A Review of PFAS Destruction Technologies
Abstract
:1. Introduction
- Electrochemical oxidation;
- Plasma;
- Photocatalysis;
- Sonolysis;
- Supercritical water oxidation;
- Thermal degradation/incineration.
2. Electrochemical Oxidation
Field Applications
3. Plasma
Field Applications
4. Photocatalysis
Field Applications
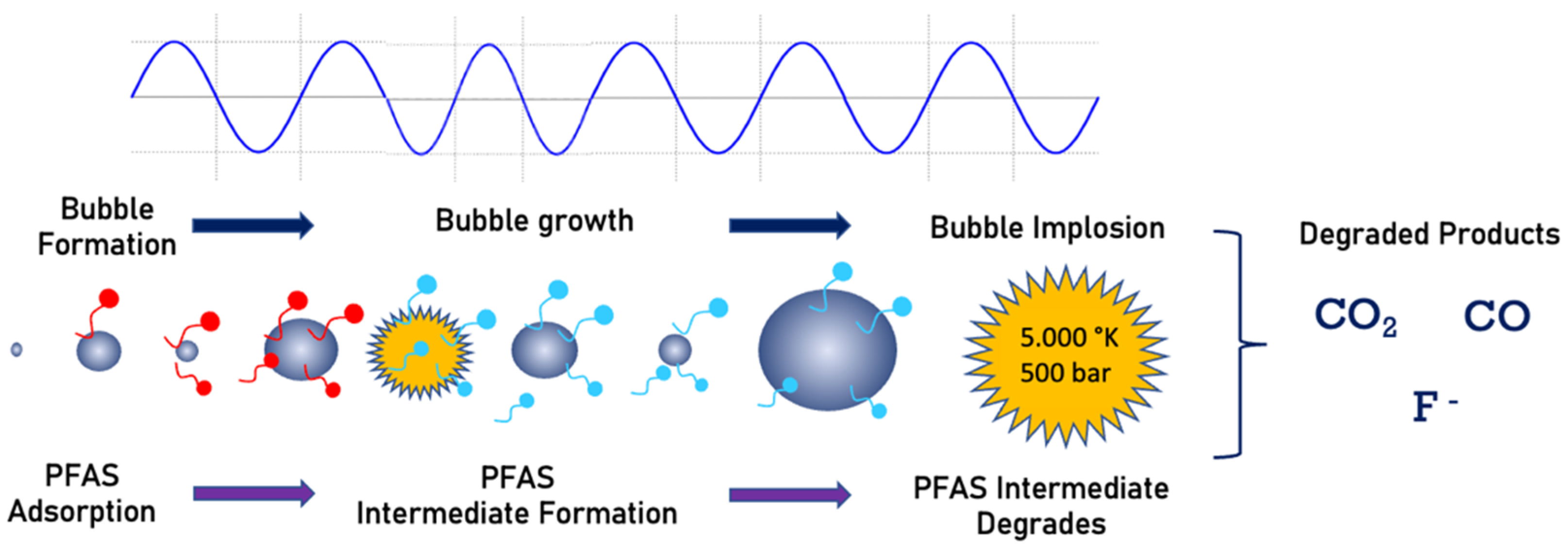
5. Sonolysis
Field Applications
6. Supercritical Water Oxidation (SCWO)
Field Applications
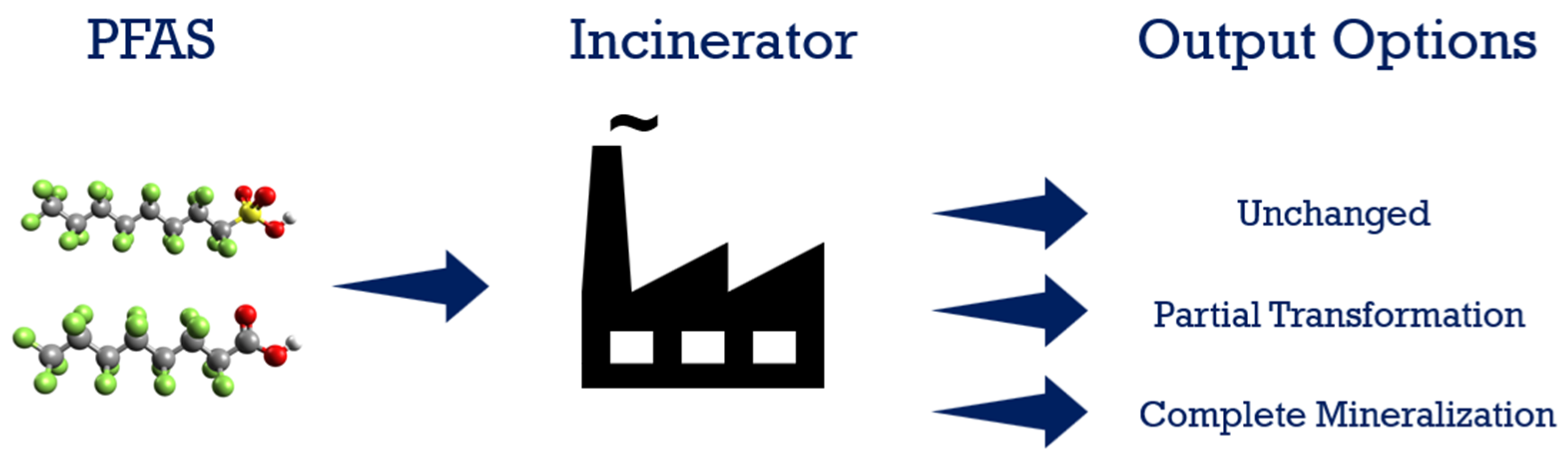
7. Thermal Degradation/Incineration
Field Applications
8. Technology Comparison
| PFAS Source | Experimental Conditions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Technology | AFFF | Still Bottom | Synthetic | PFASType | Ci (µg/L) | Cf (µg/L) | T (h) | Vol (L) | DE (%) | Energy Consumed (kWh/m3) | Mass of PFAS Destroyed (kg) | Energy per Mass (kWh/kg) | Ref. |
| Electrochemical Oxidation | Multi | 1652 | 4.2 | 10 | 2 | 99.7412 | 256 | 3.2 × 10−6 | 1.6 × 105 | [35] | |||
| PFHxA | 870,000 | 87,000 | 1.5 | 1 | 90.0000 | 15.2 | 7.8 × 10−4 | 1.9 × 101 | [52] | ||||
| x | Multi | 451 | 50.6 | 8 | 0.3 | 88.7804 | 99 | 1.0 × 10−7 | 2.5 × 105 | [45] | |||
| x | Multi | 539 | 126.4 | 8 | 0.3 | 76.5491 | 136 | 1.0 × 10−7 | 3.3 × 105 | [45] | |||
| x | PFOA | 3050 | 897 | 80 | 0.56 | 70.5902 | 0.161 | 1.21 × 10−6 | 3.98 × 10⁷ | [44] | |||
| x | PFOA | 4080 | 609 | 80 | 0.415 | 85.0735 | 0.131 | 1.44 × 10−6 | 3.33 × 10⁷ | [44] | |||
| x | PFOS | 4420 | 538 | 80 | 0.56 | 87.8281 | 0.094 | 2.17 × 10−6 | 2.21 × 10⁷ | [44] | |||
| x | PFOS | 15,200 | 361 | 80 | 0.415 | 97.6250 | 0.071 | 6.16 × 10−6 | 7.79 × 10⁶ | [44] | |||
| x | 8:2 FTS | 193 | 50 | 120 | 0.56 | 74.0933 | 0.146 | 8.01 × 10−8 | 5.99 × 10⁸ | [44] | |||
| x | 8:2 FTS | 753 | 125 | 120 | 0.415 | 83.3997 | 0.148 | 2.61 × 10−7 | 1.84 × 10⁸ | [44] | |||
| Sonochemical Degradation | x | PFOS | 5001 | 0 | 3 | 0.6 | 208 | 3.0 × 10−6 | 4.2 × 104 | [103] | |||
| x | PFOA | 4141 | 0 | 2 | 0.6 | 208 | 2.5 × 10−6 | 5.0 × 104 | [103] | ||||
| x | PFOS | 10,000 | 7200.0 | 1 | 0.1 | 28.0000 | 3333 | 1.7 × 10−7 | 1.2 × 106 | [100] | |||
| x | PFOA | 10,000 | 3700.0 | 1 | 0.1 | 63.0000 | 3333 | 3.8 × 10−7 | 5.3 × 105 | [100] | |||
| x | PFOS | 100 | 5 | 2.3 | 0.6 | 95.0000 | 448 | 5.7 × 10−8 | 4.7 × 106 | [148] | |||
| x | PFOA | 100 | 28 | 2.3 | 0.6 | 72.0000 | 1050 | 4.3 × 10−8 | 1.5 × 107 | [148] | |||
| x | PFOS | 9420 | 96 | 4 | 99.9898 | [102] | |||||||
| x | PFOS | 9420 | 133 | 4 | 99.9858 | [102] | |||||||
| x | PFOS | 9420 | 177 | 4 | 99.9812 | [102] | |||||||
| x | PFOA | 140 | 1.2 | 4 | 2 | 99.1428 | [113] | ||||||
| x | PFOS | 2.9 | 0.06 | 4 | 2 | 97.9310 | [113] | ||||||
| x | PFBS | 10.5 | 1.06 | 4 | 2 | 89.9047 | [113] | ||||||
| Plasma Reactor | x | PFOA | 7.924 | 0.012 | 0.67 | 4 | 99.8486 | [72] | |||||
| x | PFOS | 2.264 | 0.012 | 0.67 | 4 | 99.4700 | [72] | ||||||
| x | PFPeA | 10.452 | 2.432 | 1 | 4 | 76.7317 | [72] | ||||||
| x | PFBA | 3.684 | 2.253 | 1 | 4 | 38.8436 | [72] | ||||||
| x | Mulit | 261,067 | 0.82 | 6 | 0.75 | 99.9997 | 830 | [79] | |||||
| x | PFOA | 99,600 | <0.015 | 2 | >99 | 380–830 | [79] | ||||||
| x | PFOS | 70,900 | <0.035 | 2 | >99 | 380–830 | [79] | ||||||
| x | Mulit | <1 | BDL * | 1.5 | >99 | 1570–2370 | [79] | ||||||
| x | PFOA | 1.5 | <0.07 | 0.03 | >99 | 380–830 | [79] | ||||||
| x | PFOS | 1.75 | <0.07 | 0.03 | >99 | 380–830 | [79] | ||||||
| x | PFBA | 6 | 0.26 | 1.5 | 95.6667 | 1570–2370 | [79] | ||||||
| Supercritical Water Oxidation | x | Multi | 12,176 | 0 | 2 | 1085.4 | >99 | 1398 | 1.3 × 10−2 | 1.1 × 105 | [149] | ||
| x | Multi | 14,155 | 0 | 2 | 997.4 | >99 | 1506 | 1.4 × 10−2 | 1.0 × 105 | [149] | |||
| x | PFOA | 26,200 | 240 | 99.0839 | [117] a | ||||||||
| x | PFOS | 930 | 0.14 | 99.9849 | [117] a | ||||||||
| x | PFOS | 30,599 | 0.33 | 99.9989 | [117] b | ||||||||
| x | PFOS | 30,251 | 0.29 | 99.999 | [117] b | ||||||||
| x | Multi | 40,454 | 8.64 | 99.9786 | [117] b | ||||||||
| x | PFOS | 190,000 | 8.57 | 99.9954 | [117] c | ||||||||
| x | Multi | 243,000 | 9.63 | 99.9960 | [117] c | ||||||||
| x | PFOS | 28,630 | 1 | 70.0000 | [150] | ||||||||
| Photocatalysis | x | PFOA | 1000 | 690 | 1 | 31.000 | [82] | ||||||
| x | PFOS | 1000 | 670 | 1 | 33.000 | [82] | |||||||
| x | PFNA | 1000 | 0.26 | 2 | 99.974 | [82] | |||||||
| x | PFOA | 0.5 | 0.27 | 2 | 46.000 | [82] | |||||||
| x | PFOS | 0.5 | 0.06 | 2 | 88.000 | [82] | |||||||
| x | PFNA | 0.5 | 0.05 | 2 | 90.000 | [82] | |||||||
| x | PFOS | 100 | 4 | 75.000 | [84] | ||||||||
| x | PFOA | 100 | 4 | 90.000 | [86] | ||||||||
| TECHNOLOGY | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| ELECTROCHEMICAL OXIDATION |
|
|
| PLASMA |
|
|
| PHOTOCATALYSIS |
|
|
| SONOLYSIS |
|
|
| SUPERCRITICAL WATER OXIDATION |
|
|
| THERMAL DEGRADATION/ INCINERATION |
|
|
9. Summary
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- 3M Company. Fluorochemical Use, Distribution and Release Overview; 3M Company: Saint Paul, MN, USA, 1999. [Google Scholar]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K. ‘Forever chemicals’ no more? CEN Glob. Enterp. 2019, 97, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Meegoda, J.N.; Kewalramani, J.A.; Li, B.; Marsh, R.W. A review of the applications, environmental release, and remediation technologies of per- and polyfluoroalkyl substances. Int. J. Environ. Res. Public Health 2020, 17, 8117. [Google Scholar] [CrossRef] [PubMed]
- ITRC; PFAS Team. Aqueous Film-Forming Foam (AFFF) Continued; ITRC: Washington, DC, USA, 2020. [Google Scholar]
- ITRC; PFAS Team. Per- and Polyfluoroalkyl (PFAS), Technical/Regulatory Guidance. Available online: https://pfas-1.itrcweb.org/wp-content/uploads/2020/10/itrc_pfas_techreg_sept_2020_508-1.pdf (accessed on 19 February 2022).
- Anderson, R.H.; Long, G.C.; Porter, R.C.; Anderson, J.K. Occurrence of select perfluoroalkyl substances at U.S. air force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere 2016, 150, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Ebnesajjad, S. Discovery and history of fluoropolymers. In Introduction to Fluoropolymers; Elsevier: Amsterdam, The Netherlands, 2013; pp. 17–35. [Google Scholar]
- Wang, Z.; Cousins, I.T.; Scheringer, M.; Hungerbühler, K. Fluorinated alternatives to long-chain Perfluoroalkyl Carboxylic Acids (PFCAs), Perfluoroalkane Sulfonic Acids (PFSAs) and their potential precursors. Environ. Int. 2013, 60, 242–248. [Google Scholar] [CrossRef]
- Hekster, F.M.; Laane, R.W.P.M.; de Voogt, P. Environmental and toxicity effects of perfluoroalkylated substances. Rev. Environ. Contam. Toxicol. 2003, 99–121. [Google Scholar] [CrossRef]
- Kissa, E. Fluorinated Surfactants and Repellents, 2nd ed.; Marcel Dekker: New York, NY, USA, 2001; Volume 97. [Google Scholar]
- ITRC; PFAS Team. Naming Conventions for Per-and Polyfluoroalkyl Substances (PFAS); ITRC: Washington, DC, USA, 2020. [Google Scholar]
- Meegoda, J.; Kewalramani, J.; Marsh, R.; de Souza, B.B.; Dahanayake, M. Per-and polyfluoroalkyl substances. Polym. Sci. 2021. Available online: https://encyclopedia.pub/entry/6459 (accessed on 9 September 2022).
- Vierke, L.; Staude, C.; Biegel-Engler, A.; Drost, W.; Schulte, C. Perfluorooctanoic Acid (PFOA)-main concerns and regulatory developments in Europe from an environmental point of view. Environ. Sci. Eur. 2012, 24, 16. [Google Scholar] [CrossRef] [Green Version]
- Leung, S.C.E.; Shukla, P.; Chen, D.; Eftekhari, E.; An, H.; Zare, F.; Ghasemi, N.; Zhang, D.; Nguyen, N.T.; Li, Q. Emerging technologies for PFOS/PFOA degradation and removal: A review. Sci. Total Environ. 2022, 827, 153669. [Google Scholar] [CrossRef]
- Krafft, M.P.; Riess, J.G. Per- and polyfluorinated substances (PFASs): Environmental challenges. Curr. Opin. Colloid Interface Sci. 2015, 20, 192–212. [Google Scholar] [CrossRef]
- Ojo, A.F.; Peng, C.; Ng, J.C. Assessing the human health risks of per- and polyfluoroalkyl substances: A need for greater focus on their interactions as mixtures. J. Hazard Mater. 2021, 407, 124863. [Google Scholar] [CrossRef]
- Giesy, J.P.; Kannan, K. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Brusseau, M.L.; Anderson, R.H.; Guo, B. PFAS Concentrations in Soils: Background Levels versus Contaminated Sites. Sci. Total Environ. 2020, 740, 140017. [Google Scholar] [CrossRef] [PubMed]
- Rankin, K.; Mabury, S.A.; Jenkins, T.M.; Washington, J.W. A North American and global survey of perfluoroalkyl substances in surface soils: Distribution patterns and mode of occurrence. Chemosphere 2016, 161, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.; Xie, Z.; Ebinghaus, R. Distribution of perfluoroalkyl compounds in seawater from Northern Europe, Atlantic Ocean, and Southern Ocean. Chemosphere 2010, 78, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Kwok, K.Y.; Yamazaki, E.; Yamashita, N.; Taniyasu, S.; Murphy, M.B.; Horii, Y.; Petrick, G.; Kallerborn, R.; Kannan, K.; Murano, K.; et al. Transport of perfluoroalkyl substances (PFAS) from an Arctic glacier to downstream locations: Implications for sources. Sci. Total Environ. 2013, 447, 46–55. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. 2019, 177, 108648. [Google Scholar] [CrossRef]
- RIDOH; PFAS. Contamination of Water: Department of Health. Available online: https://health.ri.gov/water/about/pfas/ (accessed on 3 February 2022).
- ITRC; PFAS Team. PFAS. Available online: https://itrcweb.org/teams/active/pfas (accessed on 19 February 2022).
- Ma, D.; Zhong, H.; Lv, J.; Wang, Y.; Jiang, G. Levels, distributions, and sources of legacy and novel per- and perfluoroalkyl substances (PFAS) in the topsoil of Tianjin, China. J. Environ. Sci. 2022, 112, 71–81. [Google Scholar] [CrossRef]
- Kazwini, T.; Yadav, S.; Ibrar, I.; Al-Juboori, R.A.; Singh, L.; Ganbat, N.; Karbassiyazdi, E.; Samal, A.K.; Subbiah, S.; Altaee, A. Updated review on emerging technologies for PFAS contaminated water treatment. Chem. Eng. Res. Des. 2022, 182, 667–700. [Google Scholar] [CrossRef]
- Scheffey, J.L.; Darwin, R.L.; Leonard, J.T. Evaluating firefighting foams for aviation fire protection. Fire Technol. 1995, 31, 224–243. [Google Scholar] [CrossRef]
- Cousins, I.T.; Vestergren, R.; Wang, Z.; Scheringer, M.; McLachlan, M.S. The precautionary principle and chemicals management: The example of perfluoroalkyl acids in groundwater. Environ. Int. 2016, 94, 331–340. [Google Scholar] [CrossRef]
- Veciana, M.; Bräunig, J.; Farhat, A.; Pype, M.-L.; Freguia, S.; Carvalho, G.; Keller, J.; Ledezma, P. Electrochemical oxidation processes for PFAS removal from contaminated water and wastewater: Fundamentals, gaps and opportunities towards practical implementation. J. Hazard. Mater. 2022, 434, 128886. [Google Scholar] [CrossRef] [PubMed]
- Houtz, E.F.; Higgins, C.P.; Field, J.A.; Sedlak, D.L. Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environ. Sci. Technol. 2013, 47, 8187–8195. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Q.; Chen, F.; Sun, J.; Luo, K.; Yao, F.; Wang, X.; Wang, D.; Li, X.; Zeng, G. Photocatalytic degradation of perfluorooctanoic acid and perfluorooctane sulfonate in water: A critical review. Chem. Eng. J. 2017, 328, 927–942. [Google Scholar] [CrossRef]
- Holmquist, H.; Fantke, P.; Cousins, I.T.; Owsianiak, M.; Liagkouridis, I.; Peters, G.M. An (eco)toxicity life cycle impact assessment framework for per- and polyfluoroalkyl substances. Environ. Sci. Technol. 2020, 54, 6224–6234. [Google Scholar] [CrossRef]
- Coggan, T.L.; Moodie, D.; Kolobaric, A.; Szabo, D.; Shimeta, J.; Crosbie, N.D.; Lee, E.; Fernandes, M.; Clarke, B.O. An investigation into per- and polyfluoroalkyl substances (PFAS) in nineteen australian wastewater treatment plants (WWTPs). Heliyon 2019, 5, e02316. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Ruiz, B.; Gómez-Lavín, S.; Diban, N.; Boiteux, V.; Colin, A.; Dauchy, X.; Urtiaga, A. Efficient electrochemical degradation of poly- and perfluoroalkyl substances (PFASs) from the effluents of an industrial wastewater treatment plant. Chem. Eng. J. 2017, 322, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Dauchy, X.; Boiteux, V.; Bach, C.; Colin, A.; Hemard, J.; Rosin, C.; Munoz, J.F. Mass flows and fate of per- and polyfluoroalkyl substances (PFASs) in the wastewater treatment plant of a fluorochemical manufacturing facility. Sci. Total Environ. 2017, 576, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.M.; Higgins, C.P.; Huset, C.A.; Luthy, R.G.; Barofsky, D.F.; Field, J.A. Fluorochemical mass flows in a municipal wastewater treatment facility. Environ. Sci. Technol. 2006, 40, 7350–7357. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, E.; Kannan, K. Mass loading and fate of perfluoroalkyl surfactants in wastewater treatment plants. Environ. Sci. Technol. 2006, 40, 1408–1414. [Google Scholar] [CrossRef]
- Fuertes, I.; Gómez-Lavín, S.; Elizalde, M.P.; Urtiaga, A. Perfluorinated alkyl substances (PFASs) in Northern Spain municipal solid waste landfill leachates. Chemosphere 2017, 168, 399–407. [Google Scholar] [CrossRef]
- Ahrens, L. Polyfluoroalkyl compounds in the aquatic environment: A review of their occurrence and fate. J. Environ. Monit. 2011, 13, 20–31. [Google Scholar] [CrossRef]
- Loganathan, B.G.; Sajwan, K.S.; Sinclair, E.; Senthil Kumar, K.; Kannan, K. Perfluoroalkyl sulfonates and perfluorocarboxylates in two wastewater treatment facilities in Kentucky and Georgia. Water Res. 2007, 41, 4611–4620. [Google Scholar] [CrossRef]
- Radjenovic, J.; Sedlak, D.L. Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environ. Sci. Technol. 2015, 49, 11292–11302. [Google Scholar] [CrossRef]
- Trautmann, A.M.; Schell, H.; Schmidt, K.R.; Mangold, K.-M.; Tiehm, A. Electrochemical degradation of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in groundwater. Water Sci. Technol. 2015, 71, 1569–1575. [Google Scholar] [CrossRef]
- Liang, S.; Mora, R.; Huang, Q.; Casson, R.; Wang, Y.; Woodard, S.; Anderson, H. Field demonstration of coupling ion-exchange resin with electrochemical oxidation for enhanced treatment of per- and polyfluoroalkyl substances (PFAS) in groundwater. Chem. Eng. J. Adv. 2022, 9, 100216. [Google Scholar] [CrossRef]
- Schaefer, C.E.; Choyke, S.; Ferguson, P.L.; Andaya, C.; Burant, A.; Maizel, A.; Strathmann, T.J.; Higgins, C.P. Electrochemical transformations of perfluoroalkyl acid (PFAA) precursors and pfaas in groundwater impacted with aqueous film forming foams. Environ. Sci. Technol. 2018, 52, 10689–10697. [Google Scholar] [CrossRef]
- Schaefer, C.E.; Andaya, C.; Maizel, A.; Higgins, C.P. Assessing continued electrochemical treatment of groundwater impacted by aqueous film-forming foams. J. Environ. Eng. 2019, 145, 06019007. [Google Scholar] [CrossRef]
- Zhuo, Q.; Deng, S.; Yang, B.; Huang, J.; Wang, B.; Zhang, T.; Yu, G. Degradation of perfluorinated compounds on a boron-doped diamond electrode. Electrochim. Acta 2012, 77, 17–22. [Google Scholar] [CrossRef]
- Nzeribe, B.N.; Crimi, M.; Mededovic Thagard, S.; Holsen, T.M. Physico-chemical processes for the treatment of per- and polyfluoroalkyl substances (PFAS): A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 866–915. [Google Scholar] [CrossRef]
- Maldonado, V.Y.; Becker, M.F.; Nickelsen, M.G.; Witt, S.E. Laboratory and semi-pilot scale study on the electrochemical treatment of perfluoroalkyl acids from ion exchange still bottoms. Water 2021, 13, 2873. [Google Scholar] [CrossRef]
- Soriano, A.; Schaefer, C.; Urtiaga, A. Enhanced treatment of perfluoroalkyl acids in groundwater by membrane separation and electrochemical oxidation. Chem. Eng. J. Adv. 2020, 4, 100042. [Google Scholar] [CrossRef]
- Le, T.X.H.; Haflich, H.; Shah, A.D.; Chaplin, B.P. Energy-efficient electrochemical oxidation of perfluoroalkyl substances using a Ti4O7 reactive electrochemical membrane anode. Environ. Sci. Technol. Lett. 2019, 6, 504–510. [Google Scholar] [CrossRef]
- Soriano, Á.; Gorri, D.; Urtiaga, A. Efficient treatment of perfluorohexanoic acid by nanofiltration followed by electrochemical degradation of the NF concentrate. Water Res. 2017, 112, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soriano, Á.; Gorri, D.; Urtiaga, A. Membrane preconcentration as an efficient tool to reduce the energy consumption of perfluorohexanoic acid electrochemical treatment. Sep. Purif. Technol. 2019, 208, 160–168. [Google Scholar] [CrossRef]
- Soriano, Á.; Gorri, D.; Biegler, L.T.; Urtiaga, A. An optimization model for the treatment of perfluorocarboxylic acids considering membrane preconcentration and BDD electrooxidation. Water Res. 2019, 164, 114954. [Google Scholar] [CrossRef]
- Witt, S.; Rancis, N.; Ensch, M.; Maldonado, V. Electrochemical destruction of ‘forever chemicals’: The right solution at the right time. Electrochem. Soc. Interface 2020, 29, 73–76. [Google Scholar] [CrossRef]
- Chaplin, B.P. The prospect of electrochemical technologies advancing worldwide water treatment. Acc. Chem. Res. 2019, 52, 596–604. [Google Scholar] [CrossRef]
- Lin, H.; Niu, J.; Liang, S.; Wang, C.; Wang, Y.; Jin, F.; Luo, Q.; Huang, Q. Development of Macroporous Magnéli phase Ti4O7 ceramic materials: As an efficient anode for mineralization of poly- and perfluoroalkyl substances. Chem. Eng. J. 2018, 354, 1058–1067. [Google Scholar] [CrossRef]
- Radjenovic, J.; Duinslaeger, N.; Avval, S.S.; Chaplin, B.P. Facing the challenge of poly- and perfluoroalkyl substances in water: Is electrochemical oxidation the answer? Environ. Sci. Technol. 2020, 54, 14815–14829. [Google Scholar] [CrossRef]
- Carter, K.E.; Farrell, J. Oxidative destruction of perfluorooctane sulfonate using boron-doped diamond film electrodes. Environ. Sci. Technol. 2008, 42, 6111–6115. [Google Scholar] [CrossRef]
- Ochiai, T.; Iizuka, Y.; Nakata, K.; Murakami, T.; Tryk, D.A.; Fujishima, A.; Koide, Y.; Morito, Y. Efficient electrochemical decomposition of perfluorocarboxylic acids by the use of a boron-doped diamond electrode. Diam. Relat. Mater. 2011, 20, 64–67. [Google Scholar] [CrossRef]
- Sidnell, T.; Wood, R.J.; Hurst, J.; Lee, J.; Bussemaker, M.J. Sonolysis of per- and poly fluoroalkyl substances (PFAS): A meta-analysis. Ultrason. Sonochem. 2022, 87, 105944. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.J.; Lee, J.; Bussemaker, M.J. A parametric review of sonochemistry: Control and augmentation of sonochemical activity in aqueous solutions. Ultrason. Sonochem. 2017, 38, 351–370. [Google Scholar] [CrossRef]
- Schaefer, C.E.; Andaya, C.; Urtiaga, A.; McKenzie, E.R.; Higgins, C.P. Electrochemical treatment of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) in groundwater impacted by aqueous film forming foams (AFFFs). J. Hazard Mater. 2015, 295, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Azizi, O.; Hubler, D.; Schrader, G.; Farrell, J.; Chaplin, B.P. Mechanism of perchlorate formation on boron-doped diamond film anodes. Environ. Sci. Technol. 2011, 45, 10582–10590. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.E.H.; Rollin, J.; Iourtchouk, T. The occurrence of perchlorate during drinking water electrolysis using BDD anodes. Electrochim. Acta 2009, 54, 2102–2107. [Google Scholar] [CrossRef]
- Urtiaga, A.; Fernandez-Castro, P.; Gómez, P.; Ortiz, I. Remediation of wastewaters containing tetrahydrofuran. Study of the electrochemical mineralization on BDD electrodes. Chem. Eng. J. 2014, 239, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Boudreau, J.; Bejan, D.; Li, S.; Bunce, N.J. Competition between electrochemical advanced oxidation and electrochemical hypochlorination of sulfamethoxazole at a boron-doped diamond anode. Ind. Eng. Chem. Res. 2010, 49, 2537–2542. [Google Scholar] [CrossRef]
- Radjenovic, J.; Escher, B.I.; Rabaey, K. Electrochemical degradation of the β-blocker metoprolol by Ti/Ru0.7Ir0.3O2 and Ti/SnO2-Sb electrodes. Water Res. 2011, 45, 3205–3214. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Pierce, R.; Lin, H.; Chiang, S.Y.D.; Huang, Q. Electrochemical oxidation of PFOA and PFOS in concentrated waste streams. Remediation 2018, 28, 127–134. [Google Scholar] [CrossRef]
- Sunka, P.; Babický, V.; Clupek, M.; Lukes, P.; Simek, M.; Schmidt, J.; Cernák, M. Generation of chemically active species by electrical discharges in water. Plasma Sources Sci. Technol. 1999, 8, 258–265. [Google Scholar] [CrossRef]
- Thagard, S.M.; Takashima, K.; Mizuno, A. Chemistry of the positive and negative electrical discharges formed in liquid water and above a gas–liquid surface. Plasma Chem. Plasma Process. 2009, 29, 455–473. [Google Scholar] [CrossRef]
- Singh, R.K.; Multari, N.; Nau-Hix, C.; Anderson, R.H.; Richardson, S.D.; Holsen, T.M.; Mededovic Thagard, S. Rapid removal of poly- and perfluorinated compounds from investigation-derived waste (IDW) in a pilot-scale plasma reactor. Environ. Sci. Technol. 2019, 53, 11375–11382. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.; Richard, C.; Minella, M. State of the art and perspectives about non-thermal plasma applications for the removal of PFAS in water. Chem. Eng. J. Adv. 2022, 10, 100253. [Google Scholar] [CrossRef]
- Jovicic, V.; Khan, M.J.; Zbogar-Rasic, A.; Fedorova, N.; Poser, A.; Swoboda, P.; Delgado, A. Degradation of low concentrated perfluorinated compounds (PFCs) from water samples using non-thermal atmospheric plasma (NTAP). Energies 2018, 11, 1290. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, R.; Obo, H.; Takeuchi, N.; Yasuoka, K. Decomposition of perfluorinated compounds in water by DC plasma within oxygen bubbles. Electr. Eng. Jpn. 2015, 190, 9–16. [Google Scholar] [CrossRef]
- Yasuoka, K.; Sasaki, K.; Hayashi, R. An energy-efficient process for decomposing perfluorooctanoic and perfluorooctane sulfonic acids using DC plasmas generated within gas bubbles. Plasma Sources Sci. Technol. 2011, 20, 034009. [Google Scholar] [CrossRef]
- Mededovic Thagard, S.; Stratton, G.R.; Dai, F.; Bellona, C.L.; Holsen, T.M.; Bohl, D.G.; Paek, E.; Dickenson, E.R.V. Plasma-based water treatment: Development of a general mechanistic model to estimate the treatability of different types of contaminants. J. Phys. D Appl. Phys. 2017, 50, 014003. [Google Scholar] [CrossRef]
- Stratton, G.R.; Dai, F.; Bellona, C.L.; Holsen, T.M.; Dickenson, E.R.V.; Mededovic Thagard, S. Plasma-based water treatment: Efficient transformation of perfluoroalkyl substances in prepared solutions and contaminated groundwater. Environ. Sci. Technol. 2017, 51, 1643–1648. [Google Scholar] [CrossRef]
- Singh, R.K.; Multari, N.; Nau-Hix, C.; Woodard, S.; Nickelsen, M.; Mededovic Thagard, S.; Holsen, T.M. Removal of poly- and per-fluorinated compounds from ion exchange regenerant still bottom samples in a plasma reactor. Environ. Sci. Technol. 2020, 54, 13973–13980. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Liu, L.; Guo, Y.; Mu, Q.; Mellouki, A. Enhanced degradation of perfluorooctanoic acid using dielectric barrier discharge with La/Ce-Doped TiO2. Environ. Sci. Pollut. Res. 2017, 24, 15794–15803. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, J.; Zhang, Z.; Long, X. Sono-photocatalytic degradation of organic pollutants in water. Prog. Chem. 2008, 20, 1621–1627. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.; Lim, X.; Yang, H.; Goodson, B.M.; Liu, J. Degradation of per- and polyfluoroalkyl substances (PFAS) in wastewater effluents by photocatalysis for water reuse. J. Water Process Eng. 2022, 46, 102556. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, T.; Zhao, D.; Li, F.; Liu, W.; Wang, B.; An, B. Adsorption and solid-phase photocatalytic degradation of perfluorooctane sulfonate in water using gallium-doped carbon-modified titanate nanotubes. Chem. Eng. J. 2021, 421, 129676. [Google Scholar] [CrossRef]
- Xu, B.; Ahmed, M.B.; Zhou, J.L.; Altaee, A.; Wu, M.; Xu, G. Photocatalytic removal of perfluoroalkyl substances from water and wastewater: Mechanism, kinetics and controlling factors. Chemosphere 2017, 189, 717–729. [Google Scholar] [CrossRef]
- Li, F.; Wei, Z.; He, K.; Blaney, L.; Cheng, X.; Xu, T.; Liu, W.; Zhao, D. A concentrate-and-destroy technique for degradation of perfluorooctanoic acid in water using a new adsorptive photocatalyst. Water Res. 2020, 185, 116219. [Google Scholar] [CrossRef]
- Wang, M.; Cai, Y.; Zhou, B.; Yuan, R.; Chen, Z.; Chen, H. Science of the total environment removal of PFASs from water by carbon-based composite photocatalysis with adsorption and catalytic properties: A review. Sci. Total Environ. 2022, 836, 155652. [Google Scholar] [CrossRef]
- Xu, T.; Zhu, Y.; Duan, J.; Xia, Y.; Tong, T.; Zhang, L.; Zhao, D. Enhanced photocatalytic degradation of perfluorooctanoic acid using carbon-modified bismuth phosphate composite: Effectiveness, material synergy and roles of carbon. Chem. Eng. J. 2020, 395, 124991. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- McIntyre, H.; Minda, V.; Hawley, E.; Deeb, R.; Hart, M. Coupled photocatalytic alkaline media as a destructive technology for per- and polyfluoroalkyl substances in aqueous film-forming foam impacted stormwater. Chemosphere 2022, 291, 132790. [Google Scholar] [CrossRef]
- Tsoumachidou, S.; Kouras, A.; Poulios, I. Heterogeneous and homogeneous photocatalytic degradation of psychoactive drug fluvoxamine: Kinetic study, inorganic ions and phytotoxicity evaluation. J. Chem. Technol. Biotechnol. 2018, 93, 1705–1713. [Google Scholar] [CrossRef]
- Li, P.; Zhi, D.; Zhang, X.; Zhu, H.; Li, Z.; Peng, Y.; He, Y.; Luo, L.; Rong, X.; Zhou, Y. Research progress on the removal of hazardous perfluorochemicals: A review. J. Environ. Manag. 2019, 250, 109488. [Google Scholar] [CrossRef]
- Long, M.; Brame, J.; Qin, F.; Bao, J.; Li, Q.; Alvarez, P.J.J. Phosphate changes effect of humic acids on TiO2 photocatalysis: From inhibition to mitigation of electron–hole recombination. Environ. Sci. Technol. 2017, 51, 514–521. [Google Scholar] [CrossRef]
- Rodriguez-Freire, L.; Balachandran, R.; Sierra-Alvarez, R.; Keswani, M. Effect of sound frequency and initial concentration on the sonochemical degradation of perfluorooctane sulfonate (PFOS). J. Hazard Mater. 2015, 300, 662–669. [Google Scholar] [CrossRef]
- Fernandez, N.A.; Rodriguez-Freire, L.; Keswani, M.; Sierra-Alvarez, R. Effect of chemical structure on the sonochemical degradation of perfluoroalkyl and polyfluoroalkyl substances (PFASs). Environ. Sci. 2016, 2, 975–983. [Google Scholar] [CrossRef] [Green Version]
- Kewalramani, J.A.; Wang, B.; Marsh, R.W.; Meegoda, J.N.; Rodriguez Freire, L. Coupled high and low-frequency ultrasound remediation of PFAS-contaminated soils. Ultrason. Sonochem. 2022, 88, 106063. [Google Scholar] [CrossRef]
- Didenko, Y.T.; McNamara, W.B.; Suslick, K.S. Temperature of Multibubble Sonoluminescence in Water. J. Phys. Chem. A 1999, 103, 10783–10788. [Google Scholar] [CrossRef]
- Ciawi, E.; Rae, J.; Ashokkumar, M.; Grieser, F. Determination of temperatures within acoustically generated bubbles in aqueous solutions at different ultrasound frequencies. J. Phys. Chem. B 2006, 110, 13656–13660. [Google Scholar] [CrossRef]
- Hung, H.-M.; Hoffmann, M.R. Kinetics and mechanism of the sonolytic degradation of chlorinated hydrocarbons: Frequency effects. J. Phys. Chem. A 1999, 103, 2734–2739. [Google Scholar] [CrossRef]
- Moriwaki, H.; Takagi, Y.; Tanaka, M.; Tsuruho, K.; Okitsu, K.; Maeda, Y. Sonochemical decomposition of perfluorooctane sulfonate and perfluorooctanoic acid. Environ. Sci. Technol. 2005, 39, 3388–3392. [Google Scholar] [CrossRef]
- Meegoda, J.; Kewalramani, J. Coupled High and Low-Frequency Ultrasound Systems and Methods for Remediation of Contaminated Solids. U.S. Patent Application No. 17/154,872, 29 July 2021. [Google Scholar]
- James Wood, R.; Sidnell, T.; Ross, I.; McDonough, J.; Lee, J.; Bussemaker, M.J. Ultrasonic degradation of perfluorooctane sulfonic acid (PFOS) correlated with sonochemical and sonoluminescence characterisation. Ultrason. Sonochem. 2020, 68, 105196. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Park, H.; Cheng, J.; Mader, B.T.; Hoffmann, M.R. Kinetics and mechanism of the sonolytic conversion of the aqueous perfluorinated surfactants, perfluorooctanoate (PFOA), and perfluorooctane sulfonate (PFOS) into inorganic products. J. Phys. Chem. A 2008, 112, 4261–4270. [Google Scholar] [CrossRef] [Green Version]
- Brotchie, A.; Grieser, F.; Ashokkumar, M. Sonochemistry and sonoluminescence under dual-frequency ultrasound irradiation in the presence of water-soluble solutes. J. Phys. Chem. C 2008, 112, 10247–10250. [Google Scholar] [CrossRef]
- Gole, V.L.; Fishgold, A.; Sierra-Alvarez, R.; Deymier, P.; Keswani, M. Treatment of perfluorooctane sulfonic acid (PFOS) using a large-scale sonochemical reactor. Sep. Purif. Technol. 2018, 194, 104–110. [Google Scholar] [CrossRef]
- Pee, G.-Y.; Rathman, J.F.; Weavers, L.K. Effects of surface active properties on the cavitational degradation of surfactant contaminants. Ind. Eng. Chem. Res. 2004, 43, 5049–5056. [Google Scholar] [CrossRef]
- Son, Y.; Lim, M.; Cui, M.; Khim, J. Estimation of sonochemical reactions under single and dual frequencies based on energy analysis. Jpn. J. Appl. Phys. 2010, 49, 07HE02. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, W.; Wang, C.; Liang, Y. Sonochemical degradation of poly- and perfluoroalkyl substances—A review. Ultrason. Sonochem. 2020, 69, 105245. [Google Scholar] [CrossRef]
- Dükkancı, M.; Gündüz, G. Ultrasonic Degradation of Oxalic Acid in Aqueous Solutions. Ultrason. Sonochem. 2006, 13, 517–522. [Google Scholar] [CrossRef]
- Kanthale, P.; Ashokkumar, M.; Grieser, F. Sonoluminescence, sonochemistry (H2O2 Yield) and bubble dynamics: Frequency and power effects. Ultrason. Sonochem. 2008, 15, 143–150. [Google Scholar] [CrossRef]
- Campbell, T.; Hoffmann, M.R. Sonochemical degradation of perfluorinated surfactants: Power and multiple frequency effects. Sep. Purif. Technol. 2015, 156, 1019–1027. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Park, H.; Cheng, J.; Mader, B.T.; Hoffmann, M.R. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA). Front. Environ. Sci. Eng. China 2009, 3, 129–151. [Google Scholar] [CrossRef]
- Singh Kalra, S.; Cranmer, B.; Dooley, G.; Hanson, A.J.; Maraviov, S.; Mohanty, S.K.; Blotevogel, J.; Mahendra, S. Sonolytic destruction of per- and polyfluoroalkyl substances in groundwater, aqueous film-forming foams, and investigation derived waste. Chem. Eng. J. 2021, 425, 131778. [Google Scholar] [CrossRef]
- Laramay, F.; Crimi, M. Theoretical evaluation of chemical and physical feasibility of an in situ ultrasonic reactor for remediation of groundwater contaminated with per- and polyfluoroalkyl substances. Remediation 2020, 31, 45–58. [Google Scholar] [CrossRef]
- Supercritical Water Oxidation (SCWO)—Enviro Wiki. Available online: https://www.enviro.wiki/index.php?title=Supercritical_Water_Oxidation_(SCWO) (accessed on 22 May 2022).
- Schlosky, K.M. Supercritical phase transitions at very high pressure. J. Chem. Educ. 1989, 66, 989. [Google Scholar] [CrossRef]
- Krause, M.J.; Thoma, E.; Sahle-Damesessie, E.; Crone, B.; Whitehill, A.; Shields, E.; Gullett, B. Supercritical water oxidation as an innovative technology for PFAS destruction. J. Environ. Eng. 2022, 148, 05021006. [Google Scholar] [CrossRef]
- Berg, C.; Crone, B.; Gullett, B.; Higuchi, M.; Krause, M.J.; Lemieux, P.M.; Martin, T.; Shields, E.P.; Struble, E.; Thoma, E.; et al. Developing innovative treatment technologies for PFAS-containing wastes. J. Air. Waste Manag. Assoc. 2022, 72, 540–555. [Google Scholar] [CrossRef]
- ITRC; PFAS Team. PFAS—Per- and Polyfluoroalkyl Substances, Physical and Chemical Properties. Available online: https://pfas-1.itrcweb.org/4-physical-and-chemical-properties/#:~:text=1%20Physical%20State%2FAppearance,is%20addressed%20in%20Section%204.2 (accessed on 26 April 2022).
- EPA. Air Pollution Control Technology Fact Sheet. Available online: https://www3.epa.gov/ttncatc1/dir1/fthermal.pdf (accessed on 15 September 2022).
- EPA. Per-and Polyfluoroalkyl Substances (PFAS): Incineration to Manage PFAS Waste Streams Background. US EPA Tech. Brief. 2020. [Google Scholar] [CrossRef]
- Solo-Gabriele, H.M.; Jones, A.S.; Lindstrom, A.B.; Lang, J.R. Waste type, incineration, and aeration are associated with per- and polyfluoroalkyl levels in landfill leachates. Waste Manag. 2020, 107, 191–200. [Google Scholar] [CrossRef]
- Wang, B.; Yao, Y.; Chen, H.; Chang, S.; Tian, Y.; Sun, H. Per- and polyfluoroalkyl substances and the contribution of unknown precursors and short-chain (C2–C3) perfluoroalkyl carboxylic acids at solid waste disposal facilities. Sci. Total Environ. 2020, 705, 135832. [Google Scholar] [CrossRef]
- Winchell, L.J.; Ross, J.J.; Wells, M.J.M.; Fonoll, X.; Norton, J.W.; Bell, K.Y. Per- and polyfluoroalkyl substances thermal destruction at water resource recovery facilities: A state of the science review. Water Environ. Res. 2021, 93, 826–843. [Google Scholar] [CrossRef] [PubMed]
- Hogue, C. Incineration May Spread, Not Break down PFAS. Available online: https://cen.acs.org/environment/persistent-pollutants/Incincerators-spread-break-down-PFAS/98/web/2020/04 (accessed on 18 September 2022).
- Stoiber, T.; Evans, S.; Naidenko, O.V. Disposal of products and materials containing per- and polyfluoroalkyl substances (PFAS): A cyclical problem. Chemosphere 2020, 260, 127659. [Google Scholar] [CrossRef] [PubMed]
- Crunden, E.A. Defense Department Hits the Brakes on PFAS Incineration. Available online: https://www.eenews.net/articles/defense-department-hits-the-brakes-on-pfas-incineration/ (accessed on 18 September 2022).
- U.S.C. H.R.2591 PFAS Waste Incineration Ban Act of 2019. 2019. Available online: https://www.govinfo.gov/ (accessed on 9 September 2022).
- U.S.C. Public Law 116-92-National Defense Authorization Act for Fiscal Year 2020. 2019. Available online: https://www.govinfo.gov/ (accessed on 9 September 2022).
- Horst, J.; McDonough, J.; Ross, I.; Houtz, E. Understanding and managing the potential by-products of PFAS destruction. Groundw. Monit. Remediat. 2020, 40, 17–27. [Google Scholar] [CrossRef]
- Stockenhuber, S.; Weber, N.; Dixon, L.; Lucas, J.; Grimison, C.; Bennett, M.; Stockenhuber, M.; Mackie, J.; Kennedy, E. Thermal Degradation of Perfluorooctanoic Acid (PFOA). In Proceedings of the 16th International Conference on Environmental Science and Technology, Rhodes, Greece, 4–7 September 2019. [Google Scholar]
- Altarawneh, M.; Almatarneh, M.H.; Dlugogorski, B.Z. Thermal decomposition of perfluorinated carboxylic acids: Kinetic model and theoretical requirements for PFAS incineration. Chemosphere 2022, 286, 131685. [Google Scholar] [CrossRef]
- Sasi, P.C.; Alinezhad, A.; Yao, B.; Kubátová, A.; Golovko, S.A.; Golovko, M.Y.; Xiao, F. Effect of granular activated carbon and other porous materials on thermal decomposition of per- and polyfluoroalkyl substances: Mechanisms and implications for water purification. Water Res. 2021, 200, 117271. [Google Scholar] [CrossRef]
- Xiao, F.; Sasi, P.C.; Yao, B.; Kubátová, A.; Golovko, S.A.; Golovko, M.Y.; Soli, D. Thermal decomposition of PFAS: Response to comment on “thermal stability and decomposition of perfluoroalkyl substances on spent granular activated carbon”. Environ. Sci. Technol. Lett. 2021, 8, 364–365. [Google Scholar] [CrossRef]
- Crownover, E.; Oberle, D.; Kluger, M.; Heron, G. Perfluoroalkyl and polyfluoroalkyl substances thermal desorption evaluation. Remediat. J. 2019, 29, 77–81. [Google Scholar] [CrossRef]
- Yamada, T.; Taylor, P.H.; Buck, R.C.; Kaiser, M.A.; Giraud, R.J. Thermal degradation of fluorotelomer treated articles and related materials. Chemosphere 2005, 61, 974–984. [Google Scholar] [CrossRef]
- Taylor, P.H.; Yamada, T.; Striebich, R.C.; Graham, J.L.; Giraud, R.J. Investigation of waste incineration of fluorotelomer-based polymers as a potential source of PFOA in the environment. Chemosphere 2014, 110, 17–22. [Google Scholar] [CrossRef]
- Vidonish, J.E.; Zygourakis, K.; Masiello, C.A.; Sabadell, G.; Alvarez, P.J.J. Thermal treatment of hydrocarbon-impacted soils: A review of technology innovation for sustainable remediation. Engineering 2016, 2, 426–437. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Z.; He, X.; Song, M.; Westerhoff, P.; Doudrick, K.; Hanigan, D. Critical review of thermal decomposition of per- and polyfluoroalkyl substances: Mechanisms and implications for thermal treatment processes. Environ. Sci. Technol. 2022, 56, 5355–5370. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrov, K.; Gehrmann, H.J.; Hauser, M.; Mätzing, H.; Pigeon, D.; Stapf, D.; Wexler, M. Waste incineration of polytetrafluoroethylene (PTFE) to evaluate potential formation of per- and poly-fluorinated alkyl substances (PFAS) in Flue Gas. Chemosphere 2019, 226, 898–906. [Google Scholar] [CrossRef] [PubMed]
- García, A.N.; Viciano, N.; Font, R. Products obtained in the fuel-rich combustion of PTFE at high temperature. J. Anal. Appl. Pyrolysis 2007, 80, 85–91. [Google Scholar] [CrossRef]
- Wang, F.; Shih, K.; Lu, X.; Liu, C. Mineralization behavior of fluorine in perfluorooctanesulfonate (PFOS) during thermal treatment of lime-conditioned sludge. Environ. Sci. Technol. 2013, 47, 2621–2627. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, Y.; Li, C.; Pierce, R.; Gao, S.; Huang, Q. Degradation of perfluorooctanesulfonate by reactive electrochemical membrane composed of Magnéli phase titanium suboxide. Environ. Sci. Technol. 2019, 53, 14528–14537. [Google Scholar] [CrossRef]
- Singh, R.K.; Fernando, S.; Baygi, S.F.; Multari, N.; Thagard, S.M.; Holsen, T.M. Breakdown products from perfluorinated alkyl substances (PFAS) degradation in a plasma-based water treatment process. Environ. Sci. Technol. 2019, 53, 2731–2738. [Google Scholar] [CrossRef]
- Singh, R.K.; Brown, E.; Mededovic Thagard, S.; Holsen, T.M. Treatment of PFAS-containing landfill leachate using an enhanced contact plasma reactor. J. Hazard. Mater. 2021, 408, 124452. [Google Scholar] [CrossRef]
- Shende, T.; Andaluri, G.; Suri, R.P.S. Kinetic model for sonolytic degradation of non-volatile surfactants: Perfluoroalkyl substances. Ultrason. Sonochem. 2019, 51, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Campbell, T.Y.; Vecitis, C.D.; Mader, B.T.; Hoffmann, M.R. Perfluorinated Surfactant Chain-Length Effects on Sonochemical Kinetics. J. Phys. Chem. A 2009, 113, 9834–9842. [Google Scholar] [CrossRef]
- Cheng, J.; Vecitis, C.D.; Park, H.; Mader, B.T.; Hoffmann, M.R. Sonochemical degradation of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in groundwater: Kinetic effects of matrix inorganics. Environ. Sci. Technol. 2010, 44, 445–450. [Google Scholar] [CrossRef]
- McDonough, J.T.; Kirby, J.; Bellona, C.; Quinnan, J.A.; Welty, N.; Follin, J.; Liberty, K. Validation of supercritical water oxidation to destroy perfluoroalkyl acids. Remediat. J. 2022, 32, 75–90. [Google Scholar] [CrossRef]
- Pinkard, B.R.; Shetty, S.; Stritzinger, D.; Bellona, C.; Novosselov, I.V. Destruction of perfluorooctanesulfonate (PFOS) in a batch supercritical water oxidation reactor. Chemosphere 2021, 279, 130834. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meegoda, J.N.; Bezerra de Souza, B.; Casarini, M.M.; Kewalramani, J.A. A Review of PFAS Destruction Technologies. Int. J. Environ. Res. Public Health 2022, 19, 16397. https://doi.org/10.3390/ijerph192416397
Meegoda JN, Bezerra de Souza B, Casarini MM, Kewalramani JA. A Review of PFAS Destruction Technologies. International Journal of Environmental Research and Public Health. 2022; 19(24):16397. https://doi.org/10.3390/ijerph192416397
Chicago/Turabian StyleMeegoda, Jay N., Bruno Bezerra de Souza, Melissa Monteiro Casarini, and Jitendra A. Kewalramani. 2022. "A Review of PFAS Destruction Technologies" International Journal of Environmental Research and Public Health 19, no. 24: 16397. https://doi.org/10.3390/ijerph192416397







