Effects of Inspiratory Muscle Training on Muscle Oxygenation during Vascular Occlusion Testing in Trained Healthy Adult Males
Abstract
1. Introduction
2. Materials and Method
2.1. Sample Size
2.2. Subjects
2.3. Study Protocol
2.4. Measurements
2.4.1. Anthropometry and Autonomic Cardiac Parameters
2.4.2. Spirometry and Maximal Dynamic Inspiratory Strength
2.4.3. Physical Performance
2.4.4. Vascular Occlusion Test
2.5. Intervention
2.5.1. Concurrent Training
2.5.2. Inspiratory Muscle Training
2.6. Statistic
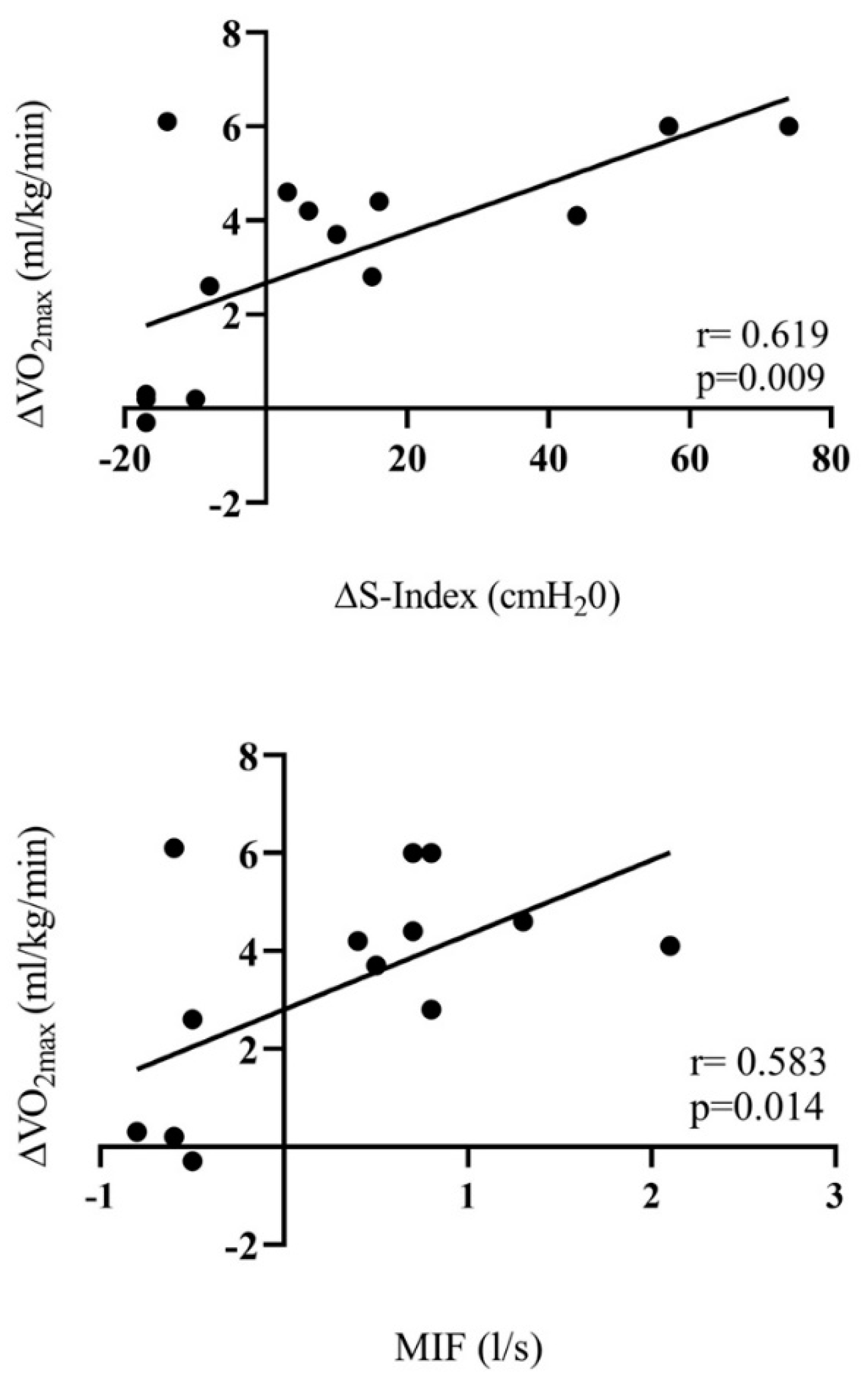
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shei, R.J. Recent Advancements in Our Understanding of the Ergogenic Effect of Respiratory Muscle Training in Healthy Humans: A Systematic Review. J. Strength Cond. Res. 2018, 32, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Bahls, M.; Ittermann, T.; Ewert, R.; Stubbe, B.; Völzke, H.; Friedrich, N.; Felix, S.B.; Dörr, M. Physical Activity and Cardiorespiratory Fitness—A Ten-Year Follow-Up. Scand. J. Med. Sci. Sport. 2021, 31, 742–751. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, R.M.; Rehder-Santos, P.; Minatel, V.; dos Santos, G.L.; Catai, A.M. Effects of Inspiratory Muscle Training on Cardiovascular Autonomic Control: A Systematic Review. Auton. Neurosci. 2017, 208, 29–35. [Google Scholar] [CrossRef]
- Craighead, D.H.; Heinbockel, T.C.; Freeberg, K.A.; Rossman, M.J.; Jackman, R.A.; Jankowski, L.R.; Hamilton, M.N.; Ziemba, B.P.; Reisz, J.A.; D’Alessandro, A.; et al. Time-Efficient Inspiratory Muscle Strength Training Lowers Blood Pressure and Improves Endothelial Function, NO Bioavailability, and Oxidative Stress in Midlife/Older Adults With Above-Normal Blood Pressure. J. Am. Heart Assoc. 2021, 10, e020980. [Google Scholar] [CrossRef] [PubMed]
- DeLucia, C.M.; De Asis, R.M.; Bailey, E.F. Daily Inspiratory Muscle Training Lowers Blood Pressure and Vascular Resistance in Healthy Men and Women. Exp. Physiol. 2018, 103, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Feriani, D.J.; Coelho-Júnior, H.J.; Scapini, K.B.; de Moraes, O.A.; Mostarda, C.; Ruberti, O.M.; Uchida, M.C.; Caperuto, É.C.; Irigoyen, M.C.; Rodrigues, B. Effects of Inspiratory Muscle Exercise in the Pulmonary Function, Autonomic Modulation, and Hemodynamic Variables in Older Women with Metabolic Syndrome. J. Exerc. Rehabil. 2017, 13, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Dennis, J.J.; Wiggins, C.C.; Smith, J.R.; Isautier, J.M.J.; Johnson, B.D.; Joyner, M.J.; Cross, T.J. Measurement of Muscle Blood Flow and O2 Uptake via Near-Infrared Spectroscopy Using a Novel Occlusion Protocol. Sci. Rep. 2021, 11, 918. [Google Scholar] [CrossRef]
- Jones, S.; Chiesa, S.T.; Chaturvedi, N.; Hughes, A.D. Recent Developments in Near-Infrared Spectroscopy (NIRS) for the Assessment of Local Skeletal Muscle Microvascular Function and Capacity to Utilise Oxygen. Artery Res. 2016, 16, 25–33. [Google Scholar] [CrossRef]
- Soares, R.N.; McLay, K.M.; George, M.A.; Murias, J.M. Differences in Oxidative Metabolism Modulation Induced by Ischemia/Reperfusion between Trained and Untrained Individuals Assessed by NIRS. Physiol. Rep. 2017, 5, e13384. [Google Scholar] [CrossRef]
- Jones, S.; Tillin, T.; Williams, S.; Rapala, A.; Chaturvedi, N.; Hughes, A.D. Skeletal Muscle Tissue Saturation Changes Measured Using Near Infrared Spectroscopy During Exercise Are Associated With Post-Occlusive Reactive Hyperaemia. Front. Physiol. 2022, 13, 1379. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of Spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- ATS/ERS. Statement on Respiratory Muscle Testing. Am. J. Respir. Crit. Care Med. 2002, 166, 518–624. [Google Scholar] [CrossRef]
- Larsen, G.E.; George, J.D.; Alexander, J.L.; Fellingham, G.W.; Aldana, S.G.; Parcell, A.C. Prediction of Maximum Oxygen Consumption from Walking, Jogging, or Running. Res. Q. Exerc. Sport 2002, 73, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Sanni, A.A.; McCully, K.K. Interpretation of Near-Infrared Spectroscopy (NIRS) Signals in Skeletal Muscle. J. Funct. Morphol. Kinesiol. 2019, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.E.; Erickson, M.L.; Brizendine, J.T.; Young, H.J.; McCully, K.K. Noninvasive Evaluation of Skeletal Muscle Mitochondrial Capacity with Near-Infrared Spectroscopy: Correcting for Blood Volume Changes. J. Appl. Physiol. 2012, 113, 175–183. [Google Scholar] [CrossRef]
- Rosenberry, R.; Nelson, M.D. Reactive Hyperemia: A Review of Methods, Mechanisms, and Considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R605–R618. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine Position Stand. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sport. Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences, 5th ed.; Mifflin, H., Ed.; Houghton Mifflin College Division: Boston, MA, USA, 2003. [Google Scholar]
- JAMOVI. The Jamovi Project. Jamovi, version 1.6; Computer Software: Sidney, Autralia, 2021. Available online: https://www.Jamovi.Org (accessed on 20 October 2022).
- Cipriano, G.F.B.; Cipriano, G.; Santos, F.V.; Chiappa, A.M.G.; Pires, L.; Cahalin, L.P.; Chiappa, G.R. Current Insights of Inspiratory Muscle Training on the Cardiovascular System: A Systematic Review with Meta-Analysis. Integr. Blood Press. Control 2019, 12, 1–11. [Google Scholar] [CrossRef]
- de Almeida, L.B.; Seixas, M.B.; Trevizan, P.F.; CamarotiLaterza, M.; da Silva, L.P.; Martinez, D.G. Los Efectos Del Entrenamiento Muscular Inspiratorio En El Control Autonómico: La Revisión Sistemática. Fisioter. Pesqui. 2018, 25, 345–351. [Google Scholar] [CrossRef]
- Task Force of The European Society of Cardiology; The North American Society of Pacing and Electrophysiology. Heart Rate Variability: Standards of Measurement, Physiological Interpretation, and Clinical Use. Eur. Heart J. 1996, 93, 354–381. [Google Scholar]
- Archiza, B.; Andaku, D.K.; Caruso, F.C.R.; Bonjorno, J.C.; de Oliveira, C.R.; Ricci, P.A.; do Amaral, A.C.; Mattiello, S.M.; Libardi, C.A.; Phillips, S.A.; et al. Effects of Inspiratory Muscle Training in Professional Women Football Players: A Randomized Sham-Controlled Trial. J. Sports Sci. 2018, 36, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Rehder-Santos, P.; Abreu, R.M.; de Signini, É.F.; da Silva, C.D.; Sakaguchi, C.A.; Dato, C.C.; Catai, A.M. Moderate- and High-Intensity Inspiratory Muscle Training Equally Improves Inspiratory Muscle Strength and Endurance-A Double-Blind Randomized Controlled Trial. Int. J. Sports Physiol. Perform. 2021, 16, 1111–1119. [Google Scholar] [CrossRef]
- Illi, S.K.; Held, U.; Frank, I.; Spengler, C.M. Effect of Respiratory Muscle Training on Exercise Performance in Healthy Individuals. Sport. Med. 2012, 42, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Witt, J.D.; Guenette, J.A.; Rupert, J.L.; Mckenzie, D.C.; Sheel, A.W. Inspiratory Muscle Training Attenuates the Human Respiratory Muscle Metaboreflex. J. Physiol. 2007, 584, 1019–1028. [Google Scholar] [CrossRef] [PubMed]

| Variables | IMTPG (n = 6) | IMTG (n = 8) | Student’s t-Test |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (years) | 25.8 ± 2.6 | 27.7 ± 2.2 | 0.163 |
| Weight (kg) | 75.6 ± 6.7 | 74.3 ± 5.9 | 0.707 |
| Height (m) | 1.71 ± 0.1 | 1.76 ± 0.1 | 0.102 |
| BMI (kg/m2) | 25.9 ± 1.3 | 24.0 ± 1.6 ** | 0.038 |
| ATT (mm) | 10.5 ± 1.9 | 9.1 ± 2.6 | 0.300 |
| Variables | IMTPG (n = 6) | IMTG (n = 8) | ANOVA Test | ||||
|---|---|---|---|---|---|---|---|
| Baseline | After | Δ | Baseline | After | Δ | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | p-Value | |||
| Cardiorespiratory fitness | |||||||
| HRrest (bpm) | 51.7 ± 5.2 | 52.7 ± 3.9 | 1.0 ± 2.8 | 54.4 ± 7.3 | 51.8 ± 5.0 | −2.6 ± 2.9 ** | 0.037 |
| SAPrest (mmHg) | 128.3 ± 9.6 | 128.8 ± 5.9 | 0.5 ± 6.2 | 124.3 ± 11.7 | 119.9 ± 8.6 | −4.4 ± 5.4 | 0.141 |
| DAPrest (mmHg) | 79.3 ± 7.1 | 77.0 ± 5.8 | −2.3 ± 3.5 | 69.9 ± 5.8 | 67.5 ± 4.9 | −2.8 ± 4.9 | 0.986 |
| 1.5-mile run(min) | 9.85 ± 0.4 | 9.57 ± 0.6 | −0.27 ± 0.4 | 9.84 ± 1.0 | 9.03 ± 0.9 * | −0.81 ± 0.2 ** | 0.006 |
| VO2max (mL/kg/min) | 52.6 ± 1.7 | 54.1 ± 3.3 | 1.51 ± 2.5 | 53.1 ± 5.2 | 57.5 ± 6.0 * | 4.48 ± 1.1 ** | 0.01 |
| Maximal dynamic inspiratory strength | |||||||
| S-index (cmH2O) | 150 ± 6.5 | 136 ± 6.7 | −13.83 ± 4.0 | 128 ± 22.9 | 156 ± 27.2 * | 28.23 ± 26.6 ** | 0.003 |
| MIF (L/s) | 8.13 ± 0.3 | 7.53 ± 0.3 * | −0.60 ± 0.1 | 7.41 ± 1.2 | 8.32 ± 1.3 * | 0.91 ± 0.6 ** | <0.001 |
| Spirometry | |||||||
| FVC (L) | 5.27 ± 0.5 | 5.15 ± 0.5 | −0.11 ± 0.1 | 5.20 ± 0.6 | 5.27 ± 0.5 | 0.07 ± 0.2 | 0.08 |
| FEV1 (L) | 4.42 ± 0.4 | 4.28 ± 0.5 | −0.15 ± 0.2 | 4.29 ± 0.6 | 4.44 ± 0.5 | 0.16 ± 0.5 | 0.214 |
| FEV1/FVC1 (%) | 83.8 ± 2.8 | 82.8 ± 2.2 | −1.00 ± 1.6 | 82.6 ± 7.6 | 84.1 ± 4.6 | 1.50 ± 8.7 | 0.506 |
| PEF (L/min) | 605 ± 84.2 | 583 ± 69.4 | −21.83 ± 41.1 | 591 ± 53.3 | 618 ± 63.8 | 26.38 ± 26.3 ** | 0.023 |
| FEF25–75% (L/s) | 4.52 ± 0.7 | 4.34 ± 0.7 | −0.18 ± 0.4 | 4.67 ± 0.8 | 4.70 ± 1.0 | 0.02 ± 0.5 | 0.415 |
| MVV (L/min) | 166 ± 16.3 | 160 ± 16.9 | −5.50 ± 5.2 | 161 ± 20.7 | 167 ± 18.3 | 5.75 ± 2.2 | 0.212 |
| Variables | IMTPG (n = 6) | IMTG (n = 8) | ANOVA Test | ||||
|---|---|---|---|---|---|---|---|
| Baseline | After | Δ | Baseline | After | Δ | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | p | |||
| TSIbaseline (%) | 70.9 ± 3.9 | 68.8 ± 1.9 | −2.13 ± 3.7 | 65.7 ± 3.9 | 68.4 ± 3.6 | 2.65 ± 3.4 | 0.028 |
| ΔTSIMB (s) | 9.37 ± 4.3 | 10.02 ± 3.4 | 0.83 ± 2.3 | 10.54 ± 2.9 | 7.18 ± 1.9 * | −3.38 ± 3.1 ** | 0.015 |
| ΔTSIMP (s) | 19.6 ± 4.8 | 23.1 ± 4.0 | 3.50 ± 6.4 | 20.3 ± 4.9 | 14.4 ± 2.0 * | −5.88 ± 3.7 ** | 0.004 |
| HHbAUC (a.u.) | 4872 ± 1502 | 4840 ± 1545 | −32.3 ± 259.3 | 5183 ± 1597 | 3847 ± 783 * | −1336.1 ± 1462.5 | 0.054 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yáñez-Sepúlveda, R.; Verdugo-Marchese, H.; Duclos-Bastías, D.; Tuesta, M.; Alvear-Ordenes, I. Effects of Inspiratory Muscle Training on Muscle Oxygenation during Vascular Occlusion Testing in Trained Healthy Adult Males. Int. J. Environ. Res. Public Health 2022, 19, 16766. https://doi.org/10.3390/ijerph192416766
Yáñez-Sepúlveda R, Verdugo-Marchese H, Duclos-Bastías D, Tuesta M, Alvear-Ordenes I. Effects of Inspiratory Muscle Training on Muscle Oxygenation during Vascular Occlusion Testing in Trained Healthy Adult Males. International Journal of Environmental Research and Public Health. 2022; 19(24):16766. https://doi.org/10.3390/ijerph192416766
Chicago/Turabian StyleYáñez-Sepúlveda, Rodrigo, Humberto Verdugo-Marchese, Daniel Duclos-Bastías, Marcelo Tuesta, and Ildefonso Alvear-Ordenes. 2022. "Effects of Inspiratory Muscle Training on Muscle Oxygenation during Vascular Occlusion Testing in Trained Healthy Adult Males" International Journal of Environmental Research and Public Health 19, no. 24: 16766. https://doi.org/10.3390/ijerph192416766
APA StyleYáñez-Sepúlveda, R., Verdugo-Marchese, H., Duclos-Bastías, D., Tuesta, M., & Alvear-Ordenes, I. (2022). Effects of Inspiratory Muscle Training on Muscle Oxygenation during Vascular Occlusion Testing in Trained Healthy Adult Males. International Journal of Environmental Research and Public Health, 19(24), 16766. https://doi.org/10.3390/ijerph192416766






