Risk Factors Associated with Preventable Hospitalisation among Rural Community-Dwelling Patients: A Systematic Review
Abstract
1. Introduction
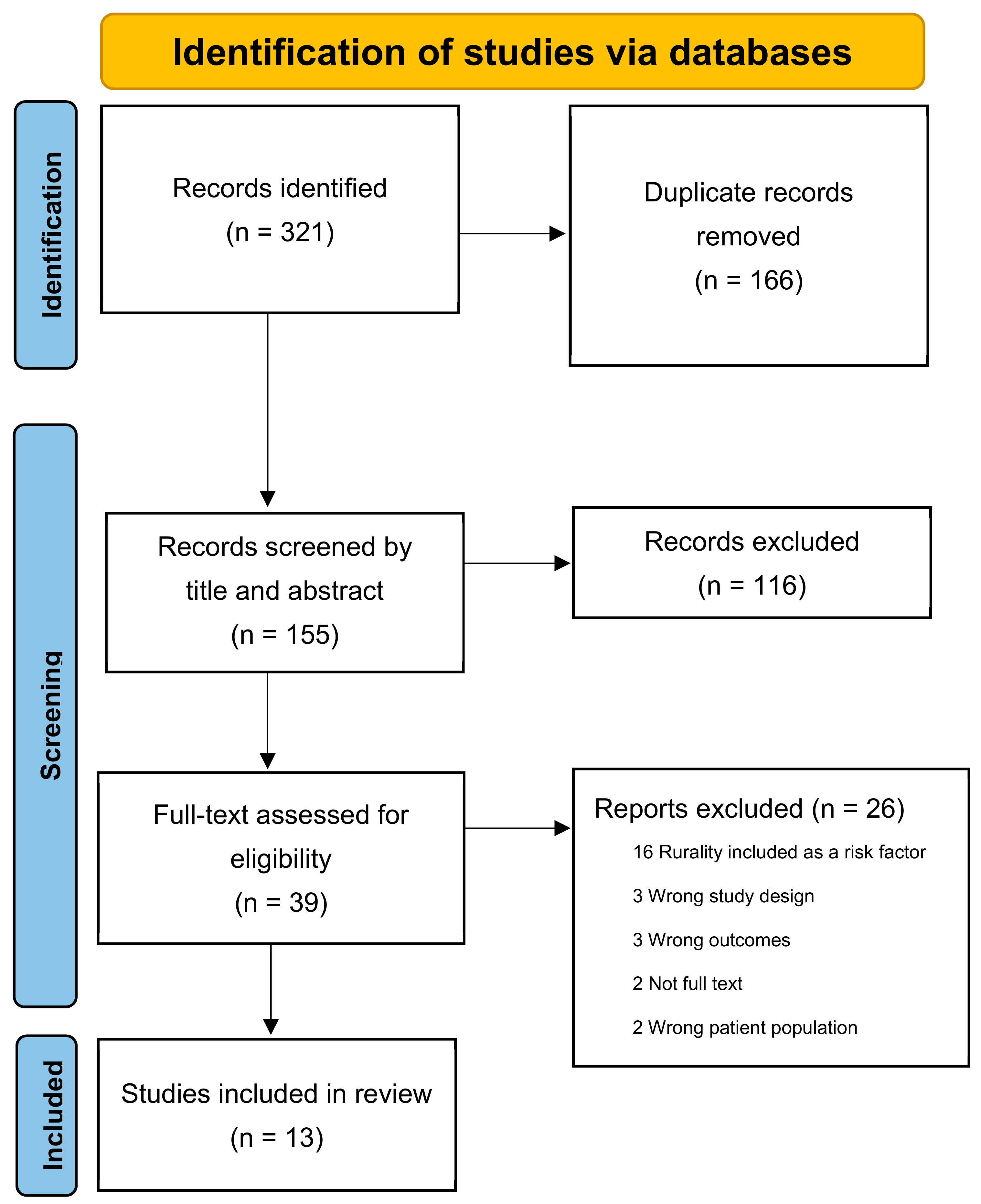
2. Method
3. Results
3.1. Location
3.2. Population
3.3. Definitions and Data Sources
3.4. Analysis Methods
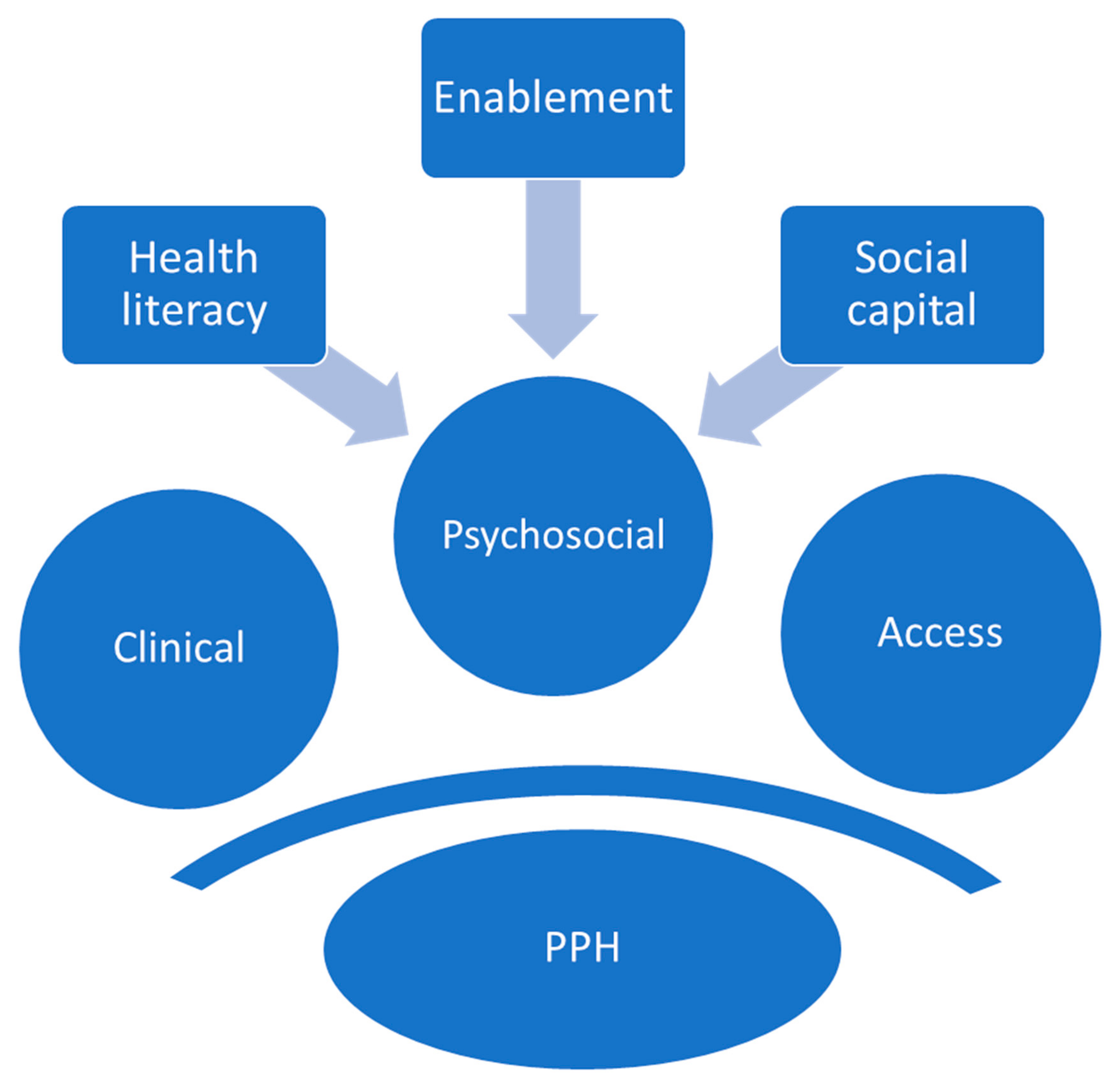
3.5. Predictors of PPH
3.6. Quality Assessment
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Research Question | “What Factors Are Associated with Preventable Hospitalisation in Rural Areas” | |||
|---|---|---|---|---|
| Concepts | Factors | Preventable Hospitalisation | Rural | |
| Synonyms | Predictor Risk Factor Cause Determinant Influence | Preventable Avoidable Unnecessary | Hospitalisation Admission | Rural Non-urban |
- 1
- (predict* or risk* or factor* or caus* or determin* or influenc*).ab,kf,ti.15023263
- 2
- risk factor/ 1212808
- 3
- predictor variable/ 29645
- 4
- 1 or 2 or 3 15231026
- 5
- (rural* or “non-urban” or “non urban”).ab,kf,ti. 193644
- 6
- rural health/or rural population/or rural area/or rural hospital/or rural health care/ 124869
- 7
- 5 or 6 220201
- 8
- ((prevent* or avoid*) adj2 (hospitali* or admission or admit*)).ab,ti. 5973
- 9
- 4 and 7 and 8 83
- 1
- (predict* or risk* or factor* or caus* or determin* or influenc*).ab,kf,ti. 11822934
- 2
- Risk Factors/ 924309
- 3
- 1 or 2 11988606
- 4
- (rural* or “non-urban” or “non urban”).ab,kf,ti. 164085
- 5
- Rural Health/or Hospitals, Rural/or Rural Population/or Rural Health Services/ 103381
- 6
- 4 or 5 190189
- 7
- ((prevent* or avoid*) adj2 (hospitali* or admission or admit*)).ab,ti. 3647
- 8
- 3 and 6 and 7 68
- S8
- S3 AND S6 AND S7 (59)
- S7
- TI ((prevent* OR avoid*) N2 (hospitali* OR admission OR admit*)) OR AB ((prevent* OR avoid*) N2 (hospitali* OR admission OR admit*)) OR MH ((prevent* OR avoid*) N2 (hospitali* OR admission OR admit*)) (3,273)
- S6
- S4 OR S5 (81,259)
- S5
- (MH “Hospitals, Rural”) OR (MH “Rural Population”) OR (MH “Rural Health Services”) OR (MH “Rural Areas”) OR (MH “Rural Health”) (51,135)
- S4
- TI (rural* OR “non-urban” OR “non urban”) OR AB (rural* OR “non-urban” OR “non urban”) OR MH (rural* OR “non-urban” OR “non urban”) (80,334)
- S3
- S1 OR S2 (2,367,920)
- S2
- (MH “Risk Factors”) (197,634)View DetailsEdit
- S1
- TI (predict* OR risk* OR factor* OR caus* OR determin* OR influenc*) OR AB (predict* OR risk* OR factor* OR caus* OR determin* OR influenc*) OR MH (predict* OR risk* OR factor* OR caus* OR determin* OR influenc*) (2,367,920)
- 4
- #3 AND #2 AND #1 111
- 3
- ((TI = ((prevent* or avoid*) near/2 (hospitali* or admission or admit*))) OR AB = ((prevent* or avoid*) near/2 (hospitali* or admission or admit*))) OR TS = ((prevent* or avoid*) near/2 (hospitali* or admission or admit*)) 5,726
- 2
- ((TI = (rural* or “non-urban” or “non urban”)) OR AB = (rural* or “non-urban” or “non urban”)) OR TS = (rural* or “non-urban” or “non urban”) 302,206
- 1
- ((TI = (predict* or risk* or factor* or caus* or determin* or influenc*)) OR AB = (predict* or risk* or factor* or caus* or determin* or influenc*)) OR TS = (predict* or risk* or factor* or caus* or determin* or influenc*) 20,632,004
References
- Australian Institute of Health and Welfare (AIHW). Australia’s Health 2018-In Brief; Australian Institute of Health and Welfare: Canberra, Australia, 2018. [Google Scholar]
- Billings, J.; Zeitel, L.; Lukomnik, J.; Carey, T.S.; Blank, A.E.; Newman, L. Impact of socioeconomic status on hospital use in New York City. Health Aff. 1993, 12, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; White, N.; Mengersen, K.; Rolfe, M.; Morgan, G.G. Joint modelling of potentially avoidable hospitalisation for five diseases accounting for spatiotemporal effects: A case study in New South Wales, Australia. PLoS ONE 2017, 12, e0183653. [Google Scholar] [CrossRef] [PubMed]
- Agency for Healthcare Research and Quality. Potentially Avoidable Hospitalizations. Available online: https://www.ahrq.gov/research/findings/nhqrdr/chartbooks/carecoordination/measure3.html (accessed on 19 September 2022).
- Lugo-Palacios, D.G.; Cairns, J. Using ambulatory care sensitive hospitalisations to analyse the effectiveness of primary care services in Mexico. Soc. Sci. Med. 2015, 144, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Falster, M.; Jorm, L. A Guide to the Potentially Preventable Hospitalisations Indicator in Australia; Centre for Big Data Research in Health, University of New South Wales in Consultation with Australian Commission on Safety and Quality in Health Care and Australian Institute of Health and Welfare: Sydney, Australia, 2017. [Google Scholar]
- Solberg, L.I.; Ohnsorg, K.A.; Parker, E.D.; Ferguson, R.; Magnan, S.; Whitebird, R.R.; Neely, C.; Brandenfels, E.; Williams, M.D.; Dreskin, M.; et al. Potentially Preventable Hospital and Emergency Department Events: Lessons from a Large Innovation Project. Perm. J. 2018, 22, 17–102. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare (AIHW). Admitted Patient Care 2017–2018: Australian Hospital Statistics; AIHW: Canberra, Australia, 2019. [Google Scholar]
- Australian Institute of Health and Welfare. Admitted Patient Safety and Quality. Available online: https://www.aihw.gov.au/reports-data/myhospitals/intersection/quality/apc (accessed on 12 September 2022).
- Australian Institute of Health and Welfare. Atlas 2017-1. Chronic Disease and Infection: Potentially Preventable Hospitalisations. Available online: https://www.safetyandquality.gov.au/our-work/healthcare-variation/atlas-2017/atlas-2017-1-chronic-disease-and-infection-potentially-preventable-hospitalisations (accessed on 12 September 2022).
- Sheringham, J.; Asaria, M.; Barratt, H.; Raine, R.; Cookson, R. Are some areas more equal than others? Socioeconomic inequality in potentially avoidable emergency hospital admissions within English local authority areas. J. Health Serv. Res. Policy 2017, 22, 83–90. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare (AIHW). National Healthcare Agreement: PI 18–Selected Potentially Preventable Hospitalisations. 2019. Available online: https://meteor.aihw.gov.au/content/index.phtml/itemId/698904 (accessed on 28 July 2022).
- Australian Institute of Health and Welfare. Rural & Remote Health. Available online: https://www.aihw.gov.au/reports/rural-remote-australians/rural-remote-health (accessed on 23 November 2021).
- Levesque, J.-F.; Harris, M.F.; Russell, G. Patient-centred access to health care: Conceptualising access at the interface of health systems and populations. Int. J. Equity Health 2013, 12, 18. [Google Scholar] [CrossRef]
- Greenwood-Ericksen, M.B.; Macy, M.L.; Ham, J.; Nypaver, M.M.; Zochowski, M.; Kocher, K.E. Are Rural and Urban Emergency Departments Equally Prepared to Reduce Avoidable Hospitalizations? West. J. Emerg. Med. 2019, 20, 477–484. [Google Scholar] [CrossRef]
- Johnston, K.J.; Wen, H.; Kotwal, A.; Joynt Maddox, K.E. Comparing Preventable Acute Care Use of Rural Versus Urban Americans: An Observational Study of National Rates During 2008–2017. J. Gen. Intern. Med. 2021, 36, 3728–3736. [Google Scholar] [CrossRef]
- Rust, G.; Baltrus, P.; Ye, J.L.; Daniels, E.; Quarshie, A.; Boumbulian, P.; Strothers, H. Presence of a Community Health Center and Uninsured Emergency Department Visit Rates in Rural Counties. J. Rural. Health 2009, 25, 8–16. [Google Scholar] [CrossRef]
- Vest, J.R.; Gamm, L.D.; Oxford, B.A.; Gonzalez, M.I.; Slawson, K.M. Determinants of preventable readmissions in the United States: A systematic review. Implement. Sci. 2010, 5, 88. [Google Scholar] [CrossRef]
- Bourke, L.; Humphreys, J.S.; Wakerman, J.; Taylor, J. Understanding drivers of rural and remote health outcomes: A conceptual framework in action. Aust. J. Rural. Health 2012, 20, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.L.; Wakerman, J.; Humphreys, J.S. Ensuring equity of access to primary health care in rural and remote Australia-what core services should be locally available? Int. J. Equity Health 2015, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Aljassim, N.; Ostini, R. Health literacy in rural and urban populations: A systematic review. Patient Educ. Couns. 2020, 103, 2142–2154. [Google Scholar] [CrossRef]
- Department of Health and Ageing. Report on the Audit of Health Workforce in Rural and Regional Australia; Commonwealth of Australia Canberra: Canberra, Australia, 2008. [Google Scholar]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Centre for Reviews and Dissemination. PROSPERO: International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/PROSPERO/ (accessed on 12 September 2022).
- Cochrane Library. Available online: https://www.cochranelibrary.com/cdsr/reviews (accessed on 12 September 2022).
- Clarivate Analytics. EndNote Version 20; Clarivate Analytics: San Francisco, CA, USA, 2018. [Google Scholar]
- Covidence Systematic Review Software. Available online: www.covidence.org (accessed on 12 September 2022).
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 12 September 2022).
- Critical Appraisal Skills Programme. CASP Qualitative Checklist. Available online: https://casp-uk.b-cdn.net/wp-content/uploads/2018/03/CASP-Qualitative-Checklist-2018_fillable_form.pdf (accessed on 26 July 2022).
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Snilstveit, B.; Oliver, S.; Vojtkova, M. Narrative approaches to systematic review and synthesis of evidence for international development policy and practice. J. Dev. Eff. 2012, 4, 409–429. [Google Scholar] [CrossRef]
- Ansari, Z.; Barbetti, T.; Carson, N.J.; Auckland, M.J.; Cicuttini, F.M. The Victorian ambulatory care sensitive conditions study: Rural and urban perspectives. Soz. Und Prav. 2003, 48, 33–43. [Google Scholar] [CrossRef]
- Johnston, K.J.; Hefei, W.; Joynt Maddox, K.E. Lack Of Access To Specialists Associated With Mortality And Preventable Hospitalizations Of Rural Medicare Beneficiaries. Health Aff. 2019, 38, 1993–2002. [Google Scholar] [CrossRef]
- Korenbrot, C.C.; Ehlers, S.; Crouch, J.A. Disparities in hospitalizations of rural American Indians. Med. Care 2003, 41, 626–636. [Google Scholar] [CrossRef]
- Laditka, J.N.; Laditka, S.B.; Probst, J.C. More may be better: Evidence of a negative relationship between physician supply and hospitalization for ambulatory care sensitive conditions. Health Serv. Res. 2005, 40, 1148–1166. [Google Scholar] [CrossRef]
- Ridge, A.; Peterson, G.; Kitsos, A.; Seidel, B.; Anderson, V.; Nash, R. Potentially Preventable Hospitalisations in rural Community-Dwelling Patients. Int. Med. J. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Slimings, C.; Moore, M. Geographic variation in health system performance in rural areas of New South Wales, Australia. Aust. J. Rural. Health 2021, 29, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.; Akiyama, J.; Potter, A.J.; Sabik, L.M.; Stehlin, G.G.; Trivedi, A.N.; Wolinsky, F.D. Health center use and hospital-based care among individuals dually enrolled in Medicare and Medicaid, 2012–2018. Health Serv. Res. 2022, 57, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Q.; Mueller, K.J.; Chen, L.W.; Conway, K. The role of rural health clinics in hospitalization due to ambulatory care sensitive conditions: A study in Nebraska. J. Rural. Health 2006, 22, 220–223. [Google Scholar] [CrossRef]
- Ridge, A.; Peterson, G.M.; Seidel, B.M.; Anderson, V.; Nash, R. Rural Patients’ Perceptions of Their Potentially Preventable Hospitalisation: A Qualitative Study. J. Patient Exp. 2022, 9. [Google Scholar] [CrossRef]
- Ridge, A.; Peterson, G.M.; Seidel, B.M.; Anderson, V.; Nash, R. Healthcare Providers’ Perceptions of Potentially Preventable Rural Hospitalisations: A Qualitative Study. Int. J. Environ. Res. Public Health 2021, 18, 12767. [Google Scholar] [CrossRef]
- Longman, J.M.; Singer, J.B.; Gao, Y.; Barclay, L.M.; Passey, M.E.; Pirotta, J.P.; Heathcote, K.E.; Ewald, D.P.; Saberi, V.; Corben, P.; et al. Community based service providers’ perspectives on frequent and/or avoidable admission of older people with chronic disease in rural NSW: A qualitative study. BMC Health Serv. Res. 2011, 11, 265. [Google Scholar] [CrossRef]
- Longman, J.; Passey, M.; Singer, J.; Morgan, G. The role of social isolation in frequent and/or avoidable hospitalisation: Rural community-based service providers’ perspectives. Aust. Health Rev. 2013, 37, 223–231. [Google Scholar] [CrossRef]
- Longman, J.; Johnston, J.; Ewald, D.; Gilliland, A.; Burke, M.; Mutonga, T.; Passey, M. What could prevent chronic condition admissions assessed as preventable in rural and metropolitan contexts? An analysis of clinicians’ perspectives from the DaPPHne study. PLoS ONE 2021, 16, e0244313. [Google Scholar] [CrossRef]
- Borda-Olivas, A.; Fernandez-Navarro, P.; Otero-Garcia, L.; Sanz-Barbero, B. Rurality and avoidable hospitalization in a Spanish region with high population dispersion. Eur. J. Public Health 2013, 23, 946–951. [Google Scholar] [CrossRef]
- Longman, J.M.; Rolfe, M.I.; Passey, M.D.; Heathcote, K.E.; Ewald, D.P.; Dunn, T.; Barclay, L.M.; Morgan, G.G. Frequent hospital admission of older people with chronic disease: A cross-sectional survey with telephone follow-up and data linkage. BMC Health Serv. Res. 2012, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.; Potter, A.J.; Trivedi, A.N.; Mueller, K.J. The Relationship Between Rural Health Clinic Use and Potentially Preventable Hospitalizations and Emergency Department Visits Among Medicare Beneficiaries. J. Rural. Health 2018, 34, 423–430. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, M.; Olajide, D.; Dusheiko, M.; Elliott, R.; Guthrie, B.; Jorm, L.; Leyland, A.H. The impact of quality and accessibility of primary care on emergency admissions for a range of chronic ambulatory care sensitive conditions (ACSCs) in Scotland: Longitudinal analysis. BMC Fam. Pract. 2019, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Elek, P.; Molnar, T.; Varadi, B. The closer the better: Does better access to outpatient care prevent hospitalization? Eur. J. Health Econ. 2019, 20, 801–817. [Google Scholar] [CrossRef]
- World Health Organisation. Human Rights and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/human-rights-and-health (accessed on 12 September 2022).
- Agency for Healthcare Research and Quality. Access and Disparities in Access to Health Care; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2018. [Google Scholar]
- Mazumdar, S.; Chong, S.; Arnold, L.; Jalaludin, B. Spatial clusters of chronic preventable hospitalizations (ambulatory care sensitive conditions) and access to primary care. J. Public Health 2019, 42, e134–e141. [Google Scholar] [CrossRef]
- Carmeiro, C.S. Hospitalisation of ambulatory care sensitive conditions and access to primary care in Portugal. Public Health 2018, 165, 117–124. [Google Scholar] [CrossRef]
- Penchansky, R.; Thomas, J.W. The Concept of Access: Definition and Relationship to Consumer Satisfaction. Med. Care 1981, 19, 127–140. [Google Scholar] [CrossRef]
- Swerissen, H.; Duckett, S.; Moran, G. Mapping Primary Care in Australia (Grattan Institute Report No. 2018–09); Grattan Institute Melbourne, Australia: Carlton, Australia, 2018. [Google Scholar]
- The Royal Australian College of General Practitioners. General Practice: Health of the Nation 2020; RACGP: East Melbourne, VIC, Australia, 2020. [Google Scholar]
- Duckett, S.; Breadon, P. Access All Areas: New Solutions for GP Shortages in Rural AUSTRALIA; Grattan Institute Melbourne, Australia: Carlton, Australia, 2013. [Google Scholar]
- Cyr, M.E.; Etchin, A.G.; Guthrie, B.J.; Benneyan, J.C. Access to specialty healthcare in urban versus rural US populations: A systematic literature review. BMC Health Serv. Res. 2019, 19, 974. [Google Scholar] [CrossRef]
- Cheek, C.; Allen, P.; Shires, L.; Parry, D.; Ruigrok, M. Low-acuity presentations to regional emergency departments: What is the issue? Emerg. Med. Australas. 2016, 28, 145–152. [Google Scholar] [CrossRef]
- Rowlands, G.; Shaw, A.; Jaswal, S.; Smith, S.; Harpham, T. Health literacy and the social determinants of health: A qualitative model from adult learners. Health Promot. Int. 2017, 32, 130–138. [Google Scholar] [CrossRef]
- Pelikan, J.M.; Ganahl, K.; Roethlin, F. Health literacy as a determinant, mediator and/or moderator of health: Empirical models using the European Health Literacy Survey dataset. Glob. Health Promot. 2018, 25, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Stormacq, C.; Van Den Broucke, S.; Wosinski, J. Does health literacy mediate the relationship between socioeconomic status and health disparities? Integrative review. Health Promot. Int. 2019, 34, e1–e17. [Google Scholar] [CrossRef] [PubMed]
- Villalonga-Olives, E.; Kawachi, I. The dark side of social capital: A systematic review of the negative health effects of social capital. Soc. Sci. Med. 2017, 194, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, A.T.; Groene, O.; Rieger, M.A.; Siegel, A. The Relationship between Health Literacy, Quality of Life, and Subjective Health: Results of a Cross-Sectional Study in a Rural Region in Germany. Int. J. Environ. Res. Public Health 2020, 17, 1683. [Google Scholar] [CrossRef]
- Berkman, N.D.; Sheridan, S.L.; Donahue, K.E.; Halpern, D.J.; Crotty, K. Low health literacy and health outcomes: An updated systematic review. Ann. Intern. Med. 2011, 155, 97–107. [Google Scholar] [CrossRef]
- Balakrishnan, M.P.; Herndon, J.B.; Zhang, J.N.; Payton, T.; Shuster, J.; Carden, D.L. The Association of Health Literacy With Preventable Emergency Department Visits: A Cross-sectional Study. Acad. Emerg. Med. 2017, 24, 1042–1050. [Google Scholar] [CrossRef]
- Schumacher, J.R.; Hall, A.G.; Davis, T.C.; Arnold, C.L.; Bennett, R.D.; Wolf, M.S.; Carden, D.L. Potentially preventable use of emergency services: The role of low health literacy. Med. Care 2013, 51, 654. [Google Scholar] [CrossRef]
- Trezona, A.; Dodson, S.; Osborne, R.H. Development of the organisational health literacy responsiveness (Org-HLR) framework in collaboration with health and social services professionals. BMC Health Serv. Res. 2017, 17, 513. [Google Scholar] [CrossRef]
- Agency for Clinical Innovation. Consumer Enablement: A Clinician’s Guide. Available online: https://aci.health.nsw.gov.au/resources/primary-health/consumer-enablement/guide (accessed on 19 September 2022).
- Batterham, R.; Osborne, R.; Mcphee, C.; Townsend, B. Consumer Enablement: An Evidence Check Rapid Review Brokered by the Sax Institute for the Agency for Clinical Innovation; Sax Institute: Ultimo, NSW, Australia, 2017. [Google Scholar]
- Kurosawa, S.; Matsushima, M.; Fujinuma, Y.; Hayashi, D.; Noro, I.; Kanaya, T.; Watanabe, T.; Tominaga, T.; Nagata, T.; Kawasaki, A.; et al. Two Principal Components, Coping and Independence, Comprise Patient Enablement in Japan: Cross Sectional Study in Tohoku Area. Tohoku J. Exp. Med. 2012, 227, 97–104. [Google Scholar] [CrossRef]
- Veazie, S.; Gilbert, J.; Winchell, K.; Paynter, R.; Guise, J.-M. Addressing Social Isolation to Improve the Health of Older Adults: A Rapid Review. Available online: https://effectivehealthcare.ahrq.gov/products/social-isolation/rapid-product (accessed on 3 December 2021).
- Edwards, M.; Wood, F.; Davies, M.; Edwards, A. ‘Distributed health literacy’: Longitudinal qualitative analysis of the roles of health literacy mediators and social networks of people living with a long-term health condition. Health Expect. 2015, 18, 1180–1193. [Google Scholar] [CrossRef]
- Valtorta, N.K.; Kanaan, M.; Gilbody, S.; Ronzi, S.; Hanratty, B. Loneliness and social isolation as risk factors for coronary heart disease and stroke: Systematic review and meta-analysis of longitudinal observational studies. Heart 2016, 102, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Cene, C.W.; Beckie, T.M.; Sims, M.; Suglia, S.F.; Aggarwal, B.; Moise, N.; Jimenez, M.C.; Gaye, B.; McCullough, L.D.; American Heart Association Social Determinants of Health Committee of the Council on Epidemiology and Prevention and Council on Quality of Care and Outcomes Research; et al. Effects of Objective and Perceived Social Isolation on Cardiovascular and Brain Health: A Scientific Statement From the American Heart Association. J. Am. Heart Assoc. 2022, 11, e026493. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Lim, M. How the COVID-19 pandemic is focusing attention on loneliness and social isolation. Public Health Res. Pract. 2020, 30, 3022008. [Google Scholar] [CrossRef]
- Duckett, S. What should primary care look like after the COVID-19 pandemic? Aust. J. Prim. Health 2020, 26, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Koh, G.C.H.; Car, J. COVID-19: A remote assessment in primary care. BMJ 2020, 368, m1182. [Google Scholar] [CrossRef] [PubMed]
- Mental Health Council of Tasmania. COVID-19: A Mental Health Response for Older Tasmanians; Mental Health Council of Tasmania: Hobart, Tasmania, 2021. [Google Scholar]
- Rumas, R.; Shamblaw, A.L.; Jagtap, S.; Best, M.W. Predictors and consequences of loneliness during the COVID-19 Pandemic. Psychiatry Res. 2021, 300, 113934. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, H.; Camic, P.; Lockyer, B.; Thomson, L. Non-clinical community interventions: A systematised review of social prescribing schemes. Arts Health 2018, 10, 97–123. [Google Scholar] [CrossRef]
- Fitzmaurice, C. Social prescribing: A new paradigm with additional benefits in rural Australia. Aust. J. Rural. Health 2022, 30, 298–299. [Google Scholar] [CrossRef]
- Tierney, S.; Wong, G.; Roberts, N.; Boylan, A.-M.; Park, S.; Abrams, R.; Reeve, J.; Williams, V.; Mahtani, K.R. Supporting social prescribing in primary care by linking people to local assets: A realist review. BMC Med. 2020, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- TASCOSS. Legislative Council Government Administration Committee A: Inquiry into Rural Health Services; Tasmanian Council of Social Service: Hobart, Tasmania, 2021. [Google Scholar]
- Department of Health. National Preventive Health Strategy 2021–2030; Commonwealth of Australia: Canberra, Australia, 2021. [Google Scholar]
- Royal Australian College of General Practitioners and Consumers Health Forum of Australia. Social Prescribing Roundtable November 2019-Report; Royal Australian College of General Practitioners: Melbourne, Australia, 2020. [Google Scholar]
- NHS England and NHS Improvement. Social Prescribing and Community-Based Support Summary Guide; NHS: London, UK, 2020. [Google Scholar]
- Marmot, M. Health equity in England: The Marmot review 10 years on. BMJ 2020, 368, m693. [Google Scholar] [CrossRef] [PubMed]
- Health Service Executive. Building the Capacity for the Evaluation of Social Prescribing: An Evaluability Assessment; Department of Health: Dublin, Ireland, 2020. [Google Scholar]
- Frankston Mornington Peninsula Primary Care Partnership. Frankston Mornington Peninsula Social Prescribing Program. Available online: https://fmppcp.org.au/fmpsocialprescribingprogram/# (accessed on 22 March 2022).
- Ingold, B.B.; Yersin, B.; Wietlisbach, V.; Burckhardt, P.; Burnand, B.; Büla, C.J. Characteristics associated with inappropriate hospital use in elderly patients admitted to a general internal medicine service. Aging Clin. Exp. Res. 2000, 12, 430–438. [Google Scholar] [CrossRef]
- Burgdorf, F.; Sundmacher, L. Potentially Avoidable Hospital Admissions in Germany. Dtsch. Arztebl. Int. 2014, 111, 215–223. [Google Scholar] [CrossRef]
- Cloutier-Fisher, D.; Penning, M.J.; Zheng, C.; Druyts, E.-B.F. The devil is in the details: Trends in avoidable hospitalization rates by geography in British Columbia, 1990–2000. BMC Health Serv. Res. 2006, 6, 104. [Google Scholar] [CrossRef]
- O’Cathain, A.; Knowles, E.; Maheswaran, R.; Pearson, T.; Turner, J.; Hirst, E.; Goodacre, S.; Nicholl, J. A system-wide approach to explaining variation in potentially avoidable emergency admissions: National ecological study. BMJ Qual. Saf. 2014, 23, 47–55. [Google Scholar] [CrossRef]
- Lynch, B.M.; Fitzgerald, T.; Corcoran, P.; Buckley, C.; Healy, O.; Browne, J. Avoidable emergency admissions for ambulatory care sensitive conditions in the Republic of Ireland: Analysis of regional determinants. Int. J. Integr. Care (IJIC) 2018, 18, 352. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
Participants/Population
| Types of studies to be excluded
|
| Study (Year) Country | Study Type | Number of Participants–Data Source | Age | Sex (% Female) | Analysis Method | Key Findings |
|---|---|---|---|---|---|---|
| Korenbrot et al. (2003) [34] USA | Cross sectional | 3920 California Department of Health Services | 20.1% > 65 years (AI/AN group) | 60.1 | risk ratio with stratification by age, sex | AI/AN status increased PPH risk ratio for men (RR 2.26; 95%CI 1.39–2.98) & women (RR 1.87; 95%CI 1.46–3.17) Rates of PPH were only significantly higher for adult males aged > 45 years & women aged < 75 years after adjusting for age-group |
| Ansari et al. (2003) [32] Australia | Cross sectional | 4,403,637 Victorian Admitted Episodes Dataset | 42.0% aged > 65 years (ACSC group) | 49.5 | random effects multilevel regression | Strong associations with ACSH were observed for the following factors:
|
| Laditka et al. (2005) [35] USA | Cross sectional | 948 counties Agency for Healthcare Research and Quality | n.s. | n.s. | multivariate ordinary least squares regression | Physician supply was not associated with ACSH in rural areas |
| Zhang et al. (2006) [39] USA | Cross sectional | 538,580 Nebraska hospital discharge data (1999–2001) | n.s. | n.s. | multilevel logistic regression | Presence of ≥1 rural health clinic in rural areas was associated with a 5.5% reduction in risk of a ACSH due to a chronic disease among elderly patients (ORadj = 0.945; 95%CI 0.893–0.997) |
| Longman et al. (2011) [42] Australia | Qualitative | 15 semi-structured interviews with healthcare providers | n.s. | 86.7 | thematic analysis | External barriers influencing PPH risk: complexity of services; availability, awareness, and ability to access services; greater care needs; patient poverty; rurality; transport Internal barriers influencing PPH risk: fear of change; “stoic” attitudes; difficulty accepting change in health status |
| Longman et al. (2013) [43] Australia | Qualitative | 15 semi-structured interviews with healthcare providers | n.s. | n.s. | thematic analysis | Social isolation consistently identified as a risk factor for PPH among older patients with chronic diseases. Dimensions of social isolation included living alone, not socialising and being isolated from family |
| Johnston et al. (2019) [33] USA | Cross sectional | 11,581 Centres for Medicare and Medicaid Services | n.s. | n.s. | weighted negative binomial regression | One or more specialist visits during the previous year was associated with a 15.9% lower preventable hospitalisation rate (explained 55% of the difference in preventable hospitalisation rates between rural and urban groups) Overall morbidity, heart failure (independently), lower income and being unmarried were all associated with a higher preventable hospitalisation risk. |
| Ridge et al. (2021) [41] Australia | Qualitative | 14 semi-structured interviews with healthcare providers | n.s. | n.s. | thematic analysis | Health literacy challenges; access to PHC; perceived convenience of hospital treatment |
| Longman et al. (2021) [44] Australia | Qualitative | 148 preventable admissions reviewed by expert panel | n.s. | n.s. | thematic analysis | System issues: community-based services inadequate or not referred to; poor connections between services; problems with specialist services Clinician issues: GP care inadequate Patient issues: adherence/self-management; patient’s engagement with existing services |
| Slimings et al. (2021) [37] Australia | Ecological | 89 LGAs Social Health Atlases of Australia | 20.8% aged > 65 years | 49.80 | multivariable analysis using generalised linear model | Remoteness, Indigenous percentage, and socioeconomic disadvantage were independently associated with preventable hospitalisation in rural NSW. Socioeconomic factors (measured by internet access) and Indigenous percentage remained significant in the adjusted model with 416.5 fewer (95%CI−597.6-−235.5; p <0.001) and 367.0 (95%CI 68.8–665.2; p = 0.041) more preventable hospitalisations per 100,000 population, respectively, between 2013–2017. |
| Ridge et al. (2021) [36] Australia | Cross sectional | 436 Admitted Patient Data Collection dataset | 62.5 years | 48.6 | multivariate logistic regression | Being single/unmarried (OR 2.43; 95%CI 1.38–4.28), greater comorbidity burden (as measured by higher Charlson Comorbidity Index scores) (OR 1.40; 95%CI 1.13–1.74) and number of general practice visits in the preceding 12 months (OR 1.09, 95%CI 1.05–1.14) were all associated with a higher risk of PPH |
| Ridge et al. (2022) [40] Australia | Qualitative | 10 semi-structured patient interviews | 68 years (range 47–91) | 40.0 | thematic analysis | Patient self-efficacy and health literacy; community support networks; access to PHC services |
| Wright et al. (2022) [38] USA | Cross sectional | 8,483,758 person-year observations Medicare claims & Master Beneficiary Summary File (2012–2018) | 75.1 years (IQR 69–80) | 68.3 | negative binomial and linear probability models | Dual-registered persons in rural areas receiving 100% of their PHC at a FQHC demonstrated a lower propensity for ACSH (marginal effect 0.3%; 95%CI 0.1–0.4) |
| Korenbrot et al. (2003) [34] | Ansari et al. (2003) [32] | Laditka et al. (2005) [35] | Zhang et al. (2006) [39] | Johnston et al. (2019) [33] | Slimings et al. (2021) [37] | Ridge et al. (2021) [36] | Wright et al. (2022) [38] | |
|---|---|---|---|---|---|---|---|---|
| Selection | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Comparability | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 1 |
| Outcome | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Longman et al. (2011) [42] | Longman et al. (2013) [43] | Longman et al. (2021) [44] | Ridge et al. (2021) [41] | Ridge et al. (2022) [40] | |
|---|---|---|---|---|---|
| Section A: Are the results valid? | |||||
| ✓ | ✓ | ✓ | ✓ | ✓ |
| ✓ | ✓ | ✓ | ✓ | ✓ |
| ✓ | ? | ✓ | ✓ | ✓ |
| ✓ | ? | ✓ | ✓ | ✓ |
| ✓ | ? | ✓ | ✓ | ✓ |
| x | x | ? | ? | ✓ |
| Section B: What are the results? | |||||
| ✓ | ? | ? | ✓ | ✓ |
| x | ? | ✓ | ✓ | ✓ |
| ✓ | ✓ | ✓ | ✓ | ✓ |
| Section C: Will the results help locally? | |||||
| 7 ✓ 2 x | 3 ✓ 5 ? 1 x | 7 ✓ 2 ? | 8 ✓ 1 ? | 9 ✓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ridge, A.; Peterson, G.M.; Nash, R. Risk Factors Associated with Preventable Hospitalisation among Rural Community-Dwelling Patients: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 16487. https://doi.org/10.3390/ijerph192416487
Ridge A, Peterson GM, Nash R. Risk Factors Associated with Preventable Hospitalisation among Rural Community-Dwelling Patients: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(24):16487. https://doi.org/10.3390/ijerph192416487
Chicago/Turabian StyleRidge, Andrew, Gregory M. Peterson, and Rosie Nash. 2022. "Risk Factors Associated with Preventable Hospitalisation among Rural Community-Dwelling Patients: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 24: 16487. https://doi.org/10.3390/ijerph192416487
APA StyleRidge, A., Peterson, G. M., & Nash, R. (2022). Risk Factors Associated with Preventable Hospitalisation among Rural Community-Dwelling Patients: A Systematic Review. International Journal of Environmental Research and Public Health, 19(24), 16487. https://doi.org/10.3390/ijerph192416487






