Evaluation of Biodegradation of BTEX in the Subsurface of a Petrochemical Site near the Yangtze River, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Sampling
2.2. Analyses of Physiochemical Properties of the Collected Samples
2.3. Analyses of Microbial Communities of the Collected Samples
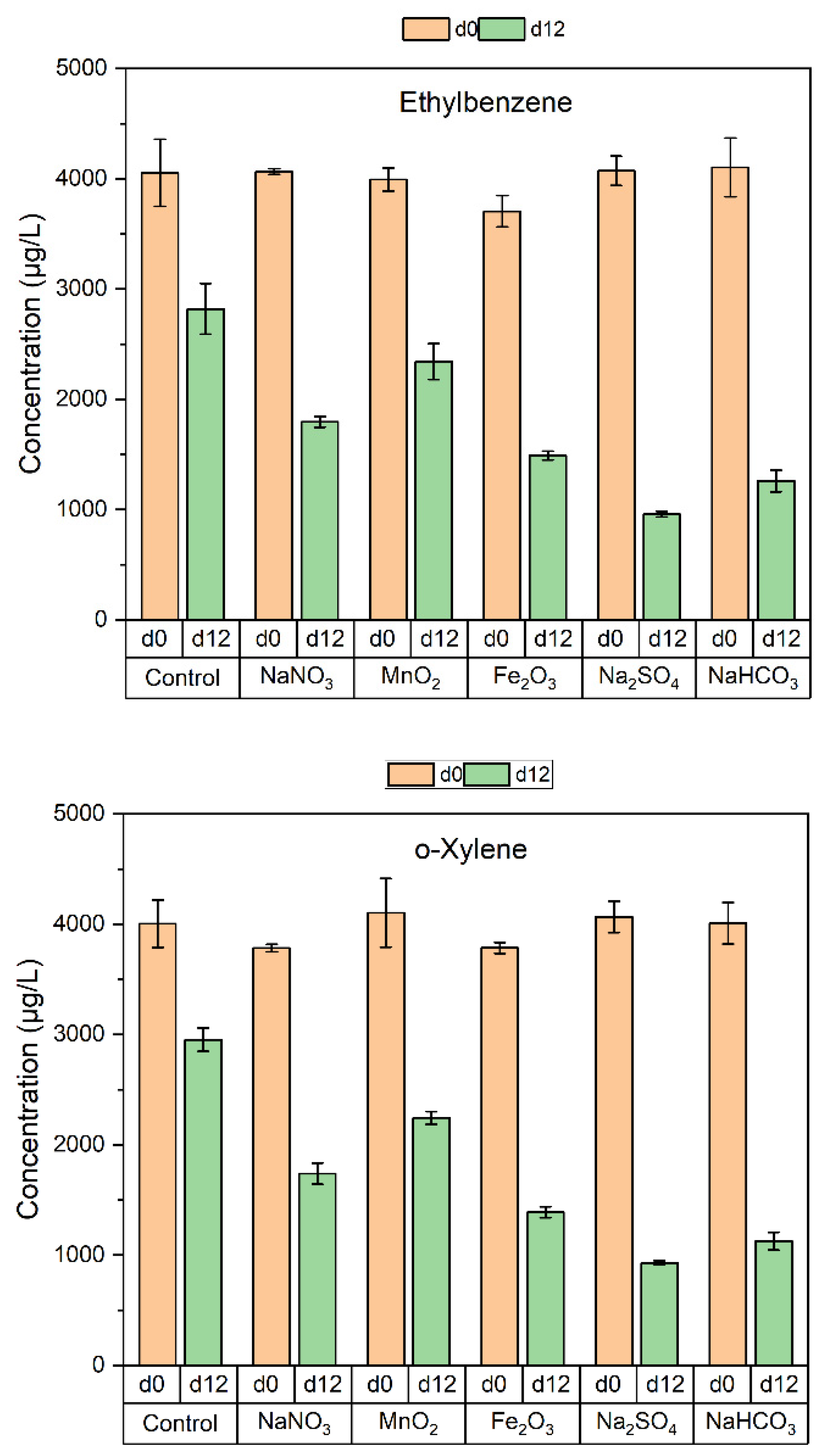
2.4. Microcosm Setup and Analyses
2.5. Data Analyses
3. Results and Discussion
3.1. Characterization of the Soil and Groundwater Samples Collected from the Site
3.1.1. Physicochemical Properties of the Samples
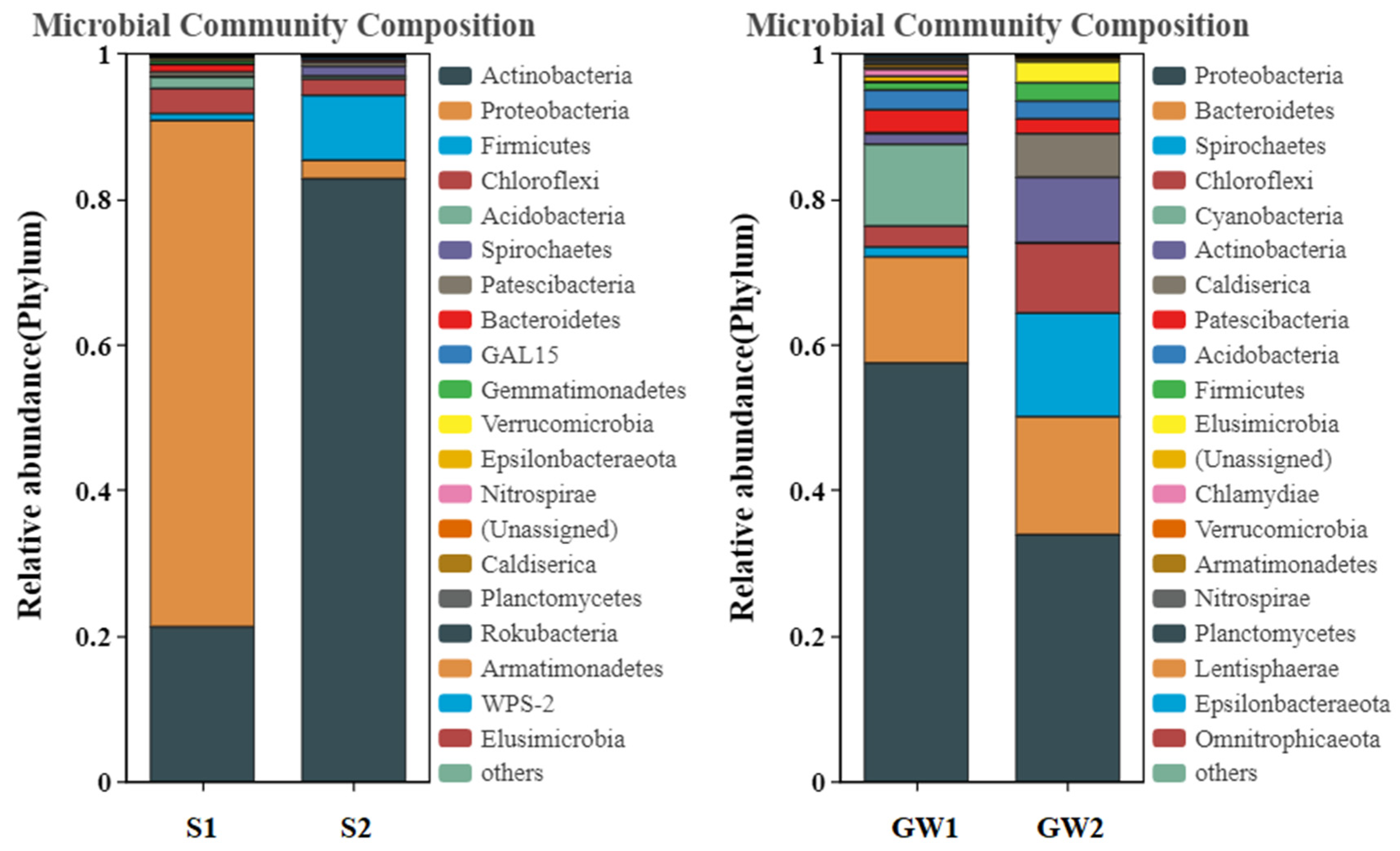
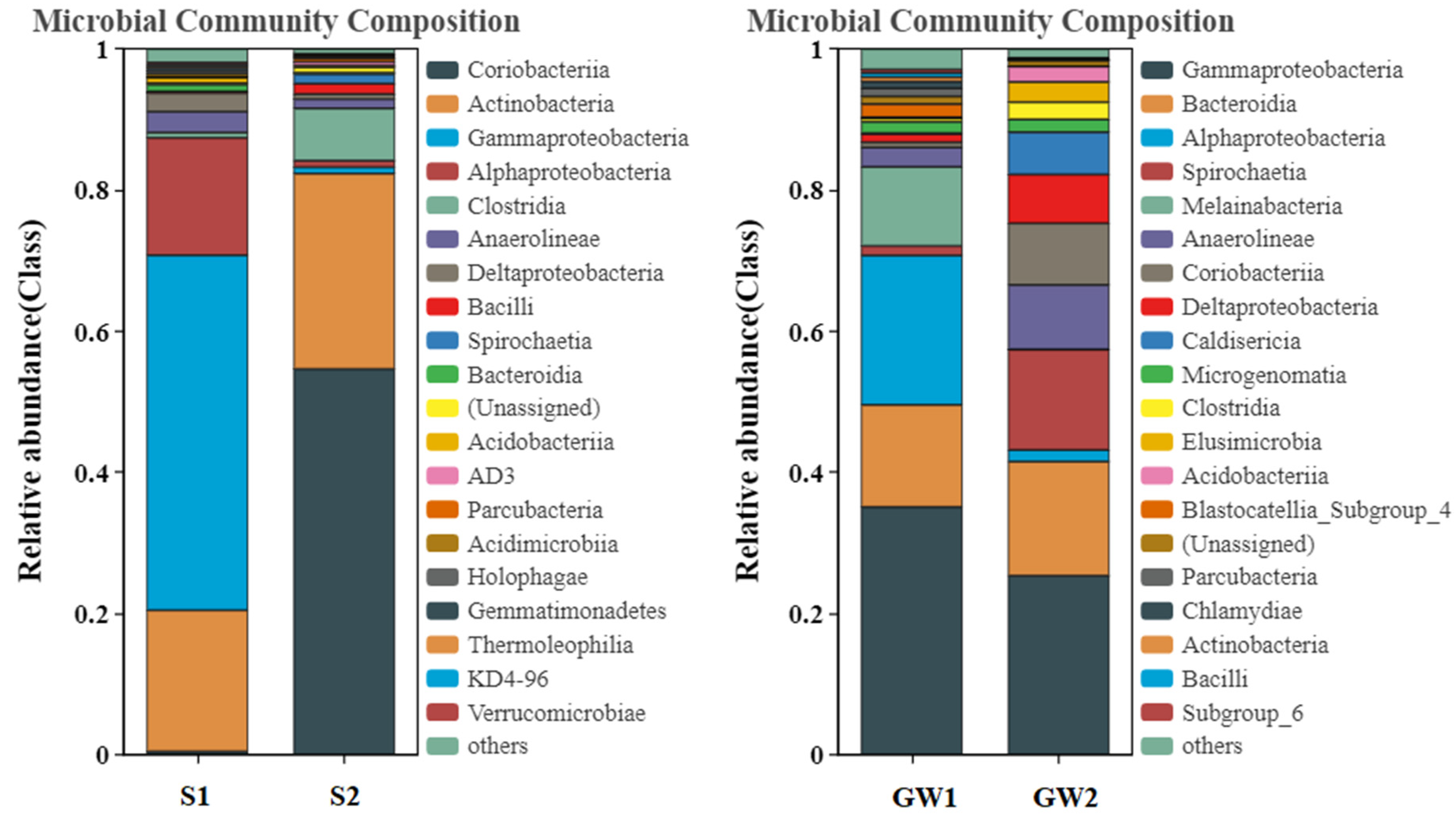
3.1.2. Bacterial Community Structures of the Samples
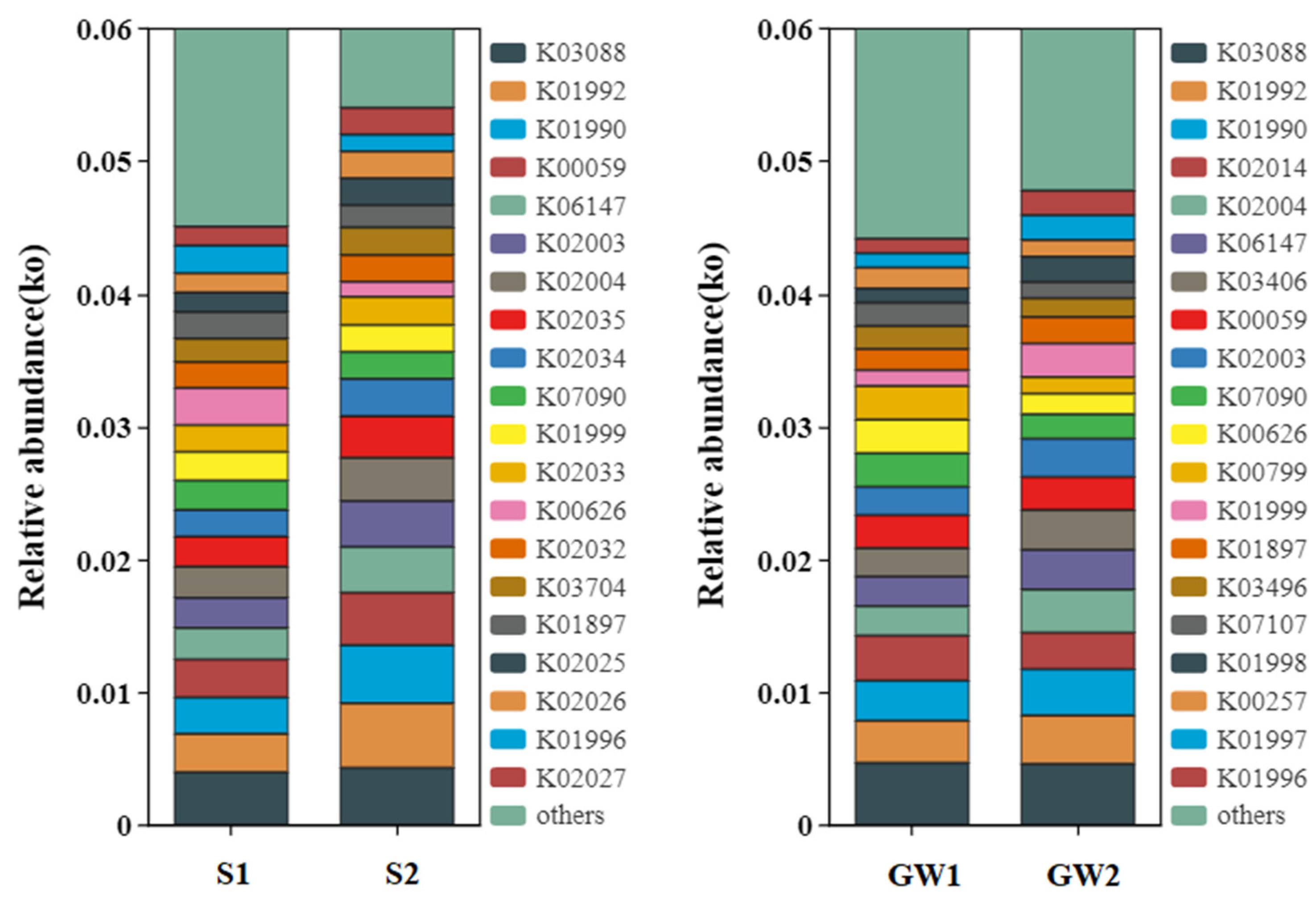
3.1.3. Functional Prediction for the Bacterial Communities
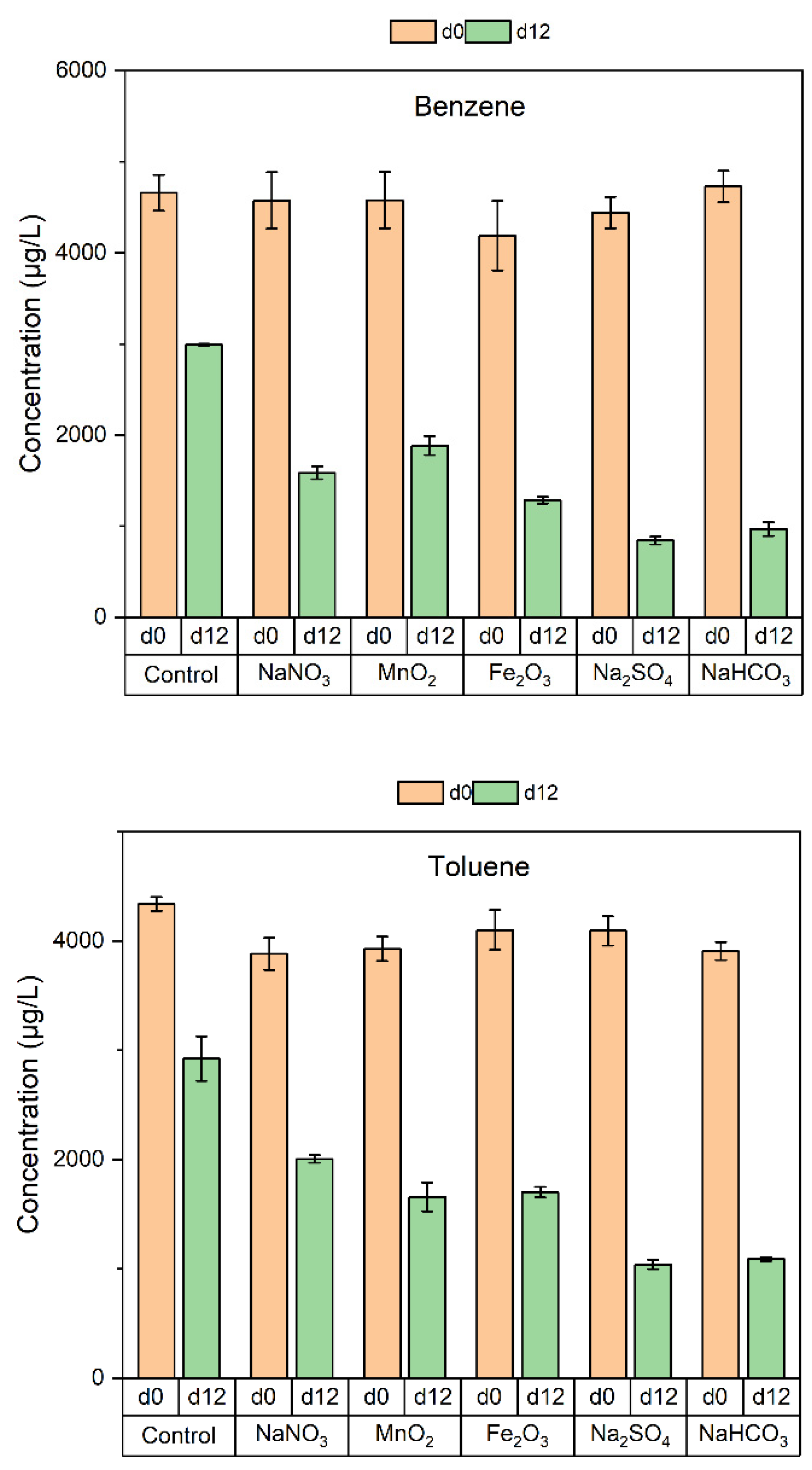
3.2. Degradation of BTEX by the Soil Microorganisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kong, H.; He, C. A Report from the Yangtze River Economic Belt—A Strong Man Breaks His Wrist to Cure the “Yangtze River Disease”. 2018. Available online: https://baijiahao.baidu.com/s?id=1609557431739492860&wfr=spider&for=pc (accessed on 1 January 2019).
- Tan, H.; Liu, T.; Cao, X.; Gu, G.; Liu, M. Consideration on soil pollution control in petroleum and petrochemical enterprises. China Pet. Chem. Std. Qual. 2019, 9, 152–154. [Google Scholar]
- Zhang, D.; Jiang, L.; Xia, T.; Jia, X.; Zheng, D.; Zhang, L.; Fan, Y.; Liu, H. The migration and biodegradation of petroleum hydrocarbons in soils-groundwater system: A review. Environ. Eng. 2015, 7, 1–6. [Google Scholar] [CrossRef]
- Ren, W.; Zhou, Q.; Wang, M. Environmental behavior of BTEX in soil: A review. Chin. J. Ecol. 2009, 28, 1647–1654. [Google Scholar] [CrossRef]
- Zhai, Y. Investigation and risk assessment study of organic contaminated soil from a petrochemical enterprise. Environ. Eng. 2019, 37 (Suppl. S389–S393), 208. (In Chinese) [Google Scholar]
- Sun, Y.; Dong, S.; Meng, F.; Liu, Z. Analysis of groundwater pollution characteristics of a coastal petrochemical enterprise. Environ. Prot. Oil. Gas Fields 2016, 26, 42–44. [Google Scholar] [CrossRef]
- Naraboyina, D.; Rastogi, A.K. Remediation techniques for BTEX contamination of groundwater—A review. Int. J. Eng. Res. Technol. 2015, 3, 1–6. [Google Scholar]
- Yeh, C.H.; Lin, C.W.; Wu, C.H. A permeable reactive barrier for the bioremediation of BTEX-contaminated groundwater: Microbial community distribution and removal efficiencies. J. Hazard. Mater. 2010, 178, 74–80. [Google Scholar] [CrossRef]
- Dhar, K.; Subashchandrabose, S.R.; Venkateswarlu, K.; Krishnan, K.; Megharaj, M. Anaerobic microbial degradation of polycyclic aromatic hydrocarbons: A comprehensive review. Rev. Environ. Contam. Toxicol. 2020, 251, 25–108. [Google Scholar] [CrossRef]
- Ulrich, A.C.; Edwards, E.A. Physiological and molecular characterization of anaerobic benzene-degrading mixed cultures. Environ. Microbiol. 2003, 5, 92–102. [Google Scholar] [CrossRef]
- van der Waals, M.J.; Atashgahi, S.; da Rocha, U.N.; van der Zaan, B.M.; Smidt, H.; Gerritse, J. Benzene degradation in a denitrifying biofilm reactor: Activity and microbial community composition. Appl. Microbiol. Biotechnol. 2017, 101, 5175–5188. [Google Scholar] [CrossRef] [Green Version]
- van der Zaan, B.M.; Saia, F.T.; Stams, A.J.M.; Plugge, C.M.; de Vos, W.M.; Smidt, H.; Langenhoff, A.A.M.; Gerritse, J. Anaerobic benzene degradation under denitrifying conditions: Peptococcaceae as dominant benzene degraders and evidence for a syntrophic process. Environ. Microbiol. 2012, 14, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.C.; Zhang, L.L.; Zhang, D.J.; Lu, P.L.; Zhang, X.T.; He, Q. Denitrification synergized with ANAMMOX for the anaerobic degradation of benzene: Performance and microbial community structure. Appl. Microbiol. Biotechnol. 2017, 101, 4315–4325. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.B.; Ramos, D.T.; Larose, C.; Fernandes, M.; Lazzarin, H.S.C.; Vogel, T.M.; Corseuil, H.X. Combined iron and sulfate reduction biostimulation as a novel approach to enhance BTEX and PAH source-zone biodegradation in biodiesel blend-contaminated groundwater. J. Hazard. Mater. 2017, 326, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ommedal, H.; Torsvik, T. Desulfotignum toluenicum sp nov., a novel toluene-degrading, sulphate-reducing bacterium isolated from an oil-reservoir model column. Int. J. Syst. Evol. Microbiol. 2007, 57, 2865–2869. [Google Scholar] [CrossRef]
- Lee, E.H.; Park, H.; Cho, K.S. Biodegradation of methane, benzene, and toluene by a consortium MBT14 enriched from a landfill cover soil. J. Environ. Sci. Health A 2013, 48, 273–278. [Google Scholar] [CrossRef]
- Abu Laban, N.; Selesi, D.; Jobelius, C.; Meckenstock, R.U. Anaerobic benzene degradation by Gram-positive sulfate-reducing bacteria. FEMS Microbiol. Ecol. 2009, 68, 300–311. [Google Scholar] [CrossRef] [Green Version]
- Dorer, C.; Vogt, C.; Neu, T.R.; Stryhanyuk, H.; Richnow, H.H. Characterization of toluene and ethylbenzene biodegradation under nitrate-, iron(III)- and manganese(IV)-reducing conditions by compound-specific isotope analysis. Environ. Pollut. 2016, 211, 271–281. [Google Scholar] [CrossRef]
- Kasai, Y.; Takahata, Y.; Manefield, M.; Watanabe, K. RNA-based stable isotope probing and isolation of anaerobic benzene-degrading bacteria from gasoline-contaminated groundwater. Appl. Environ. Microbiol. 2006, 72, 3586–3592. [Google Scholar] [CrossRef] [Green Version]
- Keller, A.H.; Kleinsteuber, S.; Vogt, C. Anaerobic benzene mineralization by nitrate-reducing and sulfate-reducing microbial consortia enriched from the same site: Comparison of community composition and degradation characteristics. Microbiol. Ecol. 2018, 75, 941–953. [Google Scholar] [CrossRef]
- Lueders, T. The ecology of anaerobic degraders of BTEX hydrocarbons in aquifers. FEMS Mcrobiol. Ecol. 2017, 93, fiw220. [Google Scholar] [CrossRef] [Green Version]
- HJ 1020-2019; Soil and Sediment—Determination of Petroleum Hydrocarbons (C6–C9)—Purge and Trap/Gas Chromatography. Ministry of Ecology and Environmental Protection: Beijing, China, 2019.
- HJ 1021-2019; Soil and Sediment—Determination of Petroleum Hydrocarbons (C10–C40)—Gas Chromatography. Ministry of Ecology and Environmental Protection: Beijing, China, 2019.
- HJ 605-2011; Soil and Sediment—Determination of Volatile Organic Compounds—Purge and Trap/Gas Chromatographymass Spectrometry. Ministry of Environmental Protection: Beijing, China, 2011.
- HJ 639-2012; Water Quality—Determination of Volatile Organic Compounds—Purge and Trap/Gas Chromatographymass Spectrometry. Ministry of Environmental Protection: Beijing, China, 2012.
- Castrillo, G.; Teixeira, P.J.P.L.; Paredes, S.H.; Law, T.F.; Lorenzo, L.; Feltcher, M.E.; Finkel, O.M.; Breakfield, N.W.; Mieczkowski, P.; Jones, C.D.; et al. Root microbiota drive direct integration of phosphate stress and immunity. Nature 2017, 543, 513–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Q.; An, S. Identifying the biogeographic patterns of rare and abundant bacterial communities using different primer sets on the loess plateau. Microorganisms 2021, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Mafei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, C.; He, J.; Wang, H. Anaerobic phenanthrene biodegradation with four kinds of electron acceptors enriched from the same mixed inoculum and exploration of metabolic pathways. Front. Environ. Sci. Eng. 2019, 13, 80. [Google Scholar] [CrossRef]
- Li, C.H.; Ye, C.; Hou, X.P.; Chen, M.H.; Zheng, X.Y.; Cai, X.Y. Isolation and characterization of polycyclic aromatic hydrocarbon-degrading bacteria with tolerance to hypoxic environments. J. Environ. Sci. Health A 2017, 52, 581–589. [Google Scholar] [CrossRef]
- Yuan, S.Y.; Chang, B.V. Anaerobic degradation of five polycyclic aromatic hydrocarbons from river sediment in Taiwan. J. Environ. Sci. Health B 2007, 42, 63–69. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, Y.; Zhao, J.; Li, S.; Schulz, S.; Deng, L. BTEX biodegradation was found linked to bacterial community assembly patterns in contaminated groundwater ecosystem. J. Hazard. Mater. 2021, 419, 126205. [Google Scholar] [CrossRef]
- KEGG: Kyoto Encyclopedia of Genes and Genomes. 2022. Available online: https://www.kegg.jp (accessed on 1 September 2022).
- Ma, Y.; Zha, J.; Yang, X.; Li, Q.; Zhang, Q.; Yin, A.; Beharry, Z.; Huang, H.; Huang, J.; Bartlett, M.; et al. Long-chain fatty acyl-CoA synthetase promotes prostate cancer progression by elevation of lipogenesis and fatty acid beta-oxidation. Oncogene 2021, 40, 1806–1820. [Google Scholar] [CrossRef]
- Castro, H.F.; Williams, N.H.; Ogram, A. Phylogeny of sulfate-reducing bacteria. FEMS Microbiol. Ecol. 2000, 31, 1–9. [Google Scholar] [CrossRef]
- Dou, J.; Liu, X.; Hu, Z. Substrate interactions during anaerobic biodegradation of BTEX by the mixed cultures under nitrate reducing conditions. J. Hazard. Mater. 2008, 158, 264–272. [Google Scholar] [CrossRef]

| S1 | S2 | |
|---|---|---|
| pH | 6.4 | 6.0 |
| Conductivity (μs/cm) | 2790 | 1210 |
| Total nitrogen (mg/kg) | 13 | 7 |
| Total phosphate (mg/kg) | 17 | 9 |
| C6–C9 a (mg/kg) | 11.57 | 1049.45 |
| C10–C40 a (mg/kg) | 9.73 | 87.57 |
| VOC b (mg/kg) | 0.083 | 138.07 |
| Benzene (mg/kg) | 0.005 | 17.47 |
| Toluene (mg/kg) | 0.002 | 1.06 |
| Ethylbenzene (mg/kg) | 0.002 | 22.41 |
| o-Xylene (mg/kg) | <0.001 | 0.12 |
| m/p-Xylene (μg/kg) | 0.002 | 10.99 |
| Parameter | GW1 | GW2 |
|---|---|---|
| pH | 7.3 | 7.2 |
| Conductivity (μs/cm) | 749 | 788 |
| ORP (mV) | −75.7 | −93.6 |
| DO (mg/L) | 0.9 | 0.5 |
| NO2− (mg/L) | 0.01 | 0.01 |
| S2− (mg/L) | 0.09 | 0.46 |
| Fe (mg/L) | <0.1 | 0.1 |
| Mn (mg/L) | 2.1 | 6.7 |
| COD (mg/L) | 2.5 | 12.2 |
| VOC (μg/L) | 6.85 | 1355.27 |
| Benzene (μg/L) | <1.00 | 644.03 |
| Toluene (μg/L) | <1.00 | 16.70 |
| Ethylbenzene (μg/L) | <1.00 | 207.79 |
| o-Xylene (μg/L) | <1.00 | 20.43 |
| m/p-Xylene (μg/L) | <1.00 | 161.03 |
| Matrix | Sample | Depth (m) | No. of Sequences | Richness c | Shannon_e |
|---|---|---|---|---|---|
| Soil | S1 | 4.0 a | 33,155 | 1387 | 4.41 |
| Soil | S2 | 4.0 a | 49,236 | 843 | 2.23 |
| Groundwater | GW1 | 3.4 b | 83,438 | 1623 | 4.19 |
| Groundwater | GW2 | 2.9 b | 76,320 | 1316 | 3.96 |
| Compound | Electron Acceptor | ||||
|---|---|---|---|---|---|
| NaNO3 | MnO2 | Fe2O3 | Na2SO4 | NaHCO3 | |
| Benzene | 0.05 | 0.03 | 0.06 | 0.10 | 0.09 |
| Toluene | 0.03 | 0.04 | 0.04 | 0.08 | 0.08 |
| Ethylbenzene | 0.04 | 0.01 | 0.05 | 0.09 | 0.07 |
| o-Xylene | 0.03 | 0.02 | 0.05 | 0.09 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhang, S.; Yi, L.; Liu, Z.; Ye, X.; Yu, B.; Shi, S.; Lu, X. Evaluation of Biodegradation of BTEX in the Subsurface of a Petrochemical Site near the Yangtze River, China. Int. J. Environ. Res. Public Health 2022, 19, 16449. https://doi.org/10.3390/ijerph192416449
Chen X, Zhang S, Yi L, Liu Z, Ye X, Yu B, Shi S, Lu X. Evaluation of Biodegradation of BTEX in the Subsurface of a Petrochemical Site near the Yangtze River, China. International Journal of Environmental Research and Public Health. 2022; 19(24):16449. https://doi.org/10.3390/ijerph192416449
Chicago/Turabian StyleChen, Xuexia, Shuai Zhang, Lijin Yi, Zhengwei Liu, Xiangyu Ye, Bo Yu, Shuai Shi, and Xiaoxia Lu. 2022. "Evaluation of Biodegradation of BTEX in the Subsurface of a Petrochemical Site near the Yangtze River, China" International Journal of Environmental Research and Public Health 19, no. 24: 16449. https://doi.org/10.3390/ijerph192416449




