COVID-19 Not Hypertension or Diabetes Increases the Risk of Preeclampsia among a High-Risk Population
Abstract
:1. Introduction
2. Materials and Methods
Statistical Methods
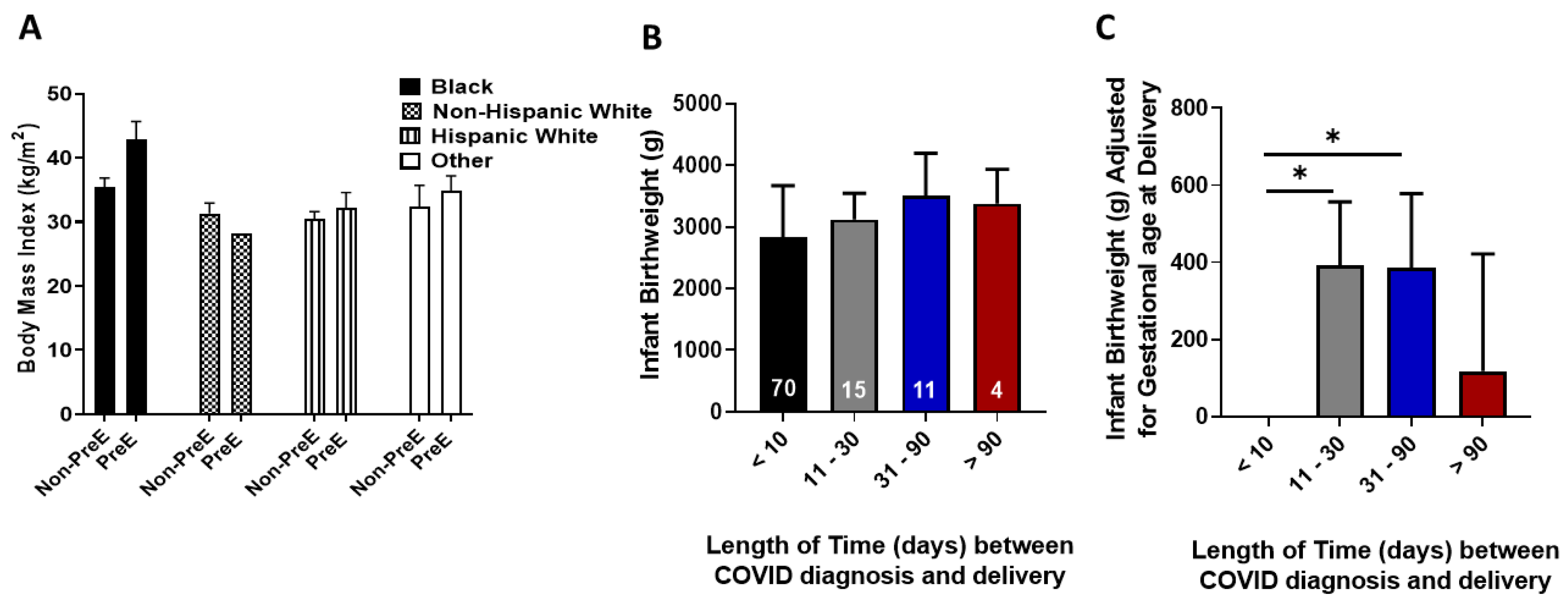
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Martino, D.; Chiaffarino, F.; Patane, L.; Prefumo, F.; Vergani, P.; Ornaghi, S.; Savasi, V.; Spinillo, A.; Cromi, A.; D’Ambrosi, F.; et al. Assessing risk factors for severe forms of COVID-19 in a pregnant population: A clinical series from Lombardy, Italy. Int. J. Gynaecol. Obstet. 2021, 152, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.; Hill, J.; Reddy, A.; Schuster, M.; Patrick, H.; Rosen, T.; Sauer, M.; Boyle, C.; Anathy, C. Epidemiology of coronavirus disease 2019 in pregnancy: Risk factors and associations with adverse maternal and neonatal outcomes. Am. J. Obstet. Gynecol. 2021, 224, 389.e1–389.e9. [Google Scholar] [CrossRef] [PubMed]
- Ellington, S.; Strid, P.; Tong, V.; Woodworth, K.; Galang, R.; Zambrano, L.; Nahabedian, J.; Anderson, K.; Gilboa, S. Characteristics of Women of Reproductive Age with Laboratory-confirmed SARS-CoV-2 Infection by Pregnancy Status - United States, January 22–June 7, 2020. MMWR Morb. Mortal. Weekly Rep. 2020, 69, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Litman, E.; Yin, Y.; Nelson, S.; Capbarat, E.; Kerchner, D.; Ahmadzia, H. Adverse Perinatal outcomes in a Large US Birth Cohort during the COVID-19 Pandemic: Adverse Perinatal Outcomes during COVID-19. Am. J. Obstet. Gynecol. 2022, 4, 10057. [Google Scholar]
- Wang, X.; Chen, X.; Zhang, K. Maternal infection with COVID-19 and increased risk of adverse pregnancy outcomes: A meta-analysis. J. Matern. Fetal Neonatal Med. 2022, 35, 9368–9375. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.; Gouyon, J.B.; Cottenet, J.; Bechraoui-Quantin, S.; Rozenberg, P.; Mariet, A.S.; Quantin, C. Impact of SARS-Cov-2 infection on risk of prematurity, birthweight and obstetrical complications: A multivariate analysis from a nationwide, population-based retrospective cohort study. Bjog 2022, 129, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ding, M.; Dong, X.; Zhang, J.; Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Chappell, L.; Cluver, C.; Kingdom, J.; Tong, S. Pre-eclampsia. Lancet 2021, 398, 341–354. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. COVID-19 Treatment Guidelines; Anti-SARS-CoV-2 Antibody Products. 2022. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 21 December 2020).
- World Health Organization. Clinical management of COVID-19: Interim Guidance. 2020. Available online: https://apps.who.int/iris/handle/10665/332196 (accessed on 27 May 2020).
- Khoury, R.; Bernstein, P.; Debolt, C.; Stone, J.; Sutton, D.; Simpson, L.; Limaye, M.; Roman, A.; Fazzari, M.; Penfield, C.; et al. Characteristics and outcomes of 241 births to women with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Infection at Five New York City Medical Centers. Obstet. Gynecol. 2020, 136, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Abedzadeh-Kalahroudi, M.; Sehat, M.; Vahedpour, Z.; Talebian, P. Maternal and neonatal outcomes of pregnant pratients with COVID-19: A prospective cohort study. Int. J. Gynaecol. Obstet. 2021, 153, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Khoury, R.; Fazzari, M.; Lambert, C.; DeBolt, C.; Stone, J.; Bianco, A.; Nathan, L.; Dolan, S.; Bernstein, P. Characteristics and outcomes of pregnant women with and without Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in New York City: A matched cohort study. Am. J. Perinatol. 2022, 39, 1261–1268. [Google Scholar] [PubMed]
- Vimercati, A.; De Nola, R.; Trerotoli, P.; Metta, M.; Cazzato, G.; Resta, L.; Malvasi, A.; Lepera, A.; Ricci, I.; Capozza, M.; et al. COVID-19 Infection in Pregnancy: Obstetrical Risk Factors and Neontal Outcomes - A Monocentric, Single-Cohort Study. Vaccines 2022, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, N.M.; Rommel, A.S.; de Witte, L.; Dolan, S.M.; Lieb, W.; Ibroci, E.; Ohrn, S.; Lynch, J.; Capuano, C.; Stadlbauer, D.; et al. SARS-CoV-2 during pregnancy and associated outcomes: Results from an ongoing prospective cohort. Paediatr. Perinat. Epidemiol. 2022, 36, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, E.; Moreno, W.; Zofkie, A.; MacDonald, L.; McIntire, D.; Collins, R.; Spong, C. Pregnancy Outcomes among women with and without severe acute respiratory syndrome coronavirus 2 Infection. JAMA Netw Open 2020, 3, e2029256. [Google Scholar] [CrossRef] [PubMed]
- Álvarez Bartolomé, A.; Abdallah Kassab, N.A.; Cruz Melguizo, S.; de la Cruz Conty, M.L.; Forcen Acebal, L.; Abascal Saiz, A.; Pintado Recarte, P.; Martinez Varea, A.; Cerrillos Gonzalez, L.; García Fernández, J.; et al. Critical Care in SARS-CoV-2 Infected Pregnant Women: A Prospective Multicenter Study. Biomedicines 2022, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Yanes-Lane, M.; Winters, N.; Fregonese, F.; Bastos, M.; Perlman-Arrow, S.; Campbell, J.R.; Menzies, D. Proportion of asymptomatic infection among COVID-19 positive persons and their transmission potential: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0241536. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.S.A.; Hamid, L.R.; Ali, A.; Salam, R.A.; Zuberi, N.; Lassi, Z.S.; Das, J.K. Differences in pregnancy and perinatal outcomes among symptomatic versus asymptomatic COVID-19-infected pregnant women: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2021, 21, 801. [Google Scholar] [CrossRef] [PubMed]
| Asymptomatic (n = 50) | Mild Illness (n = 28) | Moderate Illness (n = 7) | Severe Illness (n = 10) | Critical Illness (n = 3) | p Value | |
|---|---|---|---|---|---|---|
| BMI (kg/m2) | 32.25 ± 7.7 | 36.35 ± 10.7 | 36.77 ± 4.5 | 34.46 ± 8 | 47.33 ± 10.5 | 0.03 |
| Race/Ethnicity (n) Black White Hispanic Other | 22 4 24 0 | 16 2 9 1 | 4 0 2 1 | 7 0 2 1 | 2 0 0 1 | 0.12 |
| HTN/Diabetes (n) No Yes | 34 16 | 14 14 | 3 4 | 6 4 | 1 2 | 0.35 |
| Preeclampsia (n) No Yes | 44 6 | 22 6 | 3 4 | 7 5 | 1 2 | 0.009 |
| Preterm delivery (n) No Yes | 40 6 | 23 6 | 6 4 | 3 5 | 1 2 | 0.006 |
| Trimester at DX (n) Second Third | 4 46 | 11 17 | 0 7 | 2 8 | 0 3 | 0.009 |
| Gest. age Deliv. (wks) | 37.53 ± 3 | 37.78 ± 1.8 | 38.03 ± 1.2 | 33.75 ± 4.9 | 35.1 ± 1 | 0.02 |
| Preg. Complications (n) None PPROM PP Hemor. Shoulder dystocia AKI Other Combination | 40 2 3 2 0 1 2 | 19 0 5 0 0 3 1 | 5 0 0 0 0 2 0 | 7 0 1 0 1 0 1 | 3 0 0 0 0 0 0 | 0.35 |
| Fetal weight (grams) | 3037 ± 753 | 3180 ± 599 | 3004 ± 516 | 1992 ± 1153 | 2833 ± 504 | 0.04 |
| Fetal Death (n) No Yes | 46 4 | 27 1 | 7 0 | 9 1 | 2 1 | 0.09 |
| NICU Admission (n) No Yes | 28 21 | 17 10 | 4 3 | 2 7 | 1 2 | 0.13 |
| NICU LOS (days) | 11.85 ± 20.9 | 7.36 ± 8.5 | 3 ± 0.0 | 11.86 ± 7.9 | 6.5 ± 3.5 | 0.14 |
| Case | Control | p Value | |
|---|---|---|---|
| Maternal Age (yr) | 26.65 ± 6.4 | 26.81 ± 6.6 | 0.86 |
| Maternal Race/Ethnicity (n) Black White Hispanic Other | 52 6 37 5 | 54 6 37 5 | 0.94 |
| BMI (kg/m2) | 34.25 ± 9.2 | 34.33 ± 8.9 | 0.95 |
| Chronic HTN (n) No Yes | 76 24 | 73 27 | 0.63 |
| Diabetes (n) No Yes | 83 17 | 82 18 | 0.85 |
| Gest Age Deliv (weeks) | 36.82 ± 3.7 | 37.14 ± 3.1 | 0.51 |
| Preterm Deliv (weeks) No Yes | 75 25 | 69 31 | 0.43 |
| Mode of delivery Cesarean Section Vaginal | 44 56 | 40 60 | 0.57 |
| Preeclampsia No Yes | 76 24 | 74 26 | 0.74 |
| Pregnancy Complications (n) None PPROM PP Hemor. Shoulder dystocia AKI Other Combination | 76 2 9 2 1 6 4 | 74 3 2 3 1 10 7 | 0.31 |
| Birthweight (g) | 2965 ± 797 | 2875 ± 837 | 0.44 |
| Fetal Death No Yes | 93 7 | 98 2 | 0.17 |
| NICU admission No Yes | 52 44 | 79 18 | <.0001 |
| NICU LOS (days) | 10.2 ± 15.3 | 30.7 ± 26.8 | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morris, R.; Moustafa, A.S.Z.; Kassahun-Yimer, W.; Novotny, S.; Billsby, B.; Abbas, A.; Wallace, K. COVID-19 Not Hypertension or Diabetes Increases the Risk of Preeclampsia among a High-Risk Population. Int. J. Environ. Res. Public Health 2022, 19, 16631. https://doi.org/10.3390/ijerph192416631
Morris R, Moustafa ASZ, Kassahun-Yimer W, Novotny S, Billsby B, Abbas A, Wallace K. COVID-19 Not Hypertension or Diabetes Increases the Risk of Preeclampsia among a High-Risk Population. International Journal of Environmental Research and Public Health. 2022; 19(24):16631. https://doi.org/10.3390/ijerph192416631
Chicago/Turabian StyleMorris, Rachael, Ahmed S. Z. Moustafa, Wondwosen Kassahun-Yimer, Sarah Novotny, Brittney Billsby, Amira Abbas, and Kedra Wallace. 2022. "COVID-19 Not Hypertension or Diabetes Increases the Risk of Preeclampsia among a High-Risk Population" International Journal of Environmental Research and Public Health 19, no. 24: 16631. https://doi.org/10.3390/ijerph192416631
APA StyleMorris, R., Moustafa, A. S. Z., Kassahun-Yimer, W., Novotny, S., Billsby, B., Abbas, A., & Wallace, K. (2022). COVID-19 Not Hypertension or Diabetes Increases the Risk of Preeclampsia among a High-Risk Population. International Journal of Environmental Research and Public Health, 19(24), 16631. https://doi.org/10.3390/ijerph192416631







