The Control of Novel and Traditional Elemental Impurities: Ag, Au, Co, Cs, Li, Mo, Se, Sr, and V in Mint Tea Infusions (Peppermint, Mentha piperita L.) Available in Poland: A Health Risk Assessment
Abstract
1. Introduction
- Health risk assessment of new and traditional EI (Ag, Au, Co, Cs, Li, Mo, Se, Sr, and V) for the first time in mint tea infusions (Mentha piperita L.) available in Polish markets;
- EI profile in mint tea infusions (µg/L of infusion) as raw results;
- Estimation of the weekly intake (µg/L of infusion/week) established on weekly tea consumption (based on [23]);
- Estimation of the weekly intake per body weight (µg/L of infusion/week/bw) based on weekly tea consumption per individual (~70 kg bw) in comparison to the Provisional Tolerable Weekly Intake (PTWI) appointed by the Joint FAO/WHO Expert Committee on Food Additives (JECFA); and
- Individual health risk assessment for elements without PTWI values.
2. Materials and Methods
2.1. Samples
2.2. Chemicals
2.3. Apparatus
2.4. The Procedure of the Study
2.5. The Calibration and Quality Control
2.6. Health Risk Assessment
- The tea infusion (µg/L of infusion) using elemental impurity profile, the half violin plots, and the descriptive statistics (minimum, maximum, and mean);
- The assessment of weekly intake (µg/L of infusion/week) established on weekly tea consumption (about 6 L of tea per week, based on [23]), as seen in Equation (1):
- 3.
- Based on the weekly tea consumption per individual (at about 70 kg/bw) in comparison to the PTWI. The weekly intake was evaluated depending on body weight (µg/L of infusion/week/bw), as seen in Equation (2):
2.7. Statistical Analysis
3. Results and Discussion
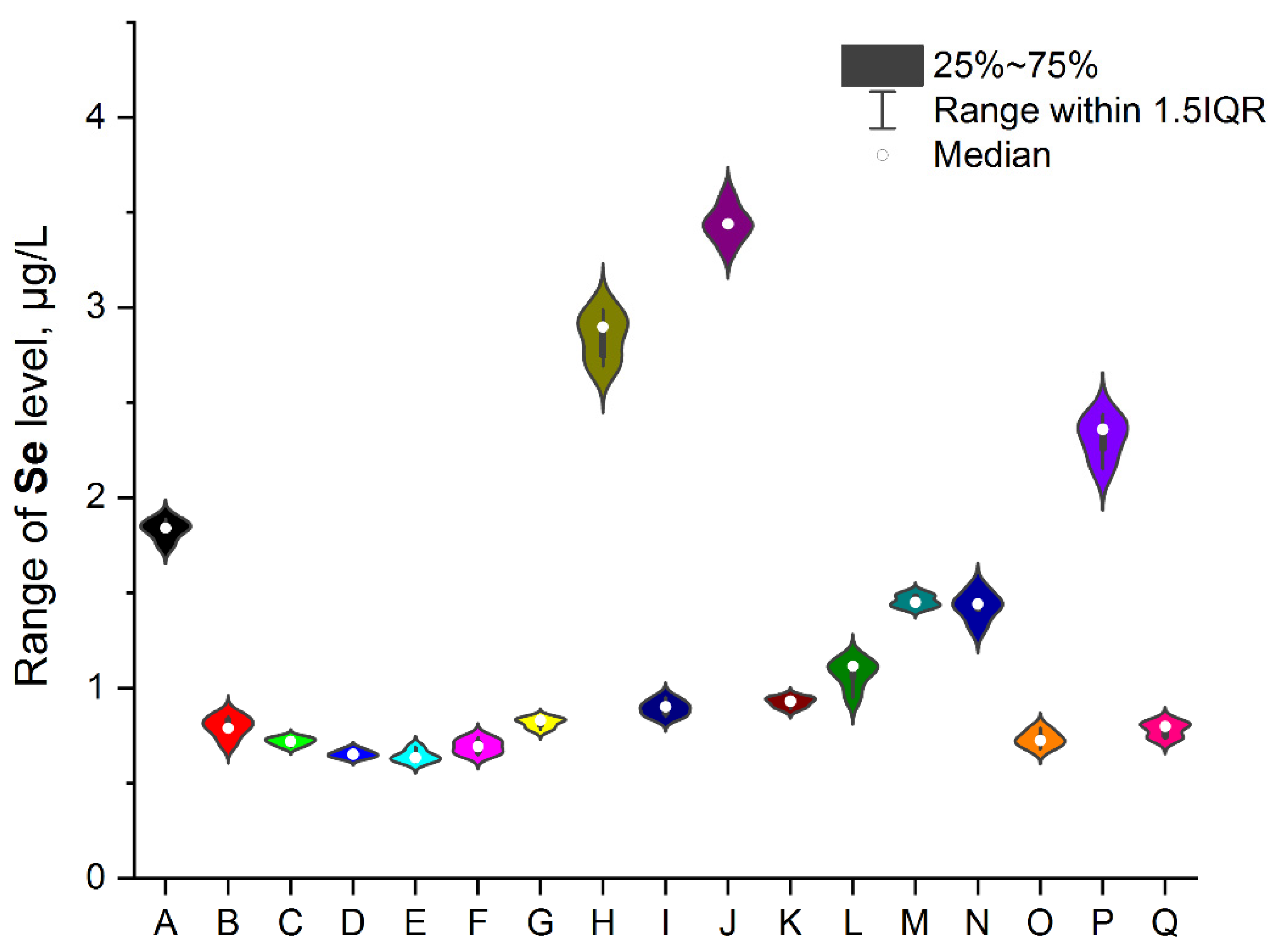
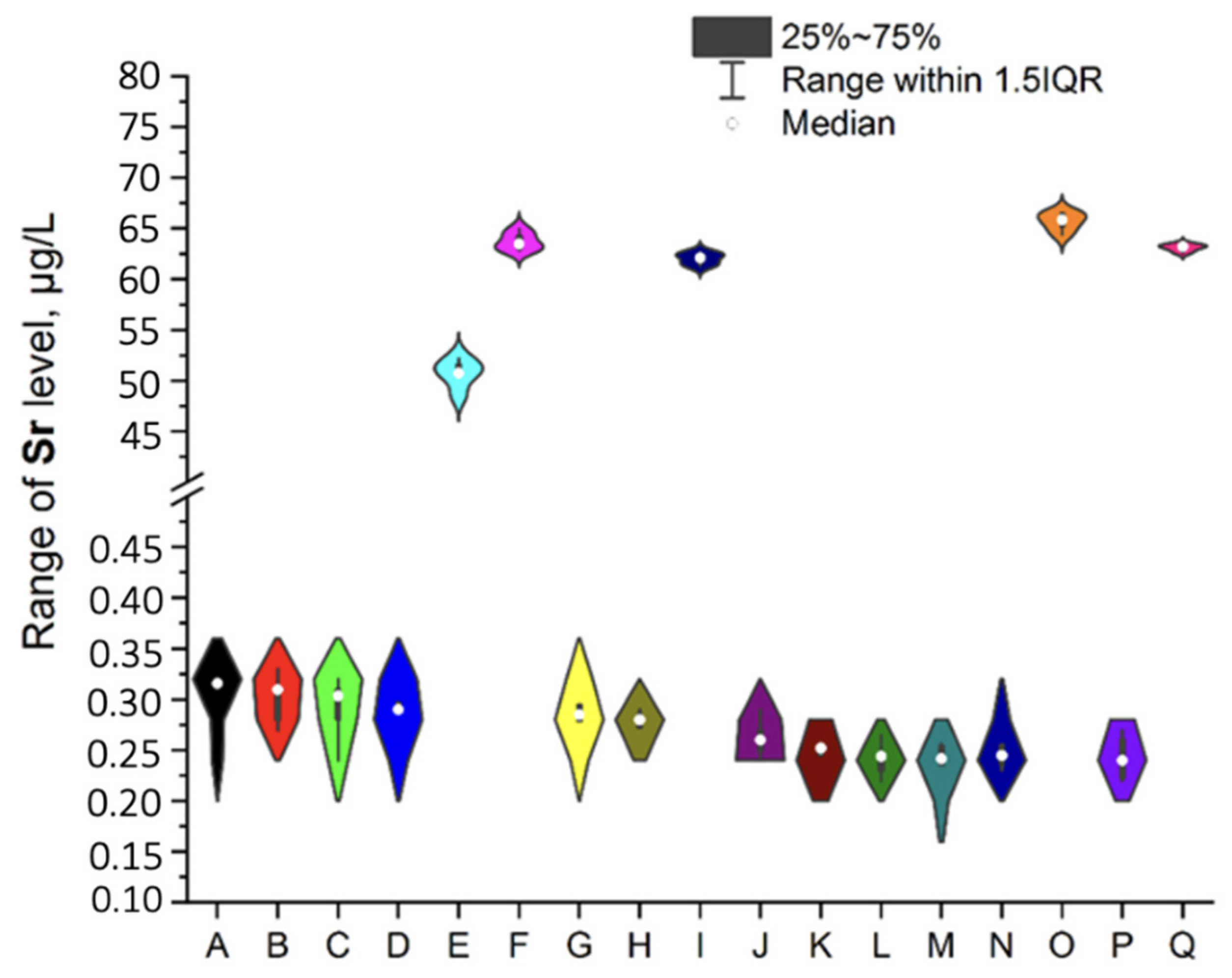
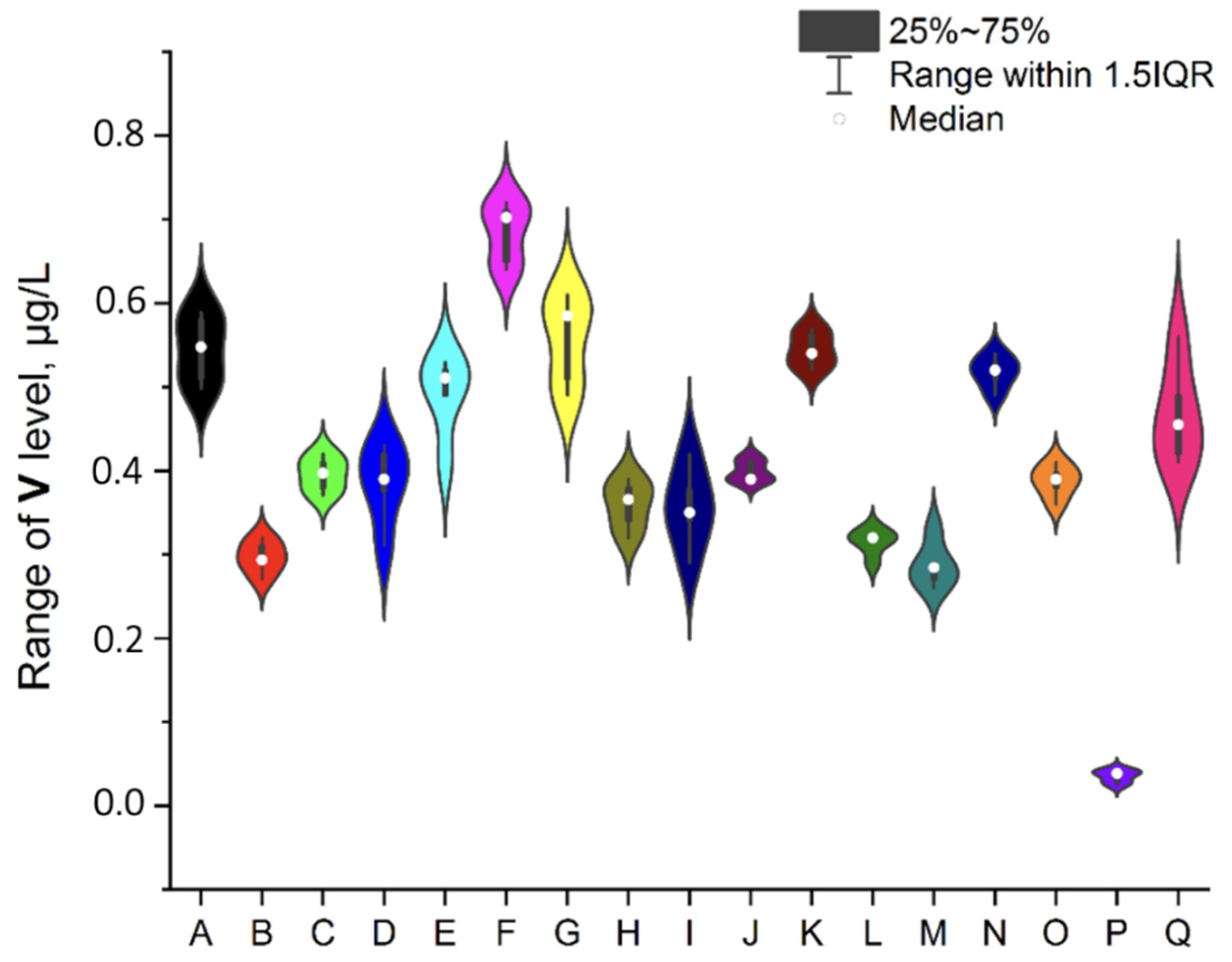
3.1. NEIs and Traditional Impurities Profile in Analysed Mint Tea Samples
3.2. The Assessment of the Weekly Exposure in Comparison with the Weekly Intake of Tea
3.3. The Assessment of the Weekly Intake of Each Element, including Body Weight Based on Weekly Consumption
3.4. Health Risk Assessment of NEIs in Analysed Teas
3.4.1. Silver
3.4.2. Gold
3.4.3. Cobalt
3.4.4. Cesium
3.4.5. Lithium
3.4.6. Molybdenum
3.4.7. Strontium
3.4.8. Vanadium
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKay, D.L.; Blumberg, J.B. A Review of the Bioactivity and Potential Health Benefits of Peppermint Tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Lin, Z.; Shao, K.; Wang, X.; Xu, J.; Zhai, H.; Wang, H.; Xu, W.; Zhao, Y. Combination of White Tea and Peppermint Demonstrated Synergistic Antibacterial and Anti-inflammatory Activities. J. Sci. Food Agric. 2021, 101, 2500–2510. [Google Scholar] [CrossRef]
- Jurowski, K.; Kondratowicz-Pietruszka, E.; Krośniak, M. The Toxicological Safety Assessment of Heavy Metal Impurities (As, Pb, and Cd) in Mint Tea Infusions (Mentha piperita L.) Available in Polish Markets. Biol. Trace Elem. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Maiga, A.; Diallo, D.; Bye, R.; Paulsen, B.S. Determination of Some Toxic and Essential Metal Ions in Medicinal and Edible Plants from Mali. J. Agric. Food Chem. 2005, 53, 2316–2321. [Google Scholar] [CrossRef] [PubMed]
- Madejón, P.; Domínguez, M.T.; Madejón, E.; Cabrera, F.; Marañón, T.; Murillo, J.M. Soil-Plant Relationships and Contamination by Trace Elements: A Review of Twenty Years of Experimentation and Monitoring after the Aznalcóllar (SW Spain) Mine Accident. Sci. Total Environ. 2018, 625, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Sarma, H.; Deka, S.; Deka, H.; Saikia, R.R. Accumulation of Heavy Metals in Selected Medicinal Plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2012; Volume 214, pp. 63–86. ISBN 978-1-4614-0667-9. [Google Scholar]
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and Environmental Risks of Nanomaterials: Challenges and Future Needs. J. Environ. Sci. Health Part C 2009, 27, 1–35. [Google Scholar] [CrossRef]
- Hadrup, N.; Sharma, A.K.; Loeschner, K. Toxicity of Silver Ions, Metallic Silver, and Silver Nanoparticle Materials after in Vivo Dermal and Mucosal Surface Exposure: A Review. Regul. Toxicol. Pharmacol. 2018, 98, 257–267. [Google Scholar] [CrossRef]
- Lansdown, A.B.G. GOLD: Human Exposure and Update on Toxic Risks. Crit. Rev. Toxicol. 2018, 48, 596–614. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Cobalt; Public Health Service, U.S. Department of Health and Human Services: Atlanta, GA, USA, 2004. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp33.pdf (accessed on 2 December 2022).
- United States Environmental Protection Agency (US EPA). Cobalt Compounds: Technology Transfer Network Air Toxics Web Site: Hazard Summary; United States Environmental Protection Agency: Washington, DC, USA, 2000. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/cobalt-compounds.pdf (accessed on 2 December 2022).
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt Toxicity in Humans—A Review of the Potential Sources and Systemic Health Effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Jarrell, W.M.; Page, A.L.; Elseewi, A.A. Molybdenum in the Environment. In Residue Reviews; Gunther, F.A., Gunther, J.D., Eds.; Springer: New York, NY, USA, 1980; pp. 1–43. ISBN 978-1-4612-6098-1. [Google Scholar]
- Hadrup, N.; Sørli, J.B.; Sharma, A.K. Pulmonary Toxicity, Genotoxicity, and Carcinogenicity Evaluation of Molybdenum, Lithium, and Tungsten: A Review. Toxicology 2022, 467, 153098. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Selenium; Public Health Service, U.S. Department of Health and Human Services: Atlanta, GA, USA, 2003. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp92.pdf (accessed on 2 December 2022).
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Vanadium; Public Health Service, U.S. Department of Health and Human Services: Atlanta, GA, USA, 2012. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp58.pdf (accessed on 2 December 2022).
- Gruzewska, K.; Michno, A.; Pawelczyk, T.; Bielarczyk, H. Essentiality and Toxicity of Vanadium Supplements in Health and Pathology. J. Physiol. Pharmacol. 2014, 65, 603–611. [Google Scholar]
- Pors Nielsen, S. The Biological Role of Strontium. Bone 2004, 35, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, E.M.; Aubry, J.-M. Lithium: Updated Human Knowledge Using an Evidence-Based Approach: Part II: Clinical Pharmacology and Therapeutic Monitoring. CNS Drugs 2009, 23, 331–349. [Google Scholar] [CrossRef] [PubMed]
- McKnight, R.F.; Adida, M.; Budge, K.; Stockton, S.; Goodwin, G.M.; Geddes, J.R. Lithium Toxicity Profile: A Systematic Review and Meta-Analysis. Lancet 2012, 379, 721–728. [Google Scholar] [CrossRef]
- Yu, D.; Morisada, S.; Kawakita, H.; Ohto, K.; Inoue, K.; Song, X.; Zhang, G. Selective Cesium Adsorptive Removal on Using Crosslinked Tea Leaves. Processes 2019, 7, 412. [Google Scholar] [CrossRef]
- Kobayashi, D.; Nishimura, N.; Hazama, A. Cesium Treatment Depresses Glycolysis Pathway in HeLa Cell. Cell. Physiol. Biochem. 2021, 55, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Matuszak, L.; Maleszka, A. Assessment of polish consumer’s exposure to lead element from teas. Zarządzanie Finans. 2012, 3, 206–223. [Google Scholar]
- Łozak, A.; Sołtyk, K.; Ostapczuk, P.; Fijałek, Z. Determination of Selected Trace Elements in Herbs and Their Infusions. Sci. Total Environ. 2002, 289, 33–40. [Google Scholar] [CrossRef]
- EFSA Scientific Committee. Guidance on Selected Default Values to Be Used by the EFSA Scientific Committee, Scientific Panels and Units in the Absence of Actual Measured Data. EFSA J. 2012, 10, 2579. [Google Scholar] [CrossRef]
- Herrman, J.L.; Younes, M. Background to the ADI/TDI/PTWI. Regul. Toxicol. Pharmacol. 1999, 30, S109–S113. [Google Scholar] [CrossRef]
- Vračko, P.; Tuomisto, J.; Grad, J.; Kunsele, E. Exposure of Children to Chemical Hazards in Food; World Health Organization: Geneva, Switzerland, 2007; Available online: https://www.euro.who.int/__data/assets/pdf_file/0004/97042/4.4.-Exposure-of-children-to-chemical-hazards-in-food-EDITED_layouted.pdf (accessed on 2 December 2022).
- World Health Organization. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), As; No. TRS 959-JECFA 72; World Health Organization: Geneva, Switzerland, 2011; Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/1863 (accessed on 2 December 2022).
- World Health Organization. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), Cd; No. TRS 983 JECFA 77; World Health Organization: Geneva, Switzerland, 2021; Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/1376 (accessed on 2 December 2022).
- World Health Organization. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), Pb; No. TRS 960-JECFA 73; World Health Organization: Geneva, Switzerland, 2011; Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/3511 (accessed on 2 December 2022).
- World Health Organization; Food and Agriculture Organization of the United Nations; Joint FAO/WHO Expert Committee on Food Additives. Meeting (72nd: 2010: Rome, Italy). Evaluation of Certain Contaminants in Food: Seventy-Second [72nd] Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2011; ISBN 9789241209595. [Google Scholar]
- World Health Organization. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), Ag; No. TRS 617-JECFA 21/22; World Health Organization: Geneva, Switzerland, 2014; Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/3411 (accessed on 2 December 2022).
- Committee for Human Medicinal Products. ICH Guideline Q3D (R1) on Elemental Impurities; European Medicines Agency: Amsterdam, The Netherlands, 2019; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-32.pdf (accessed on 2 December 2022).
- Jurowska, A.; Jurowski, K.; Szklarzewicz, J.; Buszewski, B.; Kalenik, T.; Piekoszewski, W. Molybdenum Metallopharmaceuticals Candidate Compounds—The “Renaissance” of Molybdenum Metallodrugs? CMC 2016, 23, 3322–3342. [Google Scholar] [CrossRef] [PubMed]

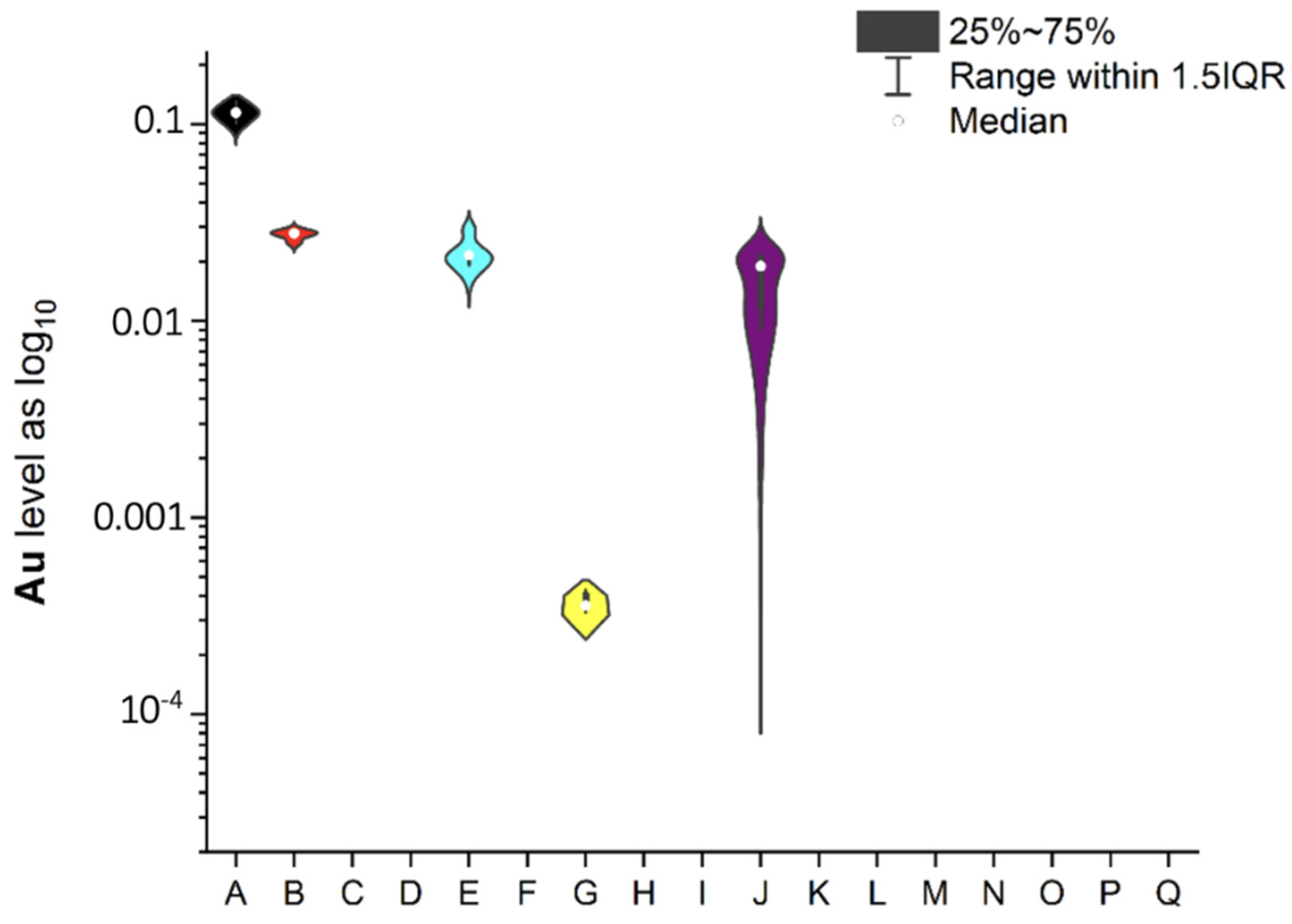
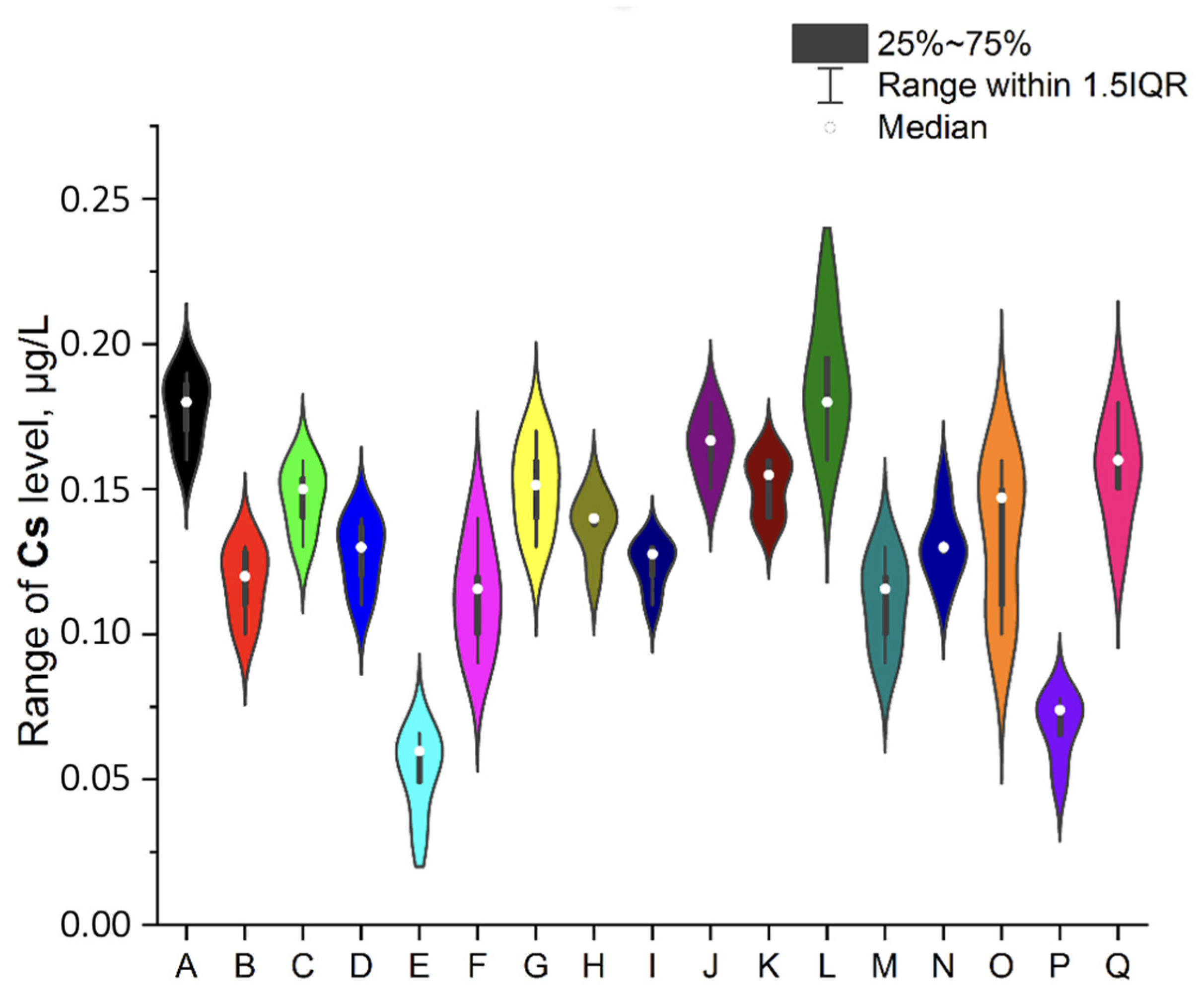
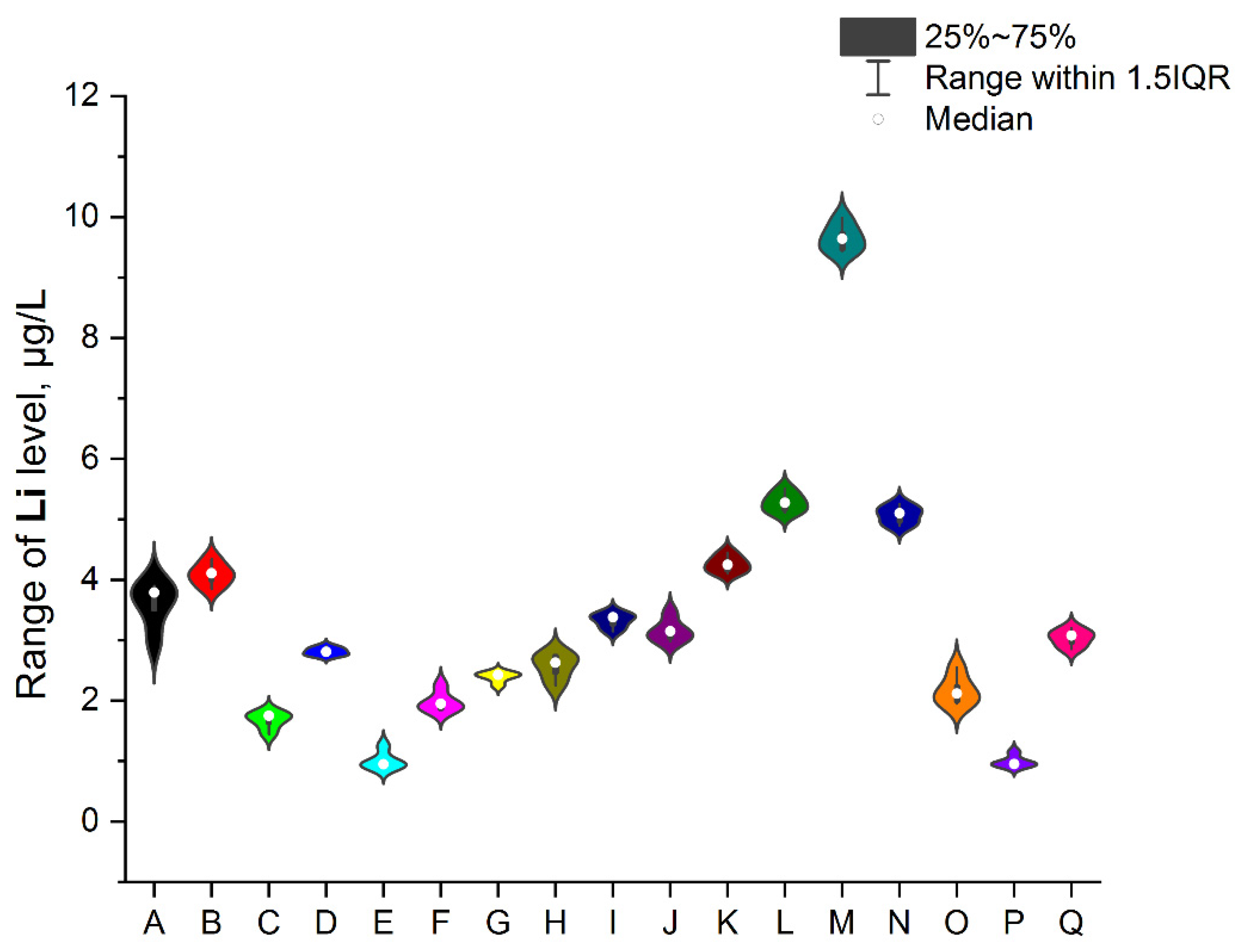
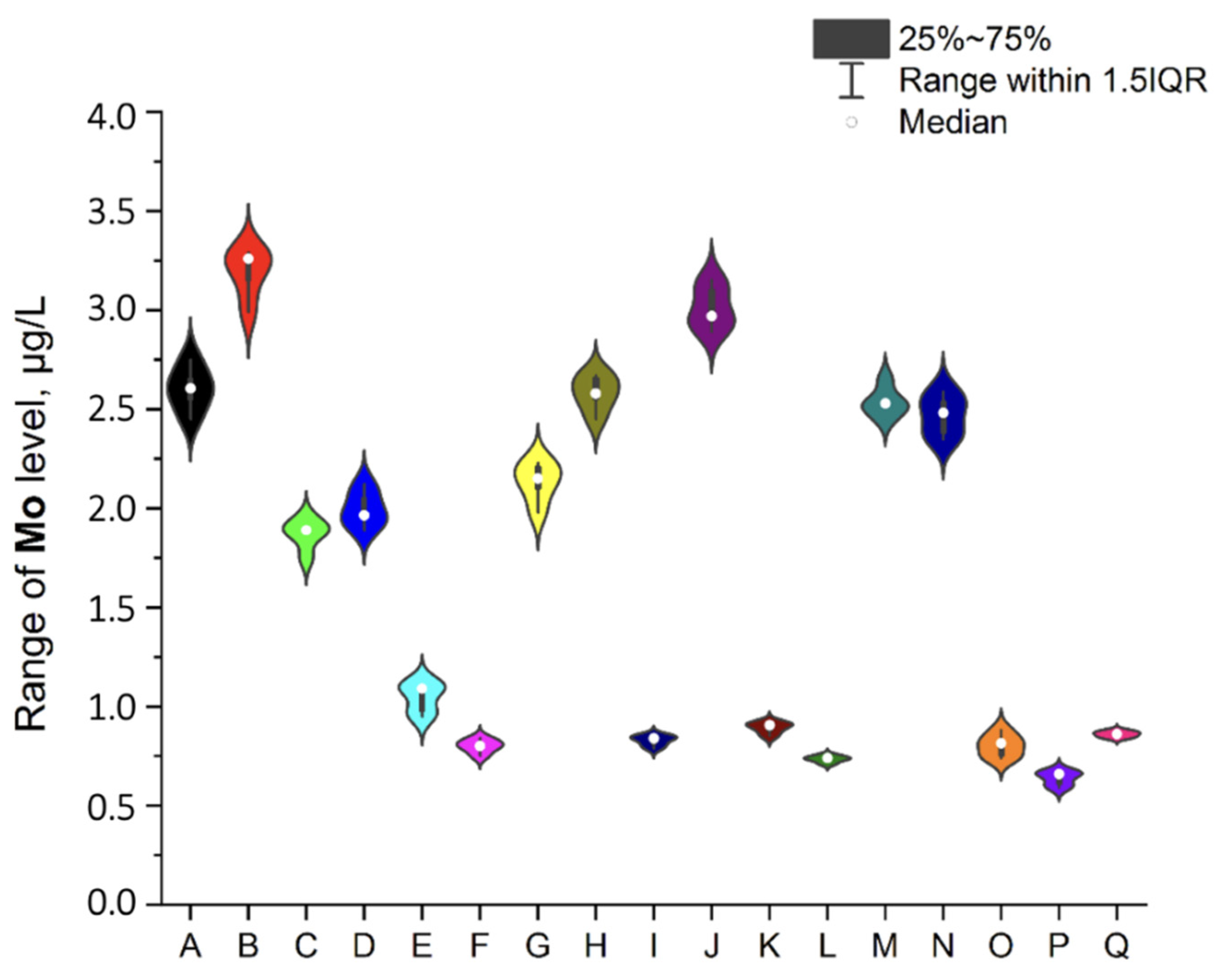
| Code of Sample | Form | Number of Raw Materials for the Infusion Process, g | Time of the Infusion Process (Brew Time), Minutes | Country of Origin | EAN |
|---|---|---|---|---|---|
| A | Tea bag | 1.5 | 8–10 | Poland | 5906881826065 |
| B | Leaf-like | 2.0 | 7–9 | Poland | nd * |
| C | Tea bag | 1.0 | 5–10 | nd * | 5900738000103 |
| D | Tea bag | 2.0 | 5–10 | Malaysia | 5051898696337 |
| E | Tea bag | 2.0 | 10 | Poland | 5900956002309 |
| F | Tea bag | 1.5 | 5–8 | Poland | 5900675006275 |
| G | Tea bag | 2.0 | 5 | Poland | 5901483060206 |
| H | Nylon tea bag | 2.0 | 8–10 | Poland | 5900396019783 |
| I | Needle-like | 1.0–1.5 (teaspoon) | 10 | Egypt | 5909000519206 |
| J | Tea bag | 2.0 | 8 | Poland | 4056489007159 |
| K | Tea bag | 2.0 | 5-8 | Poland | 5903886172371 |
| L | Tea bag | 1.5 | 5–8 | Poland | nd * |
| M | Silk tea bag | 2.0 | 10 | Poland | 5909990029303 |
| N | Needle-like | 1.0–1.5 (teaspoon) | 5–8 | Poland | 5901483130022 |
| O | Tea bag | 1.5 | 3–5 | Poland | 5900175431737 |
| P | Needle-like | 1.0–1.5 (teaspoon) | 10 | Poland | 5900956006628 |
| Q | Tea bag | 1.4 | 5–7 | Poland | 5900888010915 |
| Element | Concentration in an Applied Multi-Element Stock Solution, mg·L−1 |
|---|---|
| Ag | 10.0 |
| Au | 10.1 |
| Co | 20.0 |
| Cs | 10.0 |
| Li | 19.8 |
| Mo | 19.9 |
| Se | 101.0 |
| Sr | 9.5 |
| V | 20.0 |
| ICP-MS | Elan DRC-e Perkin Elmer (US) |
|---|---|
| Sample introduction | Scott spray chamber |
| Skimmer cone | Ni |
| Sampler cone | Ni |
| RF power, W | 1150 |
| Number of sweeps/readings | 4 |
| Number of readings/replicates | 2 |
| Number of replicates | 3 |
| Cooling gas flow rate(L·min−1) | 17 |
| Scanning mode | Peak hopping |
| Analyte | Calibration Function | R | Recovery, % | |
|---|---|---|---|---|
| Sigma A | Slope | |||
| Ag | 49.3241 | 3141.38 | 0.99986 | 98 ± 1.0 |
| Au | 4.45668 | 69.3612 | 0.99586 | 97 ± 1.3 |
| Co | 10.066 | 8651.94 | 0.99999 | 99 ± 0.7 |
| Cs | 46.851 | 14,413.5 | 0.99988 | 97 ± 1.5 |
| Li | 3.80757 | 1006.25 | 0.99998 | 98 ± 1.1 |
| Mo | 23.9563 | 3380.03 | 0.99992 | 97 ± 1.5 |
| Se | 0.79798 | 153.457 | 0.99996 | 97 ± 1.3 |
| Sr | 115.175 | 16,393.8 | 0.99992 | 96 ± 1.6 |
| V | 11.3286 | 7996.7 | 0.99999 | 98 ± 1.2 |
| Element | Minimum, μg/L | Maximum, μg/L | Mean, μg/L | RSD, % |
|---|---|---|---|---|
| Ag | 0.183 | 0.754 | 0.469 | 1.8–3.94 |
| Au | 0.000356 | 0.114 | 0.0350 | 2.37–4.17 |
| Co | 0.372 | 0.952 | 0.579 | 0.8–6.8 |
| Cs | 0.0598 | 0.195 | 0.138 | 1.1–4.3 |
| Li | 0.904 | 9.641 | 3.353 | 0.1–9.64 |
| Mo | 0.663 | 3.261 | 1.720 | 0.4–6.4 |
| Se | 0.693 | 3.417 | 1.307 | 1.9–2.84 |
| Sr | 0.223 | 64.270 | 18.202 | 0.7–5.7 |
| V | 0.284 | 0.702 | 0.420 | 1.5–10.34 |
| Element | Obtained Mean Value | |
|---|---|---|
| Obtained Mean Value, µg/L | Literature Values (Łozak et al., 2002), mg/kg | |
| Co | 0.579 ± 0.062 | 0.056 ± 0.001 |
| Cr | 13.779 ± 0.95 | 0.390 ± 0.019 |
| Li | 3.353 ± 0.36 | 0.339 ± 0.008 |
| Se | 1.307 ± 0.12 | 0.087 ± 0.033 |
| Sr | 18.202 ± 0.85 | 2.26 ± 0.06 |
| V | 0.420 ± 0.032 | 0.113 ± 0.006 |
| Product | Assessment of Weekly Intake, µg/Week | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ag | Au | Co | Cs | Li | Mo | Se | Sr | V | |
| A | 236.401 | 0.686 | 5.712 | 1.119 | 22.738 | 15.637 | 11.174 | 1.895 | 3.286 |
| B | - | 0.163 | 3.565 | 0.772 | 24.839 | 19.569 | 4.728 | 1.858 | 1.761 |
| C | - | - | 4.152 | 0.924 | 9.732 | 11.298 | 4.419 | 1.820 | 2.383 |
| D | - | - | 2.913 | 0.824 | 16.860 | 11.788 | 3.914 | 1.783 | 2.256 |
| E | - | 0.129 | 2.351 | 0.359 | 5.426 | 6.547 | 3.735 | 304.267 | 3.064 |
| F | - | - | 2.657 | 0.694 | 11.765 | 4.809 | 4.160 | 385.622 | 4.214 |
| G | - | 0.002 | 3.663 | 0.909 | 14.314 | 13.255 | 5.011 | 1.672 | 3.510 |
| H | - | - | 2.756 | 0.825 | 15.792 | 15.943 | 17.387 | 1.635 | 2.195 |
| I | - | - | 2.550 | 0.766 | 20.328 | 4.882 | 5.408 | 372.623 | 2.271 |
| J | - | 0.071 | 3.206 | 1.001 | 18.157 | 17.822 | 20.503 | 1.561 | 2.336 |
| K | - | - | 3.969 | 0.929 | 25.904 | 5.443 | 5.586 | 1.524 | 3.372 |
| L | - | - | 4.372 | 1.171 | 31.651 | 4.381 | 6.692 | 1.487 | 1.925 |
| M | - | - | 2.915 | 0.694 | 57.845 | 15.177 | 8.569 | 1.449 | 1.707 |
| N | - | - | 4.743 | 0.769 | 30.619 | 14.895 | 8.650 | 1.412 | 3.169 |
| O | 10.801 | - | 2.233 | 0.883 | 11.855 | 4.890 | 4.345 | 395.050 | 2.388 |
| P | - | - | 4.588 | 0.468 | 5.724 | 3.975 | 14.190 | 1.338 | 0.234 |
| Q | - | - | 2.737 | 0.975 | 18.477 | 5.171 | 4.792 | 379.579 | 2.730 |
| Product | Estimation of Weekly Intake, µg/Week/bw | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ag | Au | Co | Cs | Li | Mo | Se | Sr | V | |
| A | 3.377 | 0.010 | 0.082 | 0.016 | 0.325 | 0.223 | 0.160 | 0.027 | 0.047 |
| B | - | 0.002 | 0.051 | 0.011 | 0.355 | 0.280 | 0.068 | 0.027 | 0.025 |
| C | - | - | 0.059 | 0.013 | 0.139 | 0.161 | 0.063 | 0.026 | 0.034 |
| D | - | - | 0.042 | 0.012 | 0.241 | 0.168 | 0.056 | 0.025 | 0.032 |
| E | - | 0.002 | 0.034 | 0.005 | 0.078 | 0.094 | 0.053 | 4.347 | 0.044 |
| F | - | - | 0.038 | 0.010 | 0.168 | 0.069 | 0.059 | 5.509 | 0.060 |
| G | - | 0.000 | 0.052 | 0.013 | 0.204 | 0.189 | 0.072 | 0.024 | 0.050 |
| H | - | - | 0.039 | 0.012 | 0.226 | 0.228 | 0.248 | 0.023 | 0.031 |
| I | - | - | 0.036 | 0.011 | 0.290 | 0.070 | 0.077 | 5.323 | 0.032 |
| J | - | 0.001 | 0.046 | 0.014 | 0.259 | 0.255 | 0.293 | 0.022 | 0.033 |
| K | - | - | 0.057 | 0.013 | 0.370 | 0.078 | 0.080 | 0.022 | 0.048 |
| L | - | - | 0.062 | 0.017 | 0.452 | 0.063 | 0.096 | 0.021 | 0.027 |
| M | - | - | 0.042 | 0.010 | 0.826 | 0.217 | 0.122 | 0.021 | 0.024 |
| N | - | - | 0.068 | 0.011 | 0.437 | 0.213 | 0.124 | 0.020 | 0.045 |
| O | 0.154 | - | 0.032 | 0.013 | 0.169 | 0.070 | 0.062 | 5.644 | 0.034 |
| P | - | - | 0.066 | 0.007 | 0.082 | 0.057 | 0.203 | 0.019 | 0.003 |
| Q | - | - | 0.039 | 0.014 | 0.264 | 0.074 | 0.068 | 5.423 | 0.039 |
| Product | The Ratios (%) of the Obtained Values of Weekly Intake (µg/kg bw/week) to the PTWI for Each Element |
|---|---|
| A | 0.24 |
| B | 0.10 |
| C | 0.10 |
| D | 0.08 |
| E | 0.08 |
| F | 0.09 |
| G | 0.11 |
| H | 0.38 |
| I | 0.12 |
| J | 0.44 |
| K | 0.12 |
| L | 0.14 |
| M | 0.19 |
| N | 0.19 |
| O | 0.09 |
| P | 0.31 |
| Q | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milan, J.; Frydrych, A.; Noga, M.; Kondratowicz-Pietruszka, E.; Krośniak, M.; Jurowski, K. The Control of Novel and Traditional Elemental Impurities: Ag, Au, Co, Cs, Li, Mo, Se, Sr, and V in Mint Tea Infusions (Peppermint, Mentha piperita L.) Available in Poland: A Health Risk Assessment. Int. J. Environ. Res. Public Health 2022, 19, 16564. https://doi.org/10.3390/ijerph192416564
Milan J, Frydrych A, Noga M, Kondratowicz-Pietruszka E, Krośniak M, Jurowski K. The Control of Novel and Traditional Elemental Impurities: Ag, Au, Co, Cs, Li, Mo, Se, Sr, and V in Mint Tea Infusions (Peppermint, Mentha piperita L.) Available in Poland: A Health Risk Assessment. International Journal of Environmental Research and Public Health. 2022; 19(24):16564. https://doi.org/10.3390/ijerph192416564
Chicago/Turabian StyleMilan, Justyna, Adrian Frydrych, Maciej Noga, Elżbieta Kondratowicz-Pietruszka, Mirosław Krośniak, and Kamil Jurowski. 2022. "The Control of Novel and Traditional Elemental Impurities: Ag, Au, Co, Cs, Li, Mo, Se, Sr, and V in Mint Tea Infusions (Peppermint, Mentha piperita L.) Available in Poland: A Health Risk Assessment" International Journal of Environmental Research and Public Health 19, no. 24: 16564. https://doi.org/10.3390/ijerph192416564
APA StyleMilan, J., Frydrych, A., Noga, M., Kondratowicz-Pietruszka, E., Krośniak, M., & Jurowski, K. (2022). The Control of Novel and Traditional Elemental Impurities: Ag, Au, Co, Cs, Li, Mo, Se, Sr, and V in Mint Tea Infusions (Peppermint, Mentha piperita L.) Available in Poland: A Health Risk Assessment. International Journal of Environmental Research and Public Health, 19(24), 16564. https://doi.org/10.3390/ijerph192416564







