximum HR. The noodle attack: Children stand in a circle in a shallow area of the pool and hold hands. One child is in the center of the circle, and spins noodle gently on the surface of the water like a clock. The children must avoid being hit by the fry by hiding under the water. The one who is hit replaces the one in the middle. |
| Aerobic conditioning games | Duration: 70 min. Intensity: 50–70% of maximum HR (vigorous intensity). Swimming laps freestyle, backstroke, elementary backstroke, breaststroke, and only legs kicking while using a kickboard. Movement activities while standing in the shallow and running in place, jumping jacks, reciprocal arm and leg movements, hopping on one foot, jumping in place, and jumping forwards, backwards, and sideways Relay races in the shallow end running from one side of the pool to the other: (1) filling buckets with balls or other pool toys, (2) shooting a ball into a hoop, or (3) running in teams while holding onto the aquatic noodles. Obstacle courses: running in the water or swimming while going under, over, and around obstacles or retrieving dive rings. Games: playing ball by rapidly catching, throwing, and shooting baskets into a basketball net, playing keep the ball away from the coaches, and straddle sitting on the aquatic noodle and ‘‘racing the horse’’ the length of the pool |
| Cool-down and flexibility | Duration: 5 min. Intensity: stretches are held for 20–30 s and repeated twice for each side, <50% of maximum HR. Movement activities in the water include marching in place, and arm and leg circles. Performed at a slow pace to bring HR down to lower than the target range. Gently stretching of pectorals, latissimus, triceps, hamstring, quadriceps, plantar flexors, and lateral flexion trunk stretch. Performed in the shallow end of the pool using the pool wall for balance as needed. |
| Variable | Experimental Group M ± SD | Control Group M ± SD | p-Values |
|---|---|---|---|
| Age | 9.95 ± 1.31 | 9.75 ± 1.33 | 0.70 |
| Height | 1.4 ± 1.0 | 1.4 ± 0.73 | 0.15 |
| Weight | 34.95 ± 8.95 | 34.70 ± 6.60 | 0.36 |
| BMI | 17.56 ± 2.91 | 17.54 ± 2.31 | 0.36 |
| Part A Hayling test | 49.85 ± 18.97 | 45.40 ± 16.87 | 0.44 |
| Part B Hayling test | 97.69 ± 41.14 | 88.55 ± 27.72 | 0.42 |
| EsB | 22.50 ± 3.72 | 20.35 ± 3.03 | 0.05 |
| Weight (kg) | 34.95 ± 8.95 | 34.70 ± 6.60 | 0.92 |
| VO2 peak | 35.35 ± 2.09 | 35.78 ± 2.16 | 0.59 |
| Reading marks | 11.45 ± 1.46 | 11.55 ± 1.43 | 0.83 |
| Math | 5.95 ± 2.16 | 6.05 ± 2.11 | 0.88 |
| Pass marks | 10.057 ± 0.99 | 10.14 ± 0.92 | 0.78 |
| Resting HR (bpm) | 74.85 ± 4.09 | 74.85 ± 4.09 | 1.00 |
| Internalizing behavior | 19.15 ± 6.49 | 19.20 ± 7.05 | 0.98 |
| Externalizing behavior | 26.10 ± 2.86 | 24.65 ± 1.90 | 0.07 |
| Aggressive behavior | 17.10 ± 1.55 | 16.55 ± 1.57 | 0.27 |
| Delinquent behavior | 9.00 ± 1.86 | 8.10 ± 0.72 | 0.06 |
| Anxiety/depression | 7.35 ± 2.58 | 7.50 ± 2.68 | 0.86 |
| Variables | Experimental Group (n = 20) | Control Group (n = 20) | Effect Size | Level of Effect Size | ||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||
| Withdrawn | 5.50 ± 1.82 | 3.85 ± 0.59 | 5.40 ± 2.09 | 5.75 ± 2.00 | 0.283 ** | Strong |
| Somatic complaints | 6.30 ± 2.32 | 4.20 ± 0.83 | 6.30 ± 2.47 | 6.65 ± 2.50 | 0.303 ** | Strong |
| Anxious/depressed | 7.35 ± 2.58 | 4.50 ± 0.95 | 7.50 ± 2.69 | 7.60 ± 2.89 | 0.307 ** | Strong |
| Thought problems | 6.80 ± 1.44 | 4.55 ± 0.76 | 7.45 ± 2.01 | 7.55 ± 1.90 | 0.481 ** | Strong |
| Social problems | 10.70 ± 0.80 | 6.80 ± 1.24 | 10.45 ± 1.00 | 10.50 ± 0.95 | 0.801 ** | Large |
| Attention problems | 15.10 ± 0.85 | 9.35 ± 0.93 | 14.75 ± 1.02 | 14.55 ± 1.23 | 0.863 ** | Large |
| Delinquent behavior | 9.00 ± 1.86 | 4.90 ± 1.65 | 8.10 ± 0.72 | 8.35 ± 0.81 | 0.827 ** | Large |
| Aggressive behavior | 17.10 ± 1.55 | 9.75 ± 1.45 | 16.55 ± 1.57 | 16.35 ± 1.09 | 0.880 ** | Large |
| Internalizing behavior | 17.10 ± 1.55 | 12.55 ± 2.04 | 19.20 ± 7.05 | 20.00 ± 7.14 | 0.324 ** | Strong |
| Externalizing behavior | 26.10 ± 2.86 | 14.65 ± 2.85 | 24.65 ± 1.90 | 24.70 ± 1.38 | 0.908 ** | Large |
| Variables | Experimental Group (n = 20) | Control Group (n = 20) | Effect Size | Level of Effect Size | ||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||
| Reading | 11.45 ± 1.47 | 13.80 ± 1.36 | 11.55 ± 1.43 | 11.25 ± 1.16 | 0.368 ** | Strong |
| Math | 5.95 ± 2.16 | 9.20 ± 1.57 | 6.05 ± 2.11 | 5.85 ± 1.46 | 0.595 ** | Strong |
| Pass mark | 10.06 ± 1.00 | 11.70 ± 0.96 | 10.14 ± 0.91 | 10.18 ± 0.91 | 0.396 ** | Strong |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattabi, S.; Forte, P.; Kukic, F.; Bouden, A.; Have, M.; Chtourou, H.; Sortwell, A. A Randomized Trial of a Swimming-Based Alternative Treatment for Children with Attention Deficit Hyperactivity Disorder. Int. J. Environ. Res. Public Health 2022, 19, 16238. https://doi.org/10.3390/ijerph192316238
Hattabi S, Forte P, Kukic F, Bouden A, Have M, Chtourou H, Sortwell A. A Randomized Trial of a Swimming-Based Alternative Treatment for Children with Attention Deficit Hyperactivity Disorder. International Journal of Environmental Research and Public Health. 2022; 19(23):16238. https://doi.org/10.3390/ijerph192316238
Chicago/Turabian StyleHattabi, Soukaina, Pedro Forte, Filip Kukic, Asma Bouden, Mona Have, Hamdi Chtourou, and Andrew Sortwell. 2022. "A Randomized Trial of a Swimming-Based Alternative Treatment for Children with Attention Deficit Hyperactivity Disorder" International Journal of Environmental Research and Public Health 19, no. 23: 16238. https://doi.org/10.3390/ijerph192316238
APA StyleHattabi, S., Forte, P., Kukic, F., Bouden, A., Have, M., Chtourou, H., & Sortwell, A. (2022). A Randomized Trial of a Swimming-Based Alternative Treatment for Children with Attention Deficit Hyperactivity Disorder. International Journal of Environmental Research and Public Health, 19(23), 16238. https://doi.org/10.3390/ijerph192316238










