Primary Clear Cell Adenocarcinoma of the Uterine Cervix in a 14-Year-Old Virgin Girl: Case Report
Abstract
1. Introduction
2. Case Presentation
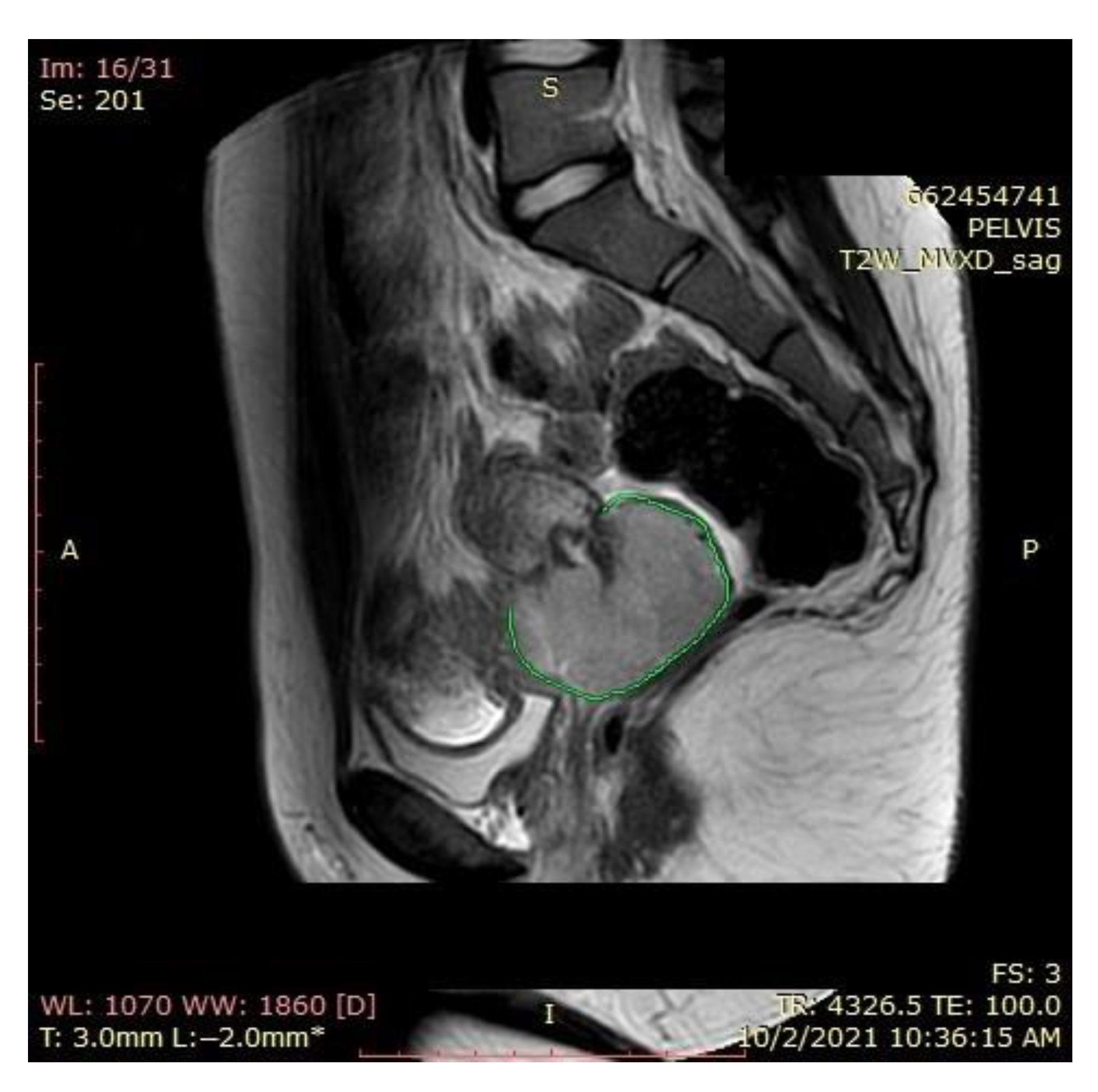
2.1. Magnetic Resonance Imaging (MRI) Findings
2.2. Computer Tomography (CT) Findings
2.3. Supplementary Investigations
2.4. Histopathology Findings
2.5. Case Management
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- McNall, R.Y.; Nowicki, P.D.; Miller, B.; Billups, C.A.; Liu, T.; Daw, N.C. Adenocarcinoma of the cervix and vagina in pediatric patients. Pediatr. Blood Cancer 2004, 43, 289–294. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Dainty, L.A.; Rose, G.S.; Krivak, T.; Mchale, M.T.; Olsen, C.H.; Elkas, J.C. Gynecologic malignancies in women aged less than 25 years. Obstet. Gynecol. 2005, 105, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.C.; Santos, D.Z.; Campos, C.S.; Vale, D.B.; Bragança, J.F.; Zeferino, L.C. Cervical cancer in women under 25 years of age and outside the screening age: Diagnosis profile and long-term outcomes. Int. J. Gynaecol. Obstet. 2021, 154, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Salib, M.Y.; Russell, J.H.B.; Stewart, V.R.; Sudderuddin, S.A.; Barwick, T.D.; Rockall, A.G.; Bharwani, N. 2018 FIGO Staging Classification for Cervical Cancer: Added Benefits of Imaging. Radiographics 2020, 40, 1807–1822. [Google Scholar] [CrossRef] [PubMed]
- Herbst, A.L.; Ulfelder, H.; Poskanzer, D.C. Adenocarcinoma of the vagina: Association of maternal stilbestrol therapy with tumor appearance in young women. N. Engl. J. Med. 1971, 284, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Herbst, A.L.; Anderson, D. Clear cell adenocarcinoma of the vagina and cervix secondary to intrauterine exposure to diethylstilbestrol. Semin. Surg. Oncol. 1990, 6, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Hanselaar, A.; Van Loosbroek, M.; Schuurbiers, O.; Helmerhorst, T.; Bulten, J.; Bernhelm, J. Clear cell adenocarcinoma of the vagina and cervix. An update of the Central Netherlands Registry showing twin age incidence peaks. Cancer 1997, 79, 2229–2236. [Google Scholar] [CrossRef]
- Seki, H.; Takada, T.; Sodemoto, T.; Hoshino, H.; Saitoh, K.; Uekusa, T. A young woman with clear cell adenocarcinoma of the uterine cervix. Int. J. Clin. Oncol. 2003, 8, 399–404. [Google Scholar] [CrossRef]
- Thomas, M.B.; Wright, J.D.; Leiser, A.L.; Chi, D.C.; Mutch, D.G.; Podratz, K.C.; Dowdy, S.C. Clear cell carcinoma of the cervix: A multi-institutional review in the post-DES era. Gynecol. Oncol. 2008, 109, 335–339. [Google Scholar] [CrossRef]
- Arora, A.; Rastogi, A.; Neyaz, A.; Husain, N. Clear cell adenocarcinoma of cervix in 1-year-old girl without in utero exposure to diethylstilbestrol: An uncommon tumour at an uncommon age and site. BMJ Case Rep. 2017, 2017, bcr2016218730. [Google Scholar] [CrossRef] [PubMed]
- Noller, K.L.; Decker, D.G.; Dockerty, M.B.; Lanier, A.P.; Smith, R.A.; Symmonds, R.E. Mesonephric (clear cell) carcinoma of the vagina and cervix. A retrospective analysis. Obstet. Gynecol. 1974, 43, 640–644. [Google Scholar]
- Wesolowski, J.A.; Adelson, M.D. Therapeutic considerations in an 8-year-old girl with clear cell adenocarcinoma of the cervix. J. Gynecol. Surg. 1997, 13, 31–34. [Google Scholar] [CrossRef]
- Ding, D.; Chang, F.; Yu, M. Huge clear cell carcinoma of the cervix in teenager not associated with diethylstilbestrol: A brief case report. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 117, 115–116. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Su, W.; Levine, D.A.; Boyd, J.; Sonoda, Y.; Laquaglia, M.P. Pediatric radical abdominal trachelectomy for cervical clear cell carcinoma: A novel surgical approach. Gynecol. Oncol. 2005, 97, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, W.A.; Barron-Rodriguez, L.P.; McKee, M.; Rivkees, S.; Reyes-Mugica, M. Clear cell adenocarcinoma of the cervix in a child without in utero exposure to diethylstilbestrol: A case report and review of the literature. Pediatr. Dev. Pathol. 2005, 8, 690–695. [Google Scholar] [CrossRef]
- Chan, K.K.L.; Ip, P.; Kwong, P.; Tam, K.F.; Ngan, H.Y.S. A combination of chemoirradiation and chemotherapy for treatment of advanced clear cell adenocarcinoma of the cervix. Int. J. Gynecol. Cancer 2008, 18, 559–563. [Google Scholar] [CrossRef]
- Yabushita, H.; Kanyama, K.; Sekiya, R.; Noguchi, M.; Wakatsuki, A. Clear-cell adenocarcinoma of the uterine cervix in a 17-year-old adolescent. Int. J. Clin. Oncol. 2008, 13, 552–554. [Google Scholar] [CrossRef]
- Lester, F.C.; Farmer, D.L.; Rabban, J.T.; Chen, L.M. Radical trachelectomy for clear cell carcinoma of the cervix in a 6-year old: A case report, review, and description of the surgical technique. J. Pediatr. Surg. 2010, 45, E1–E5. [Google Scholar] [CrossRef]
- Singh, P.; Nicklin, J.; Hassall, T. Neoadjuvant chemotherapy followed by radical vaginal trachelectomy and adjuvant chemotherapy for clear cell cancer of the cervix: A feasible approach and review. Int. J. Gynecol. Cancer 2011, 21, 137–140. [Google Scholar] [CrossRef]
- Romero-Duran, E.; Chavez-Bravo, N.C.; Garcia-Rodriguez, A.S. Clear cells cervical adenocarcinoma in a girl no exposed to dietiletilbestrol. Rev. Med. Inst. Mex. Seguro Soc. 2012, 50, 549–552. [Google Scholar] [PubMed]
- Ansari, D.O.; Horowitz, I.R.; Katzenstein, H.M.; Durham, M.M.; Esiashvili, N. Successful treatment of an adolescent with locally advanced cervicovaginal clear cell adenocarcinoma using definitive chemotherapy and radiotherapy. J. Pediatr. Hematol. Oncol. 2012, 34, e174–e176. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Kim, J.E.; Kim, H.S.; Choi, H.Y. Clear cell adenocarcinoma of the uterine cervix in a 15-year-old girl: A case report. Korean J. Radiol. 2013, 69, 321–325. [Google Scholar] [CrossRef]
- Jiang, X.; Jin, Y.; Li, Y.; Huang, H.F.; Wu, M.; Shen, K.; Pan, L.Y. Clear cell carcinoma of the uterine cervix: Clinical characteristics and feasibility of fertility-preserving treatment. Onco Targets Ther. 2014, 7, 111–116. [Google Scholar] [CrossRef]
- Ronneberg, E.T.; Lidang, M.; Palle, C. Clear cell adenocarcinoma of the uterine cervix in a 17-year-old girl. Ugeskr. Laeger 2014, 176, V04140255. [Google Scholar]
- Baykara, M.; Benekli, M.; Erdem, O.; Taskiran, C.; Demirci, U.; Vargol, E.; Gunaydin, Y.; Coskun, U.; Ozet, A.; Buyukberber, S. Clear cell adenocarcinoma of the uterine cervix: A case report and review of the literature. J. Pediatr. Hematol. Oncol. 2014, 36, e131–e133. [Google Scholar] [CrossRef]
- Andi Asri, A.A.; Lim, B.K.; Lim, Y.K.; Latiff, L.A. Clear cell adenocarcinoma of the cervix in a ten-year-old girl without prenatal diethylstilbestrol exposure. Singapore Med. J. 2016, 57, 470. [Google Scholar] [CrossRef]
- Singh, D.; Kamal, L.; Rai, P.; Biswas, R.; Shukla, S. Clear Cell Adenocarcinoma of Uterine Cervix in an Adolescent: An uncommon tumor at an uncommon site and Age. AWCH 2016, 2, c15–c19. [Google Scholar]
- Tantitamit, T.; Hamontri, S.; Rangsiratanakul, L. Clear cell adenocarcinoma of the cervix in second generation young women who are without maternal exposure to diethylstilbestrol: A case report. Gynecol. Oncol. Rep. 2017, 20, 34–36. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, C.; Hou, W.; Liou, Y.; Chen, Y.; Xie, Y.; Zhang, D.; Ji, P.; Chen, R.; Jiang, G.; et al. Fertility-preserving local excision under a hysteroscope with combined chemotherapy in a 6-year-old child with clear cell adenocarcinoma of the cervix: A case report and review of the literature. Medicine 2020, 99, e18646. [Google Scholar] [CrossRef]
- Levinson, A.; Lee, A.G.; Martell, H.J.; Breese, M.R.; Zaloudek, C.; Van Ziffle, J.; Laguna, B.; Leung, S.G.; Chen, M.D.; Chen, L.M.; et al. Complete Response to PD-1 Inhibition in an Adolescent with Relapsed Clear Cell Adenocarcinoma of the Cervix Predicted by Neoepitope Burden and APOBEC Signature. JCO Precis. Oncol. 2020, 4, PO.20.00132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Zhang, Q.Y.; Wang, T.; Wang, J.; Shi, F.; Su, J.; Gong, T.T. Successful treatment of a 19-year-old patient with locally advanced clear cell adenocarcinoma of the uterine cervix using recombinant human adenovirus type 5 (Oncorine) combined with chemoradiotherapy: A case report. Ann. Transl. Med. 2021, 9, 1747. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, R.Y.; Wei, B.B.; Liu, J.; Hou, M.M.; Liu, H. Clear cell carcinoma of cervix in a 12-year-old girl: A case report. Sichuan Da Xue Xue Bao Yi Xue Ban 2021, 52, 534–538. [Google Scholar] [CrossRef]
- Matias-Guiu, X.; Lerma, E.; Prat, J. Clear cell tumors of the female genital tract. Semin. Diagn. Pathol. 1997, 14, 233–239. [Google Scholar]
- Gaspari, L.; Paris, F.; Cassel-Knipping, N.; Villeret, J.; Verschuur, A.; Soyer-Gobillard, M.O.; Carcopino-Tusoli, X.; Hamamah, S.; Kalfa, N.; Sultan, C. Diethylstilbestrol exposure during pregnancy with primary clear cell carcinoma of the cervix in an 8-year-old granddaughter: A multigenerational effect of endocrine disruptors? Hum. Reprod. 2021, 36, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Melnick, S.; Cole, P.; Anderson, D.; Herbst, A. Rates and risks of diethylstilbestrol-related clear-cell adenocarcinoma of the vagina and cervix. An update. N. Engl. J. Med. 1987, 316, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.; Takahashi, H.; Waggoner, S.E.; Jones, L.A.; Hajek, R.A.; Wharton, J.T.; Liu, F.S.; Fujino, T.; Barrett, J.C.; McLachlan, J.A. Molecular genetic analysis of clear cell adenocarcinomas of the vagina and cervix associated and unassociated with diethylstilbestrol exposure in utero. Cancer 1996, 77, 507–513. [Google Scholar] [CrossRef]
- Fujiwara, H.; Mitchell, M.F.; Arseneau, J.; Hale, R.J.; Wright, T.C., Jr. Clear cell adenosquamous carcinoma of the cervix. An aggressive tumor associated with human papillomavirus-18. Cancer 1995, 9, 1591–1600. [Google Scholar] [CrossRef]
- Waggoner, S.E.; Anderson, S.M.; Luce, M.C.; Takahashi, H.; Boyd, J. p53 protein expression and gene analysis in clear cell adenocarcinoma of the vagina and cervix. Gynecol. Oncol. 1996, 60, 339–344. [Google Scholar] [CrossRef]
- Reich, O.; Tamussino, K.; Lahousen, M.; Pickel, H.; Haas, J.; Winter, R. Clear cell carcinoma of the uterine cervix: Pathology and prognosis in surgically treated stage IB IIB disease in women not exposed in utero to diethylstilbestrol. Gynecol. Oncol. 2000, 76, 331–335. [Google Scholar] [CrossRef]
- Green, J.; Kirwan, J.; Tierney, J.; Vale, C.; Symonds, P.; Fresco, L.; Williams, C.; Collingwood, M. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst. Rev. 2005, 3, CD002225. [Google Scholar] [CrossRef] [PubMed]
- Green, D.M.; Sklar, C.A.; Boice, J.D., Jr.; Mulvihill, J.J.; Whitton, J.A.; Stovall, M.; Yasui, Y. Ovarian failure and reproductive outcomes after childhood cancer treatment: Results from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2009, 27, 2374–2381. [Google Scholar] [CrossRef] [PubMed]
- Santaballa, A.; Márquez-Vega, C.; Rodríguez-Lescure, Á.; Rovirosa, Á.; Vázquez, L.; Zeberio-Etxetxipia, I.; Andrés, M.; Bassas, L.; Ceballos-Garcia, E.; Domingo, J.; et al. Multidisciplinary consensus on the criteria for fertility preservation in cancer patients. Clin. Transl. Oncol. 2022, 24, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.; Lee, S.J.; Partridge, A.H.; Runowicz, C.D. Preserving fertility after cancer. CA Cancer J. Clin. 2005, 55, 211–228. [Google Scholar] [CrossRef] [PubMed]













| Report (Author and Years of Publication) | No. of Cases | Age (Years) | Stage of FIGO |
|---|---|---|---|
| Noller et al. (1974) [12] | 4 | 7 10 13 14 | IA IIB IB IIA |
| Wesolowski et al. (1997) [13] | 1 | 8 | IB |
| Seki et al. (2003) [9] | 1 | 18 | IB2 |
| Ding et al. (2004) [14] | 1 | 19 | IB2 |
| Abu-Rustum et al. (2005) [15] | 2 | 6 8 | IB1 IB1 |
| Ahrens et al. (2005) [16] | 1 | 6 | IB1 |
| Chan et al. (2008) [17] | 1 | 14 | IIIA |
| Yabushita et al. (2008) [18] | 1 | 17 | IB1 |
| Lester et al. (2010) [19] | 1 | 6 | IB1 |
| Singh et al. (2011) [20] | 1 | 13 | IB1 |
| Romero-Duran et al. (2012) [21] | 1 | 11 | unknown |
| Ansari et al. (2012) [22] | 1 | 14 | IIIA |
| Choi et al. (2013) [23] | 1 | 15 | IIA |
| Jiang et al. (2014) [24] | 2 | 19 20 | IIA2 IB1 |
| Ronneberg et al. (2014) [25] | 1 | 17 | IB1 |
| Baykara et al. (2014) [26] | 2 | 14 16 | IB2 IB1 |
| Andi Asri et al. (2016) [27] | 1 | 10 | IB2 |
| Singh et al. (2016) [28] | 1 | 14 | primary |
| Arora et al. (2017) [11] | 1 | 1 | I |
| Tantitamit et al. (2017) [29] | 1 | 19 | IB1 |
| Su et al. (2020) [30] | 1 | 6 | IIA1 |
| Levinson et al. (2020) [31] | 1 | 15 | relapsed |
| Zhang et al. (2021) [32] | 1 | 19 | IIIB |
| Liu et al. (2021) [33] | 1 | 12 | IIIC1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bujor, I.E.; Lozneanu, L.; Ursache, A.; Cristofor, A.; Scurtu, A.-M.; Plamadeala, P.; Gireada, R.; Mandici, C.E.; Găină, M.A.; Matasariu, D.R. Primary Clear Cell Adenocarcinoma of the Uterine Cervix in a 14-Year-Old Virgin Girl: Case Report. Int. J. Environ. Res. Public Health 2022, 19, 16652. https://doi.org/10.3390/ijerph192416652
Bujor IE, Lozneanu L, Ursache A, Cristofor A, Scurtu A-M, Plamadeala P, Gireada R, Mandici CE, Găină MA, Matasariu DR. Primary Clear Cell Adenocarcinoma of the Uterine Cervix in a 14-Year-Old Virgin Girl: Case Report. International Journal of Environmental Research and Public Health. 2022; 19(24):16652. https://doi.org/10.3390/ijerph192416652
Chicago/Turabian StyleBujor, Iuliana Elena, Ludmila Lozneanu, Alexandra Ursache, Alexandra Cristofor, Ana-Maria Scurtu, Petru Plamadeala, Roxana Gireada, Cristina Elena Mandici, Marcel Alexandru Găină, and Daniela Roxana Matasariu. 2022. "Primary Clear Cell Adenocarcinoma of the Uterine Cervix in a 14-Year-Old Virgin Girl: Case Report" International Journal of Environmental Research and Public Health 19, no. 24: 16652. https://doi.org/10.3390/ijerph192416652
APA StyleBujor, I. E., Lozneanu, L., Ursache, A., Cristofor, A., Scurtu, A.-M., Plamadeala, P., Gireada, R., Mandici, C. E., Găină, M. A., & Matasariu, D. R. (2022). Primary Clear Cell Adenocarcinoma of the Uterine Cervix in a 14-Year-Old Virgin Girl: Case Report. International Journal of Environmental Research and Public Health, 19(24), 16652. https://doi.org/10.3390/ijerph192416652







