Regular Exercise Rescues Heart Function Defects and Shortens the Lifespan of Drosophila Caused by dMnM Downregulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drosophila Strains and Groupings
2.2. Exercise Programs
2.3. Semi-Intact Drosophila Heart Preparation and Cardiac Function
2.4. qRT-qPCR
2.5. Phalloidine
2.6. ROS Staining
2.7. Climbing and Lifespan Statistics
2.8. Statistical Analyses
3. Results
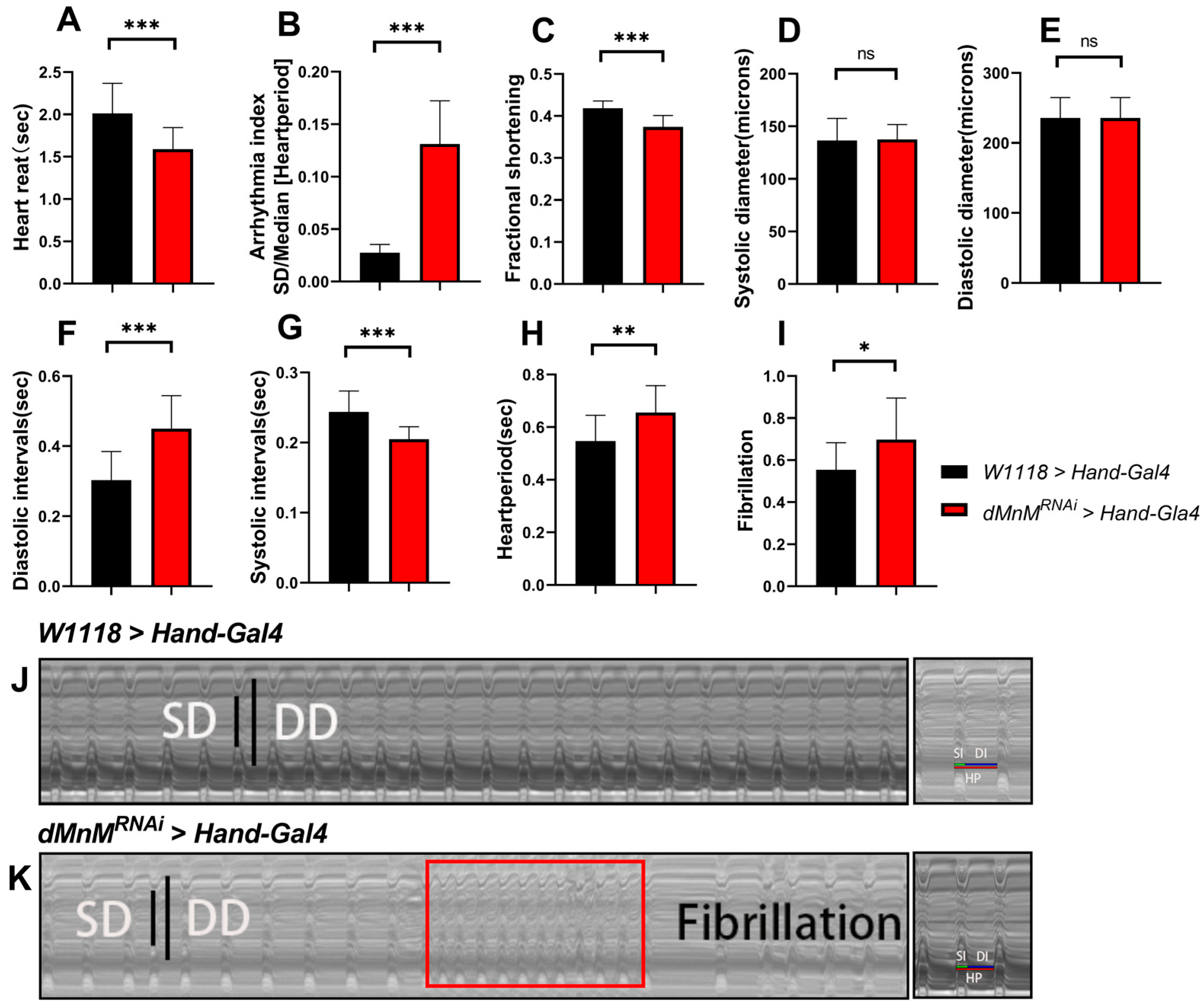
3.1. Drosophila Cardiomyocyte dMnM Knockdown Causes Cardiac Function Defects
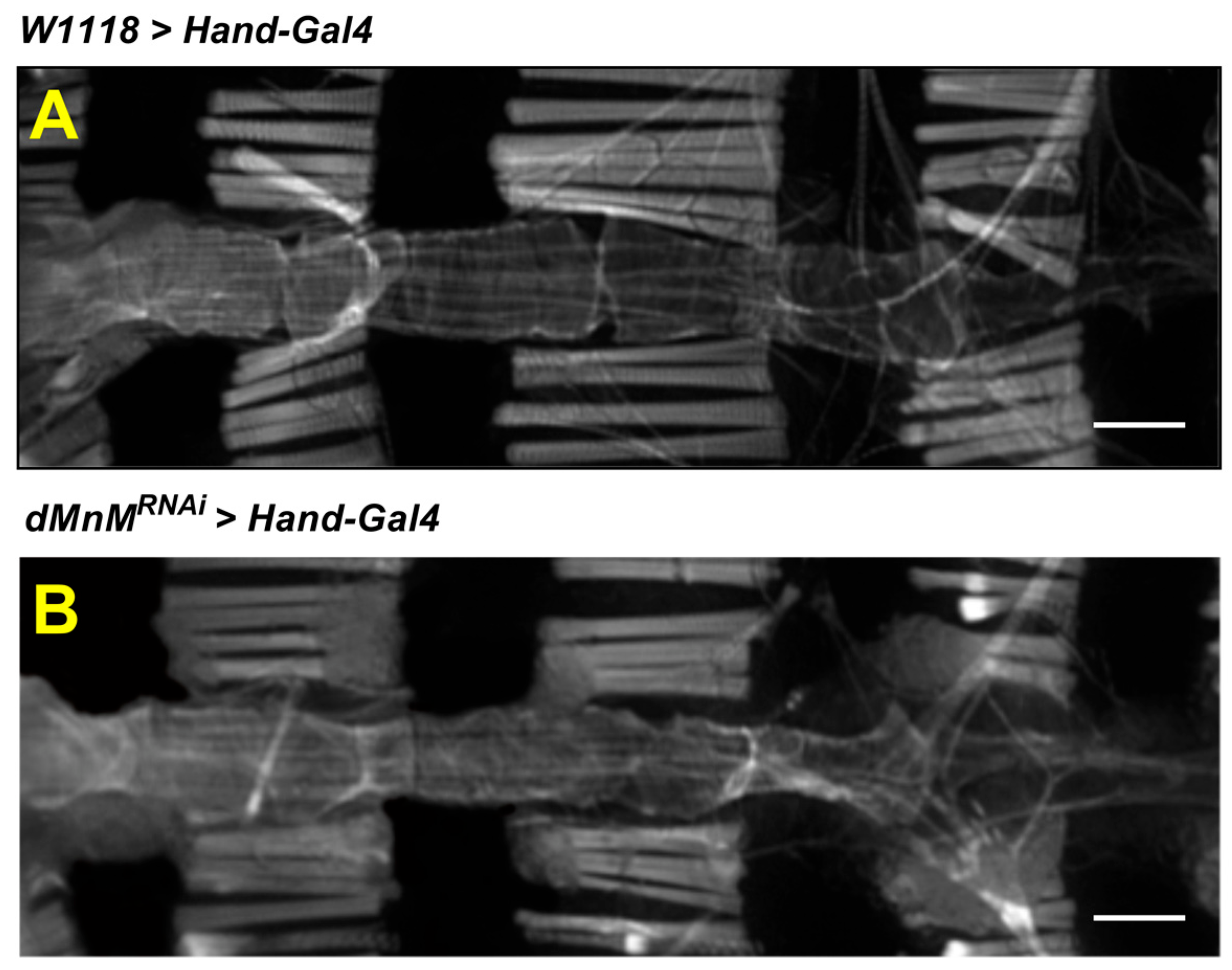
3.2. dMnM Knockdown in Drosophila Cardiomyocytes Causes Myofibril Destruction
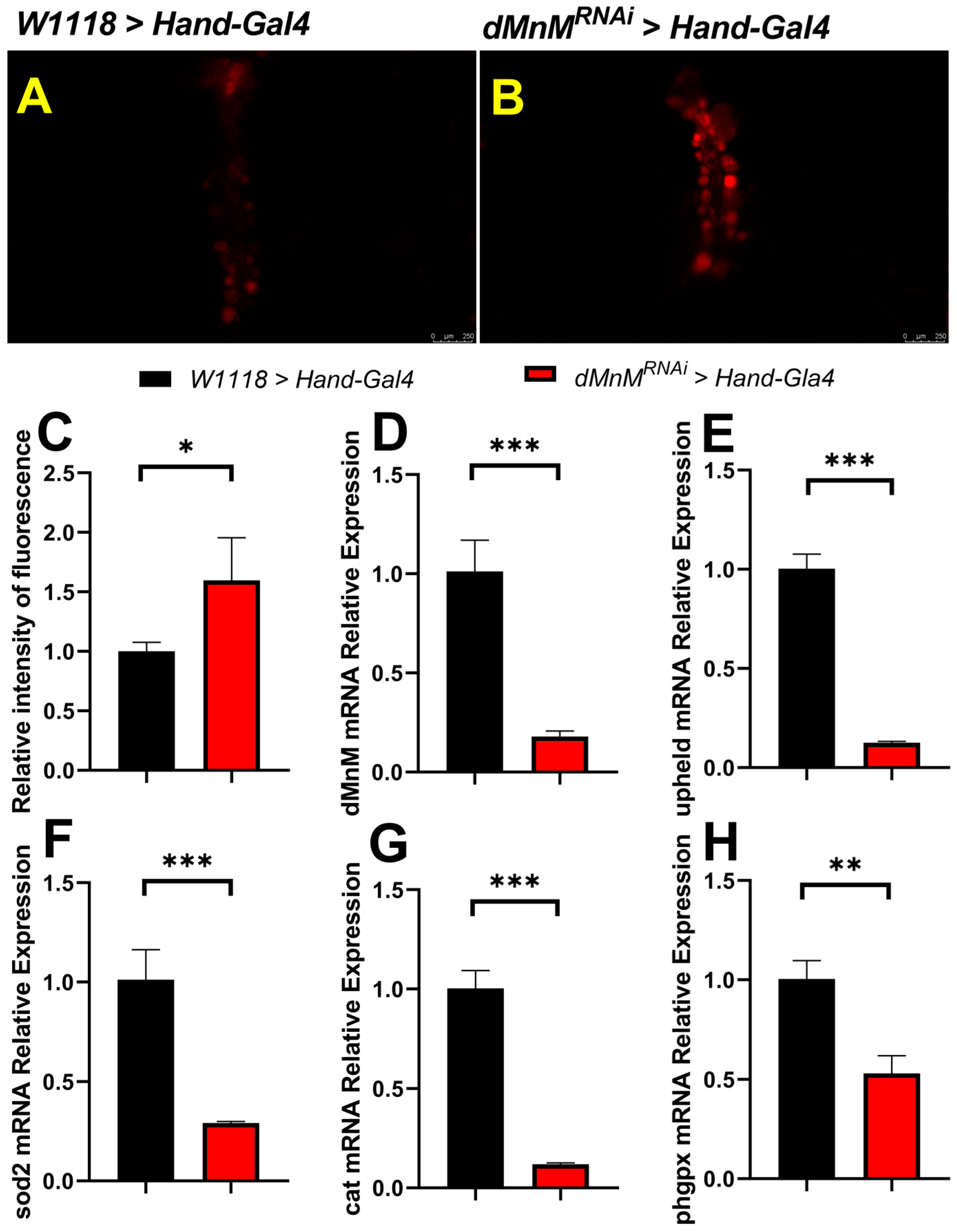
3.3. dMnM Knockdown in Drosophila Cardiomyocytes Increases Cardiac ROS Production and Decreases mRNA Expression of dMnM, Upheld, sod2, Cat, and phgpx
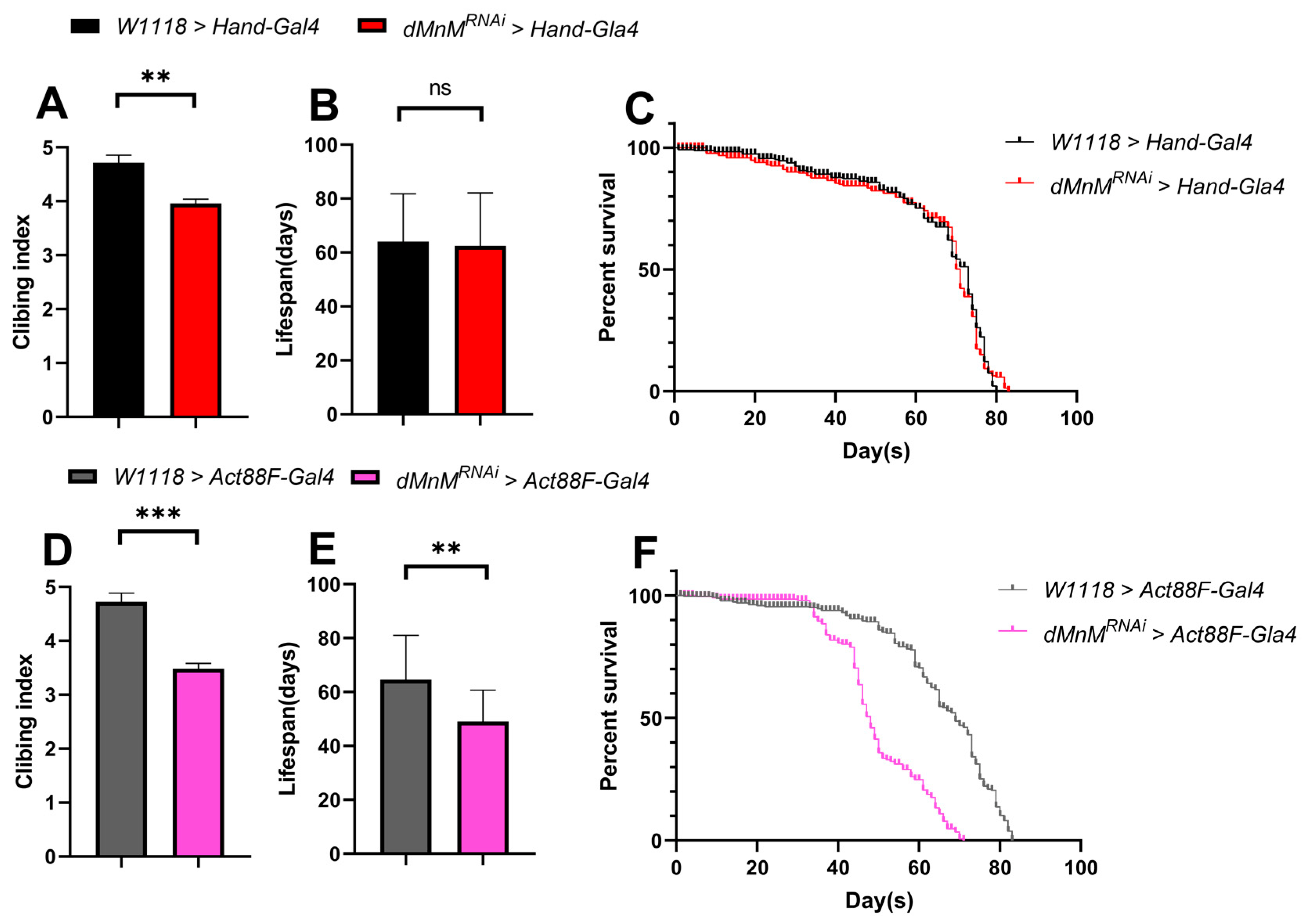
3.4. Effects of dMnM Knockdown in Cardiomyocytes and IFM on Climbing and Lifespan of Drosophila
3.5. Regular Exercise Ameliorates Cardiac Function Defects Caused by dMnM Knockdown in Drosophila Cardiomyocytes
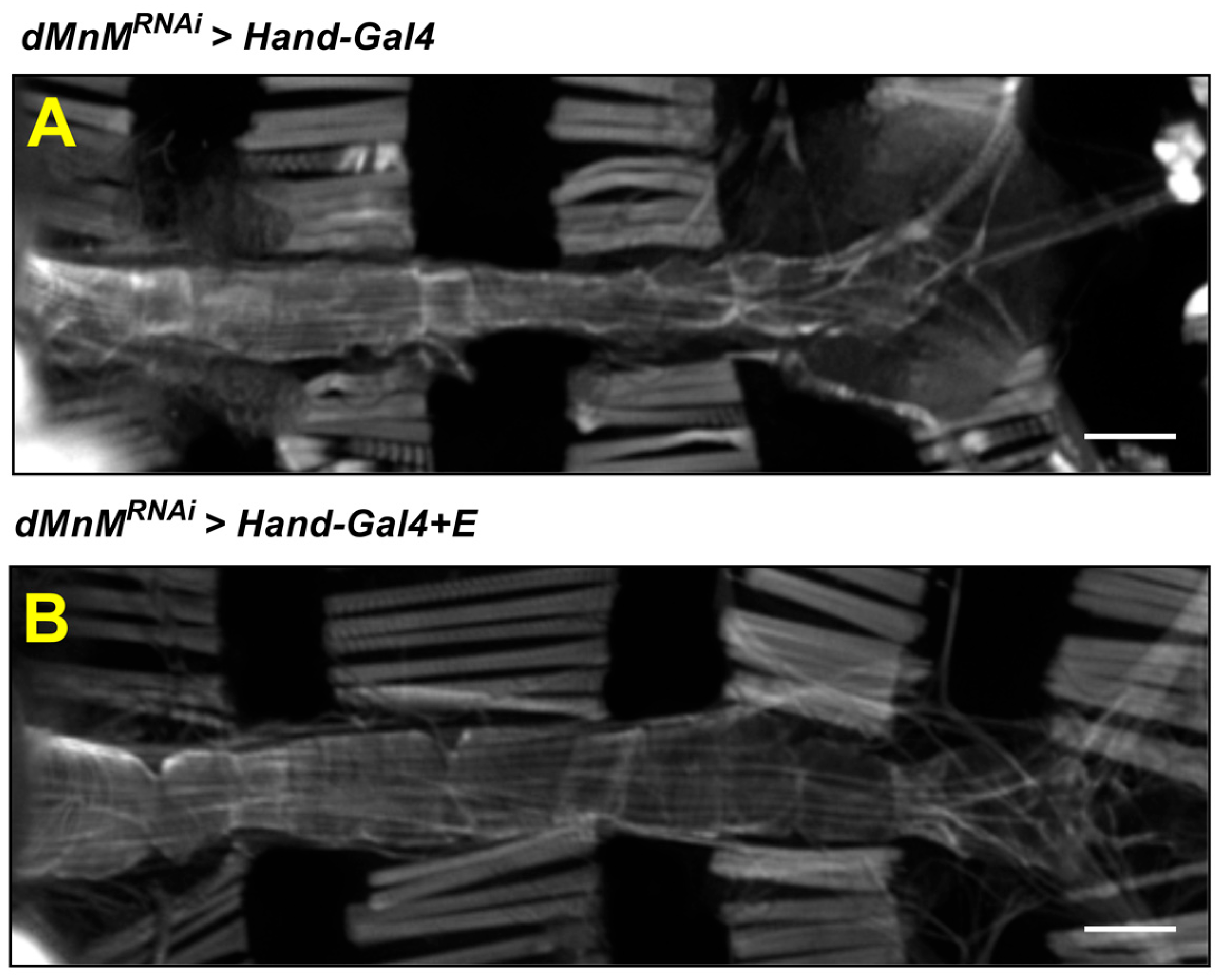
3.6. Regular Exercise Improves Myocardial Myogenic Fiber Destruction Caused by Cardiomyocyte dMnM Knockdown
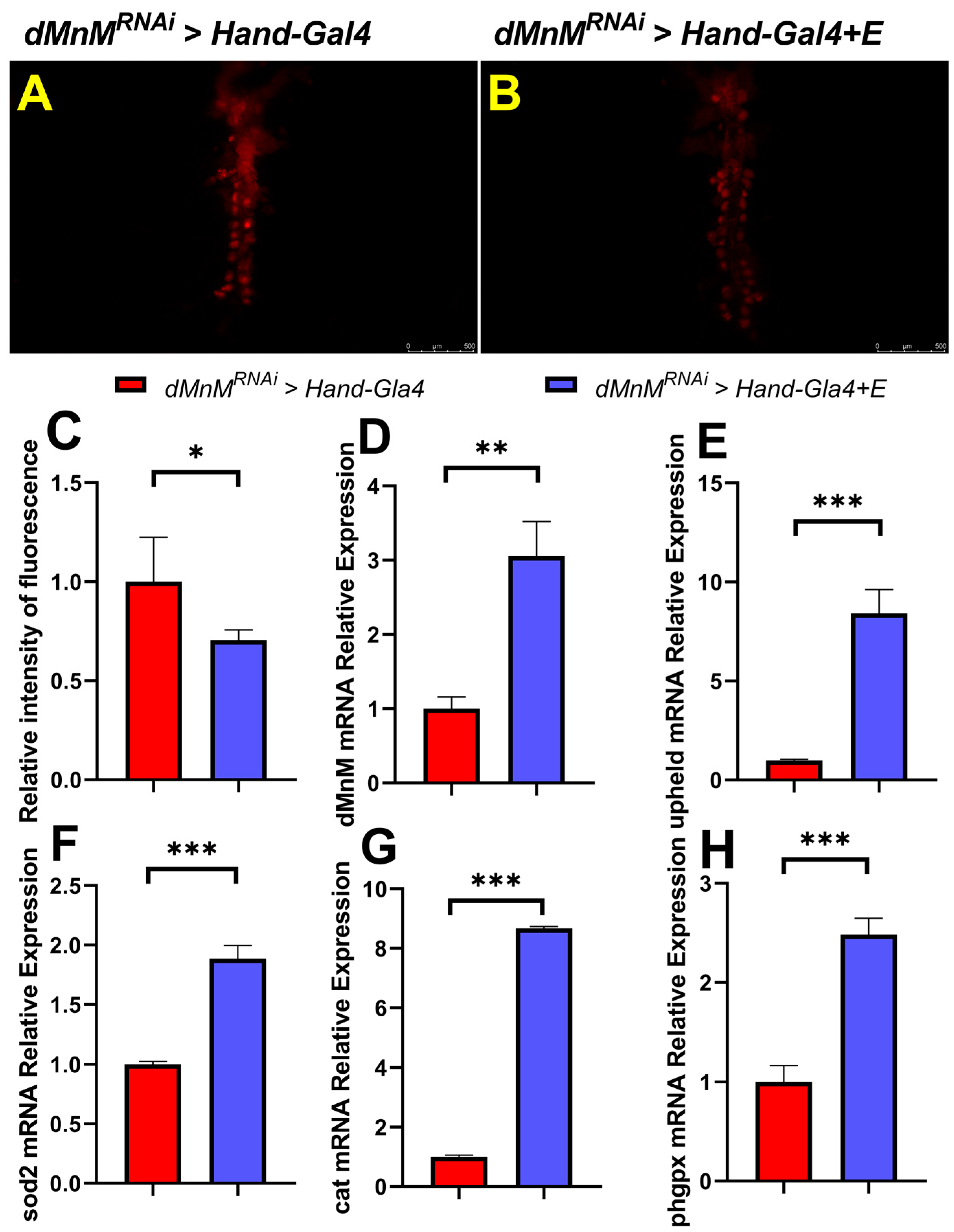
3.7. Regular Exercise Reduces ROS Production and Upregulates mRNA Expression of dMnM, Upheld, phgpx, sod2, and Cat in Cardiomyocytes after dMnM Knockdown
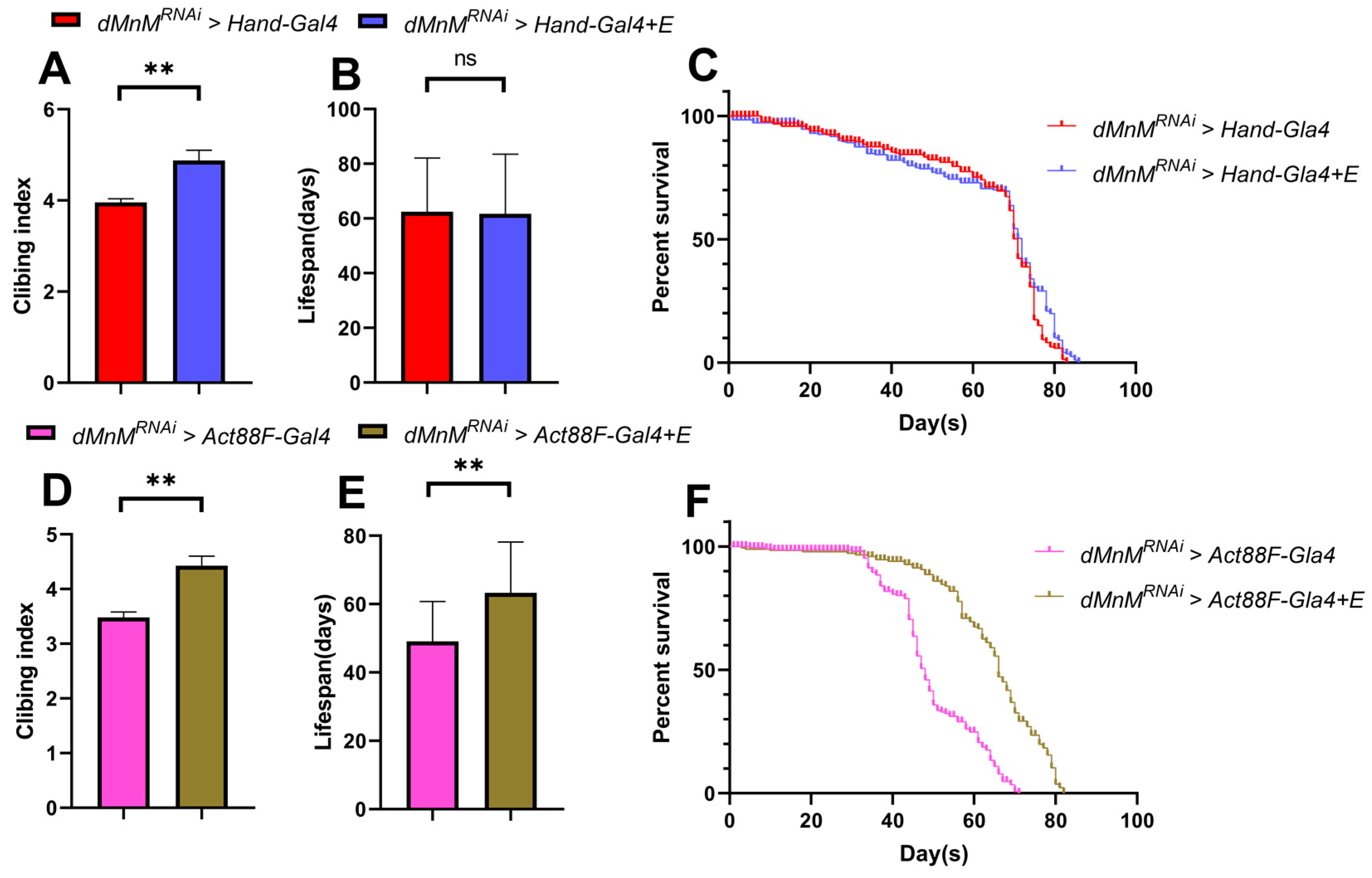
3.8. Effects of Regular Exercise on Climbing and Lifespan after dMnM Knockdown in Cardiac Myocytes and IFM of Drosophila
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nichols, M.; Townsend, N.; Scarborough, P.; Rayner, M. Cardiovascular disease in Europe 2014: Epidemiological update. Eur. Heart J. 2014, 35, 2950–2959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J. Executive summary: Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation 2014, 129, 399–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maron, B.J.; Maron, M.S. Hypertrophic cardiomyopathy. Lancet 2013, 381, 242–255. [Google Scholar] [CrossRef]
- Semsarian, C.; Ingles, J.; Maron, M.S.; Maron, B.J. New perspectives on the prevalence of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, 1249–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spudich, J.A. Three perspectives on the molecular basis of hypercontractility caused by hypertrophic cardiomyopathy mutations. Pflugers Arch. 2019, 471, 701–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auxerre-Plantie, E.; Nielsen, T.; Grunert, M.; Olejniczak, O.; Perrot, A.; Ozcelik, C.; Harries, D.; Matinmehr, F.; Dos Remedios, C.; Muhlfeld, C.; et al. Identification of MYOM2 as a candidate gene in hypertrophic cardiomyopathy and Tetralogy of Fallot, and its functional evaluation in the Drosophila heart. Dis. Model. Mech. 2020, 13, dmm045377. [Google Scholar] [CrossRef]
- Vinkemeier, U.; Obermann, W.; Weber, K.; Furst, D.O. The globular head domain of titin extends into the center of the sarcomeric M band. cDNA cloning, epitope mapping and immunoelectron microscopy of two titin-associated proteins. J. Cell Sci. 1993, 106 Pt 1, 319–330. [Google Scholar] [CrossRef]
- Agarkova, I.; Perriard, J.C. The M-band: An elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol. 2005, 15, 477–485. [Google Scholar] [CrossRef]
- Kenny, P.A.; Liston, E.M.; Higgins, D.G. Molecular evolution of immunoglobulin and fibronectin domains in titin and related muscle proteins. Gene 1999, 232, 11–23. [Google Scholar] [CrossRef]
- Grove, B.K.; Cerny, L.; Perriard, J.C.; Eppenberger, H.M. Myomesin and M-protein: Expression of two M-band proteins in pectoral muscle and heart during development. J Cell Biol. 1985, 101, 1413–1421. [Google Scholar] [CrossRef]
- Obermann, W.M.; Gautel, M.; Steiner, F.; van der Ven, P.F.; Weber, K.; Furst, D.O. The structure of the sarcomeric M band: Localization of defined domains of myomesin, M-protein, and the 250-kD carboxy-terminal region of titin by immunoelectron microscopy. J. Cell Biol. 1996, 134, 1441–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenauer, R.; Lange, S.; Hirschy, A.; Ehler, E.; Perriard, J.C.; Agarkova, I. Myomesin 3, a novel structural component of the M-band in striated muscle. J. Mol. Biol. 2008, 376, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Mishra, S.S. Studies on the preparation of rinderpest antigen for use in conglutinating complement absorption test. Indian Vet. J. 1978, 55, 643–647. [Google Scholar] [PubMed]
- Gautel, M.; Djinovic-Carugo, K. The sarcomeric cytoskeleton: From molecules to motion. J. Exp. Biol. 2016, 219, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Gao, J.; Li, J.; Xue, L.; Clark, K.J.; Ekker, S.C.; Du, S.J. Functional analysis of slow myosin heavy chain 1 and myomesin-3 in sarcomere organization in zebrafish embryonic slow muscles. J. Genet. Genom. 2012, 39, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Diop, S.B.; Bodmer, R. Gaining Insights into Diabetic Cardiomyopathy from Drosophila. Trends Endocrinol. Metab. 2015, 26, 618–627. [Google Scholar] [CrossRef] [Green Version]
- Bier, E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat. Rev. Genet. 2005, 6, 9–23. [Google Scholar] [CrossRef]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef] [Green Version]
- Van Voorhies, W.A. Metabolic function in Drosophila melanogaster in response to hypoxia and pure oxygen. J. Exp. Biol. 2009, 212, 3132–3141. [Google Scholar] [CrossRef] [Green Version]
- Gargano, J.W.; Martin, I.; Bhandari, P.; Grotewiel, M.S. Rapid iterative negative geotaxis (RING): A new method for assessing age-related locomotor decline in Drosophila. Exp. Gerontol 2005, 40, 386–395. [Google Scholar] [CrossRef]
- Bothe, I.; Baylies, M.K. Drosophila myogenesis. Curr Biol 2016, 26, R786–R791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madan, A.; Thimmaiya, D.; Franco-Cea, A.; Aiyaz, M.; Kumar, P.; Sparrow, J.C.; Nongthomba, U. Transcriptome analysis of IFM-specific actin and myosin nulls in Drosophila melanogaster unravels lesion-specific expression blueprints across muscle mutations. Gene 2017, 631, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Cammarato, A.; Hatch, V.; Saide, J.; Craig, R.; Sparrow, J.C.; Tobacman, L.S.; Lehman, W. Drosophila muscle regulation characterized by electron microscopy and three-dimensional reconstruction of thin filament mutants. Biophys. J. 2004, 86, 1618–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pai, V.B.; Nahata, M.C. Cardiotoxicity of chemotherapeutic agents: Incidence, treatment and prevention. Drug Saf 2000, 22, 263–302. [Google Scholar] [CrossRef]
- Arkat, S.; Umbarkar, P.; Singh, S.; Sitasawad, S.L. Mitochondrial Peroxiredoxin-3 protects against hyperglycemia induced myocardial damage in Diabetic cardiomyopathy. Free Radic. Biol. Med. 2016, 97, 489–500. [Google Scholar] [CrossRef]
- Faria, A.; Persaud, S.J. Cardiac oxidative stress in diabetes: Mechanisms and therapeutic potential. Pharmacol. Ther. 2017, 172, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wu, Y.; Wang, L.; Qiu, P.; Zha, W.; Yu, W. Prokineticin 2 (PK2) Rescues Cardiomyocytes from High Glucose/High Palmitic Acid-Induced Damage by Regulating the AKT/GSK3beta Pathway In Vitro. Oxid. Med. Cell Longev. 2020, 2020, 3163629. [Google Scholar] [CrossRef]
- Zheng, D.; Ma, J.; Yu, Y.; Li, M.; Ni, R.; Wang, G.; Chen, R.; Li, J.; Fan, G.C.; Lacefield, J.C.; et al. Silencing of miR-195 reduces diabetic cardiomyopathy in C57BL/6 mice. Diabetologia 2015, 58, 1949–1958. [Google Scholar] [CrossRef]
- Steiner, J.L.; Lang, C.H. Etiology of alcoholic cardiomyopathy: Mitochondria, oxidative stress and apoptosis. Int. J. Biochem. Cell Biol. 2017, 89, 125–135. [Google Scholar] [CrossRef]
- Szygula-Jurkiewicz, B.; Szczurek-Wasilewicz, W.; Osadnik, T.; Frycz-Kurek, A.M.; Maciol-Skurk, K.; Malyszek-Tumidajewicz, J.; Skrzypek, M.; Romuk, E.; Gasior, M.; Banach, M.; et al. Oxidative Stress Markers in Hypertrophic Cardiomyopathy. Medicina 2021, 58, 31. [Google Scholar] [CrossRef]
- Nakamura, K.; Kusano, K.F.; Matsubara, H.; Nakamura, Y.; Miura, A.; Nishii, N.; Banba, K.; Nagase, S.; Miyaji, K.; Morita, H.; et al. Relationship between oxidative stress and systolic dysfunction in patients with hypertrophic cardiomyopathy. J. Card. Fail. 2005, 11, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Nocon, M.; Hiemann, T.; Muller-Riemenschneider, F.; Thalau, F.; Roll, S.; Willich, S.N. Association of physical activity with all-cause and cardiovascular mortality: A systematic review and meta-analysis. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Shiroma, E.J.; Lee, I.M. Physical activity and cardiovascular health: Lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation 2010, 122, 743–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klempfner, R.; Kamerman, T.; Schwammenthal, E.; Nahshon, A.; Hay, I.; Goldenberg, I.; Dov, F.; Arad, M. Efficacy of exercise training in symptomatic patients with hypertrophic cardiomyopathy: Results of a structured exercise training program in a cardiac rehabilitation center. Eur. J. Prev. Cardiol. 2015, 22, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Saberi, S.; Wheeler, M.; Bragg-Gresham, J.; Hornsby, W.; Agarwal, P.P.; Attili, A.; Concannon, M.; Dries, A.M.; Shmargad, Y.; Salisbury, H.; et al. Effect of Moderate-Intensity Exercise Training on Peak Oxygen Consumption in Patients With Hypertrophic Cardiomyopathy: A Randomized Clinical Trial. JAMA 2017, 317, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Boardman, N.T.; Hafstad, A.D.; Lund, J.; Rossvoll, L.; Aasum, E. Exercise of obese mice induces cardioprotection and oxygen sparing in hearts exposed to high-fat load. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H1054–H1062. [Google Scholar] [CrossRef]
- Kesherwani, V.; Chavali, V.; Hackfort, B.T.; Tyagi, S.C.; Mishra, P.K. Exercise ameliorates high fat diet induced cardiac dysfunction by increasing interleukin 10. Front. Physiol. 2015, 6, 124. [Google Scholar] [CrossRef] [Green Version]
- Wen, D.T.; Zheng, L.; Yang, F.; Li, H.Z.; Hou, W.Q. Endurance exercise prevents high-fat-diet induced heart and mobility premature aging and dsir2 expression decline in aging Drosophila. Oncotarget 2018, 9, 7298–7311. [Google Scholar] [CrossRef] [Green Version]
- Wen, D.T.; Zheng, L.; Lu, K.; Hou, W.Q. Activation of cardiac Nmnat/NAD+/SIR2 pathways mediates endurance exercise resistance to lipotoxic cardiomyopathy in aging Drosophila. J. Exp. Biol. 2021, 224, jeb242425. [Google Scholar] [CrossRef]
- Lu, J.; Liu, J.; Zhang, L.; Wang, X.; Zhang, Y.; Tang, Q. Morphological and functional characterization of diabetic cardiomyopathy in db/db mice following exercise, metformin alone, or combination treatments. Biochem. Biophys. Res. Commun. 2021, 584, 80–86. [Google Scholar] [CrossRef]
- de Souza, S.L.B.; Mota, G.A.F.; Gregolin, C.S.; do Nascimento, M.; Luvizotto, R.A.M.; Bazan, S.G.Z.; Sugizaki, M.M.; Barbisan, L.F.; Cicogna, A.C.; do Nascimento, A.F. Exercise Training Attenuates Cirrhotic Cardiomyopathy. J. Cardiovasc. Transl. Res. 2021, 14, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Feng, Y.; Wen, D.T.; Wang, H.; Wu, X.S. Fatiguing exercise initiated later in life reduces incidence of fibrillation and improves sleep quality in Drosophila. Age 2015, 37, 9816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogler, G.; Ocorr, K. Visualizing the beating heart in Drosophila. J. Vis. Exp. 2009, 31, e1425. [Google Scholar] [CrossRef] [Green Version]
- Fink, M.; Callol-Massot, C.; Chu, A.; Ruiz-Lozano, P.; Izpisua Belmonte, J.C.; Giles, W.; Bodmer, R.; Ocorr, K. A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. Biotechniques 2009, 46, 101–113. [Google Scholar] [CrossRef]
- Lam, A.; Karekar, P.; Shah, K.; Hariharan, G.; Fleyshman, M.; Kaur, H.; Singh, H.; Gururaja Rao, S. Drosophila Voltage-Gated Calcium Channel alpha1-Subunits Regulate Cardiac Function in the Aging Heart. Sci. Rep. 2018, 8, 6910. [Google Scholar] [CrossRef] [Green Version]
- Zhikrevetskaya, S.; Peregudova, D.; Danilov, A.; Plyusnina, E.; Krasnov, G.; Dmitriev, A.; Kudryavtseva, A.; Shaposhnikov, M.; Moskalev, A. Effect of Low Doses (5-40 cGy) of Gamma-irradiation on Lifespan and Stress-related Genes Expression Profile in Drosophila melanogaster. PLoS ONE 2015, 10, e0133840. [Google Scholar] [CrossRef] [Green Version]
- Ding, M.; Li, Q.F.; Yin, G.; Liu, J.L.; Jan, X.Y.; Huang, T.; Li, A.C.; Zheng, L. Effects of Drosophila melanogaster regular exercise and apolipoprotein B knockdown on abnormal heart rhythm induced by a high-fat diet. PLoS ONE 2022, 17, e0262471. [Google Scholar] [CrossRef]
- Zheng, L.; Li, Q.F.; Ni, L.; Wang, H.; Ruan, X.C.; Wu, X.S. Lifetime regular exercise affects the incident of different arrhythmias and improves organismal health in aging female Drosophila melanogaster. Biogerontology 2017, 18, 97–108. [Google Scholar] [CrossRef]
- Kaushik, G.; Zambon, A.C.; Fuhrmann, A.; Bernstein, S.I.; Bodmer, R.; Engler, A.J.; Cammarato, A. Measuring passive myocardial stiffness in Drosophila melanogaster to investigate diastolic dysfunction. J. Cell Mol. Med. 2012, 16, 1656–1662. [Google Scholar] [CrossRef] [Green Version]
- Kass, D.A.; Bronzwaer, J.G.; Paulus, W.J. What mechanisms underlie diastolic dysfunction in heart failure? Circ. Res. 2004, 94, 1533–1542. [Google Scholar] [CrossRef]
- Borbely, A.; Papp, Z.; Edes, I.; Paulus, W.J. Molecular determinants of heart failure with normal left ventricular ejection fraction. Pharmacol Rep 2009, 61, 139–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudiger, A.; Singer, M. Mechanisms of sepsis-induced cardiac dysfunction. Crit. Care Med. 2007, 35, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, M.C.; Kaushik, G.; Engler, A.J.; Lehman, W.; Cammarato, A. A Drosophila melanogaster model of diastolic dysfunction and cardiomyopathy based on impaired troponin-T function. Circ. Res. 2014, 114, e6–e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laughlin, M.H.; Bowles, D.K.; Duncker, D.J. The coronary circulation in exercise training. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H10–H23. [Google Scholar] [CrossRef]
- Morris, J.N.; Everitt, M.G.; Pollard, R.; Chave, S.P.; Semmence, A.M. Vigorous exercise in leisure-time: Protection against coronary heart disease. Lancet 1980, 2, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Sturek, M. Ca2+ regulatory mechanisms of exercise protection against coronary artery disease in metabolic syndrome and diabetes. J. Appl. Physiol. (1985) 2011, 111, 573–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2181–H2190. [Google Scholar] [CrossRef] [Green Version]
- Godo, S.; Shimokawa, H. Divergent roles of endothelial nitric oxide synthases system in maintaining cardiovascular homeostasis. Free Radic. Biol. Med. 2017, 109, 4–10. [Google Scholar] [CrossRef]
- Shin, S.K.; Cho, H.W.; Song, S.E.; Song, D.K. Catalase and nonalcoholic fatty liver disease. Pflugers Arch. 2018, 470, 1721–1737. [Google Scholar] [CrossRef]
- Ogino, S.; Ogino, N.; Tomizuka, K.; Eitoku, M.; Okada, Y.; Tanaka, Y.; Suganuma, N.; Ogino, K. SOD2 mRNA as a potential biomarker for exercise: Interventional and cross-sectional research in healthy subjects. J. Clin. Biochem. Nutr. 2021, 69, 137–144. [Google Scholar] [CrossRef]
- Zhang, J.; Bi, J.; Ren, Y.; Du, Z.; Li, T.; Wang, T.; Zhang, L.; Wang, M.; Wei, S.; Lv, Y.; et al. Involvement of GPX4 in irisin’s protection against ischemia reperfusion-induced acute kidney injury. J. Cell Physiol. 2021, 236, 931–945. [Google Scholar] [CrossRef] [PubMed]
- French, J.P.; Hamilton, K.L.; Quindry, J.C.; Lee, Y.; Upchurch, P.A.; Powers, S.K. Exercise-induced protection against myocardial apoptosis and necrosis: MnSOD, calcium-handling proteins, and calpain. FASEB J. 2008, 22, 2862–2871. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, W.; Li, Q.; Ding, M.; Cao, Y.; Wang, T.; Zhang, S.; Feng, J.; Li, H.; Zheng, L. Regular Exercise Rescues Heart Function Defects and Shortens the Lifespan of Drosophila Caused by dMnM Downregulation. Int. J. Environ. Res. Public Health 2022, 19, 16554. https://doi.org/10.3390/ijerph192416554
Gu W, Li Q, Ding M, Cao Y, Wang T, Zhang S, Feng J, Li H, Zheng L. Regular Exercise Rescues Heart Function Defects and Shortens the Lifespan of Drosophila Caused by dMnM Downregulation. International Journal of Environmental Research and Public Health. 2022; 19(24):16554. https://doi.org/10.3390/ijerph192416554
Chicago/Turabian StyleGu, Wenzhi, Qiufang Li, Meng Ding, Yurou Cao, Tongquan Wang, Shihu Zhang, Jiadong Feng, Hongyu Li, and Lan Zheng. 2022. "Regular Exercise Rescues Heart Function Defects and Shortens the Lifespan of Drosophila Caused by dMnM Downregulation" International Journal of Environmental Research and Public Health 19, no. 24: 16554. https://doi.org/10.3390/ijerph192416554
APA StyleGu, W., Li, Q., Ding, M., Cao, Y., Wang, T., Zhang, S., Feng, J., Li, H., & Zheng, L. (2022). Regular Exercise Rescues Heart Function Defects and Shortens the Lifespan of Drosophila Caused by dMnM Downregulation. International Journal of Environmental Research and Public Health, 19(24), 16554. https://doi.org/10.3390/ijerph192416554





