New Models for Estimating the Sorption of Sulfonamide and Tetracycline Antibiotics in Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physical and Chemical Properties of Antibiotics
2.2. Data Collection
2.3. Sorption Isotherms
2.4. Statistics and Modeling
2.5. Model Evaluation
3. Results
3.1. Distribution of Soil Properties
3.2. Distribution of Antibiotic Sorption
3.3. Correlations between Antibiotic Sorption and Soil Properties
3.4. Model Development and Validation
4. Discussion
4.1. Governing Factors and Mechanisms of Antibiotic Sorption
4.2. Model Performance
4.3. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.Y.; Ma, W.J.; An, B.Y.; Zhou, K.X.; Mi, K.; Huo, M.X.; Liu, H.Y.; Wang, H.Y.; Liu, Z.L.; Cheng, G.Y.; et al. Adsorption/desorption and degradation of doxycycline in three agricultural soils. Ecotox. Environ. Saf. 2021, 224, 112675. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.D.; Bruland, G.L.; Agrawal, S.G.; Vasudevan, D. Factors influencing the sorption of oxytetracycline to soils. Environ. Toxicol. Chem. 2010, 24, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Bartikova, H.; Podlipna, R.; Skalova, L. Veterinary drugs in the environment and their toxicity to plants. Chemosphere 2016, 144, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xu, L.; Rysz, M.; Wang, Y.; Zhang, H.; Alvarez, P.J. Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River Basin, China. Environ. Sci. Technol. 2011, 45, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Conde-Cid, M.; Ferreira-Coelho, G.; Fernández-Calviño, D.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Arias-Estévez, M.; Álvarez-Rodríguez, E. Single and simultaneous adsorption of three sulfonamides in agricultural soils: Effects of pH and organic matter content. Sci. Total Environ. 2020, 744, 140872. [Google Scholar] [CrossRef]
- Teixidó, M.; Granados, M.; Prat, M.D.; Beltran, J.L. Sorption of tetracyclines onto natural soils: Data analysis and prediction. Environ. Sci. Pollut. Res. 2012, 19, 3087–3095. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S.; Aust, M.O. Effects of pig slurry on the sorption of sulfonamide antibiotics in soil. Arch. Environ. Contam. Toxicol. 2004, 47, 31–39. [Google Scholar] [CrossRef]
- Tang, W.; Jing, F.Q.; Laurent, Z.; Liu, Y.Y.; Chen, J.W. High-temperature and freeze-thaw aged biochar impacts on sulfonamide sorption and mobility in soil. Chemosphere 2021, 276, 130106. [Google Scholar] [CrossRef]
- Hamscher, G.; Pawelzick, H.T.; Hoper, H.; Nau, H. Different behavior of tetracyclines and sulfonamides in sandy soils after repeated fertilization with liquid manure. Environ. Toxicol. Chem. 2005, 24, 861–868. [Google Scholar] [CrossRef]
- Kodešová, R.; Grabic, R.; Kočárek, M.; Klement, A.; Golovko, O.; Fér, M.; Jakšík, O. Pharmaceuticals’ sorptions relative to properties of thirteen different soils. Sci. Total Environ. 2015, 511, 435–443. [Google Scholar] [CrossRef]
- Sassman, S.; Lee, L. Sorption of three tetracyclines by several soils: Assessing the role of pH and cation exchange. Environ. Sci. Technol. 2005, 39, 7452–7459. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Sarmah, A.K.; Manley-Harris, M. Sorption of selected veterinary antibiotics onto dairy farming soils of contrasting nature. Sci. Total Environ. 2014, 472, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Conde-Cid, M.; Fernández-Calviño, D.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Arias-Estévez, M.; Álvarez-Rodríguez, E. Estimation of adsorption/desorption Freundlich’s affinity coefficients for oxytetracycline and chlortetracycline from soil properties: Experimental data and pedotransfer functions. Ecotox. Environ. Saf. 2020, 196, 110584. [Google Scholar] [CrossRef]
- Leal, R.M.P.; Alleoni, L.R.F.; Tornisielo, V.L.; Regitano, J.B. Sorption of fluoroquinolones and sulfonamides in 13 Brazilian soils. Chemosphere 2013, 92, 979–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franklin, A.M.; Williams, C.; Andrews, D.M.; Watson, J.E. Sorption and desorption behavior of four antibiotics at concentrations simulating wastewater reuse in agricultural and forested soils. Chemosphere 2022, 289, 133038. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, B.; Li, H.; Ma, L.Q. Effects of pH and ionic strength on sulfamethoxazole and ciprofloxacin transport in saturated porous media. J. Contam. Hydrol. 2011, 126, 29–36. [Google Scholar] [CrossRef]
- Chen, Y.X.; Hu, C.Y.; Deng, D.H.; Li, Y.G.; Luo, L. Factors affecting sorption behaviors of tetracycline to soils: Importance of soil organic carbon, pH and Cd contamination. Ecotox. Environ. Saf. 2020, 197, 110572. [Google Scholar] [CrossRef]
- Álvarez-Esmorís, C.; Rodríguez-López, L.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Fernández-Calviño, D.; Arias-Estévez, M. Influence of pH on the adsorption-desorption of doxycycline, enrofloxacin, and sulfamethoxypyridazine in soils with variable surface charge. Environ. Res. 2022, 214, 114071. [Google Scholar] [CrossRef]
- Pils, J.R.; Laird, D.A. Sorption of tetracycline and chlortetracycline on K– and Ca–saturated soil clays, humic substances, and clay-humic complexes. Environ. Sci. Technol. 2007, 41, 1928–1933. [Google Scholar] [CrossRef]
- Park, J.Y.; Huwe, B. Effect of pH and soil structure on transport of sulfonamide antibiotics in agricultural soils. Environ. Pollut. 2016, 213, 561–570. [Google Scholar] [CrossRef]
- Vasudevan, D.; Bruland, G.L.; Torrance, B.S.; Upchurch, V.G.; MacKay, A.A. pH-dependent ciprofloxacin sorption to soils: Interaction mechanisms and soil factors influencing sorption. Geoderma 2009, 151, 68–76. [Google Scholar] [CrossRef]
- Zhao, Z.; Nie, T.; Yang, Z.; Zhou, W. The role of soil components in the sorption of tetracycline and heavy metals in soils. RSC Adv. 2018, 8, 32178–32187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.L.; Huang, L.D.; Chung, R.S.; Fok, K.H.; Zhang, Y.S. Sorption and dissipation of tetracyclines in soils and compost. Pedosphere 2010, 20, 807–816. [Google Scholar] [CrossRef]
- Ter Laak, T.L.; Gebbink, W.A.; Tolls, J. Estimation of soil sorption coefficients of veterinary pharmaceuticals from soil properties. Environ. Toxicol. Chem. 2006, 25, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.J.; Wilkinson, J.L.; Boxall, A.B.A. Evaluation of existing models to estimate sorption coefficients for ionisable pharmaceuticals in soils and sludge. Toxics 2020, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Droge, S.T.J.; Goss, K.U. Development and evaluation of a new sorption model for organic cations in soil: Contributions from organic matter and clay minerals. Environ. Sci. Technol. 2013, 47, 14233–14241. [Google Scholar] [CrossRef]
- Franco, A.; Trapp, S. Estimation of the soil–water partition coefficient normalized to organic carbon for ionizable organic chemicals. Environ. Toxicol. Chem. 2008, 27, 1995–2004. [Google Scholar] [CrossRef]
- Barron, L.; Havel, J.; Purcell, M.; Szpak, M.; Kelleher, B.; Paull, B. Predicting sorption of pharmaceuticals and personal care products onto soil and digested sludge using artificial neural networks. Analyst 2009, 134, 663–670. [Google Scholar] [CrossRef]
- Li, X.; Song, Y.; Jia, M.; Wang, F.; Bian, Y.; Jiang, X. Sorption and desorption characteristics of sulfamethazine in three different soils before and after removal of organic matter. Pedosphere 2021, 31, 796–806. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernández-Calviño, D.; Nóvoa-Muñoz, J.C.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Arias-Estévez, M.; Álvarez-Rodríguez, E. Experimental data and model prediction of tetracycline adsorption and desorption in agricultural soils. Environ. Res. 2019, 177, 108607. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Nóvoa-Muñoz, J.C.; Fernández-Calviño, M.J.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Arias-Estévez, M. Pedotransfer functions to estimate the adsorption and desorption of sulfadiazine in agricultural soils. Sci. Total Environ. 2019, 691, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Conde-Cid, M.; Nóvoa-Muñoz, J.C.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Arias-Estévez, M.; Álvarez-Rodríguez, E. Experimental data and modeling for sulfachloropyridazine and sulfamethazine adsorption/desorption on agricultural acid soils. Microporous Mesoporous Mat. 2019, 288, 109601. [Google Scholar] [CrossRef]
- Chu, B.; Goyne, K.W.; Anderson, S.H.; Lin, C.H.; Lerch, R.N. Sulfamethazine sorption to soil: Vegetative management, pH, and dissolved organic matter effects. J. Environ. Qual. 2013, 42, 794–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaffer, M.; Licha, T. A framework for assessing the retardation of organic molecules in groundwater: Implications of the species distribution for the sorption-influenced transport. Sci. Total Environ. 2015, 524–525, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Han, Y.; Jing, M.; Chen, J. Sorption and transport of sulfonamides in soils amended with wheat straw-derived biochar: Effects of water pH, coexistence copper ion, and dissolved organic matter. J. Soils Sediments 2015, 17, 771–779. [Google Scholar] [CrossRef]
- ElSayed, E.M.; Prasher, S.O. Sorption/desorption behavior of oxytetracycline and sulfachloropyridazine in the soil water surfactant system. Environ. Sci. Pollut. Res. 2014, 21, 3339–3350. [Google Scholar] [CrossRef]
- Jiang, Y.F.; Zhang, Q.; Deng, X.R.; Nan, Z.J.; Liang, X.R.; Wen, H.; Huang, K.; Wu, Y.Q. Single and competitive sorption of sulfadiazine and chlortetracycline on loess soil from Northwest China. Environ. Pollut. 2020, 263, 114650. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Gschwend, P.M.; Imboden, D.M. Environmental Organic Chemistry; J Wiley: New York, NY, USA, 1993; Volume 8, pp. 245–274. [Google Scholar]
- Weber, J.B.; Wilkerson, G.G.; Reinhardt, C.F. Calculating pesticide sorption coefficients (Kd) using selected soil properties. Chemosphere 2004, 55, 157–166. [Google Scholar] [CrossRef]
- Bao, Y.; Zhou, Q.; Wan, Y.; Yu, Q.; Xie, X. Effects of soil/solution ratios and cation types on adsorption and desorption of tetracycline in soils. Soil Sci. Soc. Am. J. 2010, 74, 1553–1561. [Google Scholar] [CrossRef] [Green Version]
- Conde-Cid, M.; Fernández-Calviño, D.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Arias-Estévez, M. Adsorption/desorption and transport of sulfadiazine, sulfachloropyridazine, and sulfamethazine, in acid agricultural soils. Chemosphere 2019, 234, 978–986. [Google Scholar] [CrossRef]
- Guo, X.Y.; Shen, X.F.; Zhang, M.; Zhang, H.Y.; Chen, W.X.; Wang, H.; Koelmans, A.A.; Cornelissen, G.; Tao, S.; Wang, X.L. Sorption mechanisms of sulfamethazine to soil humin and its subfractions after sequential treatments. Environ. Pollut. 2017, 221, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Cook, R.; Tao, S.; Xing, B.S. Sorption of organic contaminants by biopolymers: Role of polarity, structure and domain spatial arrangement. Chemosphere 2007, 66, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Goyne, K.W.; Anderson, S.H.; Lin, C.H.; Udawatta, R.P. Veterinary antibiotic sorption to agroforestry buffer, grass buffer and cropland soils. Agroforest. Syst. 2010, 79, 67–80. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, W.; Ma, Q.; Zhou, H. Interactive effects of sulfadiazine and Cu(II) on their sorption and desorption on two soils with different characteristics. Chemosphere 2015, 138, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.P.; Rath, S.; Fostier, A.H. Sorption of sulfachloropyridazine in Brazilian soils. J. Braz. Chem. Soc. 2017, 28, 158–167. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Hao, X. Impacts of soil organic matter, iron-aluminium oxides and pH on adsorption-desorption behaviors of oxytetracycline. Res. J. Biotechnol. 2016, 11, 121–131. [Google Scholar]
- He, Y.; Liu, C.; Tang, X.Y.; Xian, Q.S.; Zhang, J.Q.; Guan, Z. Biochar impacts on sorption-desorption of oxytetracycline and florfenicol in an alkaline farmland soil as affected by field ageing. Sci. Total Environ. 2019, 671, 928–936. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, S.K.; Wang, Y.N.; Yang, C.Y.; Chen, Y.Y.; Wang, R.Z.; Wang, Z.Z.; Yuan, X.Y.; Wang, W.K. Adsorption characteristics of oxytetracycline by different fractions of organic matter in sedimentary soil. Environ. Sci. Pollut. Res. 2019, 26, 5668–5679. [Google Scholar] [CrossRef]
- Jiang, W.T.; Chang, P.H.; Wang, Y.S.; Tsai, Y.; Jean, J.S.; Li, Z. Sorption and desorption of tetracycline on layered manganese dioxide birnessite. Int. J. Environ. Sci. Technol. 2015, 12, 1695–1704. [Google Scholar] [CrossRef] [Green Version]
- Kong, W.; Li, C.; Dolhi, J.M.; Li, S.; He, J.; Qiao, M. Characteristics of oxytetracycline sorption and potential bioavailability in soils with various physical-chemical properties. Chemosphere 2012, 87, 542–548. [Google Scholar] [CrossRef]
- Dollinger, J.; Dagès, C.; Voltz, M. Glyphosate sorption to soils and sediments predicted by pedotransfer functions. Environ. Chem. Lett. 2015, 13, 293–307. [Google Scholar] [CrossRef]
- Doretto, K.M.; Peruchi, L.M.; Rath, S. Sorption and desorption of sulfadimethoxine, sulfaquinoxaline and sulfamethazine antimicrobials in Brazilian soils. Sci. Total. Environ. 2014, 476–477, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Ter Laak, T.L.; Gebbink, W.A.; Tolls, J. The effect of pH and ionic strength on the sorption of sulfachloropyridazine, tylosin, and oxytetracycline to soil. Environ. Toxicol. Chem. 2006, 25, 904–911. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, X.; Thiele-Bruhn, S. Interaction of pig manure-derived dissolved organic matter with soil affects sorption of sulfadiazine, caffeine and atenolol pharmaceuticals. Environ. Geochem. Health. 2021, 43, 4299–4313. [Google Scholar] [CrossRef]
- Lertpaitoonpan, W.; Ong, S.K.; Moorman, T.B. Effect of organic carbon and pH on soil sorption of sulfamethazine. Chemosphere 2009, 76, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Fostier, A.H.; Pereira, L.A.; Dionisio, A.C.; Ferreira, F.O.; Doretto, K.M.; Peruchi, L.M.; Viera, A.; Neto, O.F.O.; Bosco, S.M.D.; et al. Sorption behaviors of antimicrobial and antiparasitic veterinary drugs on subtropical soils. Chemosphere 2019, 214, 111–122. [Google Scholar] [CrossRef]
- Srinivasan, P.; Sarmah, A.K. Assessing the sorption and leaching behaviour of three sulfonamides in pasture soils through batch and column studies. Sci. Total Environ. 2014, 493, 535–543. [Google Scholar] [CrossRef]
- Wegst-Uhrich, S.R.; Navarro, D.A.G.; Zimmerman, L.; Aga, D.S. Assessing antibiotic sorption in soil: A literature review and new case studies on sulfonamides and macrolides. Chem. Cent. J. 2014, 8, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.Q.; Zhang, Y.; Shen, G.X.; Zhang, H.C.; Yuan, Z.J.; Zhang, W. Adsorption/desorption behavior and mechanisms of sulfadiazine and sulfamethoxazole in agricultural soil systems. Soil Tillage Res. 2019, 186, 233–241. [Google Scholar] [CrossRef]
- Tolls, J. Sorption of veterinary pharmaceuticals in soils: A review. Environ. Sci. Technol. 2001, 35, 3397–3406. [Google Scholar] [CrossRef]
- Kah, M.; Sigmund, G.; Xiao, F.; Hofmann, T. Sorption of ionizable and ionic organic compounds to biochar, activated carbon and other carbonaceous materials. Water Res. 2017, 124, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.Y.; Ye, Z.L.; Chen, S.H.; Ye, X.; Deng, Y.J.; Zhang, J.Q. Sorption behavior of tetracyclines on suspended organic matters originating from swine wastewater. J. Environ. Sci. 2018, 65, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.A.; Pedersen, J.A. Adsorption of sulfonamide antimicrobial agents to clay minerals. Environ. Sci. Technol. 2005, 39, 9509–9516. [Google Scholar] [CrossRef]
- Kahle, M.; Stamm, C. Time and pH-dependent sorption of the veterinary antimicrobial sulfathiazole to clay minerals and ferrihydrite. Chemosphere 2007, 68, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Sithole, B.B.; Guy, R.D. Models for tetracycline in aquatic environments. I. interaction with bentonite clay systems. Water Air Soil Pollut. 1987, 32, 303–314. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H. Adsorption behavior of antibiotic in soil environment: A critical review. Front. Environ. Sci. Eng. 2015, 9, 565–574. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, J.; Wang, X.; Gu, X.; Gao, S. Tetracycline adsorption on kaolinite: pH, metal cations and humic acid effects. Ecotoxicology 2011, 20, 1141–1147. [Google Scholar] [CrossRef]
- Xu, Y.B.; Yu, X.Q.; Xu, B.L.; Peng, D.; Guo, X.T. Sorption of pharmaceuticals and personal care products on soil and soil components: Influencing factors and mechanisms. Sci. Total Environ. 2021, 753, 141891. [Google Scholar] [CrossRef]
- Figueroa, R.A.; Leonard, A.; MacKay, A.A. Modeling tetracycline antibiotic sorption to clays. Environ. Sci. Technol. 2004, 38, 476–483. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, S.; Han, M.; Cho, J. Sorption characteristics of oxytetracycline, amoxicillin, and sulfathiazole in two different soil types. Geoderma 2012, 185–186, 97–101. [Google Scholar] [CrossRef]
- Kurwadkar, S.T.; Adams, K.C.; Meyer, M.T.; Kolpin, D.W. Effects of sorbate speciation on sorption of selected sulfonamides in three loamy soils. J. Agric. Food Chem. 2007, 55, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Carter, L.J.; Boxall, A.B.A. Evaluation and development of models for estimating the sorption behaviour of pharmaceuticals in soils. J. Hazard. Mater. 2020, 392, 122469. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wilkinson, J.L.; Boxall, A.B.A. Use of a large dataset to develop new models for estimating the sorption of active pharmaceutical ingredients in soils and sediments. J. Hazard. Mater. 2021, 415, 125688. [Google Scholar] [CrossRef] [PubMed]
- Anna, B.B.; Joanna, M.; Wojciech, M.; Agata, B.; Marta, K.; Richard, P.; Piotr, S.; Jolanta, K. Sulfadimethoxine and sulfaguanidine: Their sorption potential on natural soils. Chemosphere 2012, 86, 1059–1065. [Google Scholar]
- Chen, H.; Zhang, J.Q.; Zhong, M.; Li, S.S.; Dong, Y.H. Adsorption of sulfonamides on paddy soil of Taihu Lake regeion. China Environ. Sci. 2008, 28, 309–312. [Google Scholar]
- Zeng, Q.H.; Wang, Y.W.; Li, L. Effect of acidification on adsorption behavior of sulfachloropyridazine (SCP) by black soil. Soils 2019, 51, 359–365. [Google Scholar]
- Fan, Z.; Casey, F.X.M.; Hakk, H.; Larsen, G.L.; Khan, E. Sorption, fate, and mobility of sulfonamides in soils. Water Air Soil Pollut. 2010, 218, 49–61. [Google Scholar] [CrossRef]
- Liu, Z.F. Study on Sorption-Desorption and Transportation of Typical Antibiotics in Soils. Master’s Thesis, China University of Geosciences, Beijing, China, 2016. [Google Scholar]
- Mutavdzic Pavlovic, D.; Curkovic, L.; Blazek, D.; Zupan, J. The sorption of sulfamethazine on soil samples: Isotherms and error analysis. Sci. Total Environ. 2014, 497–498, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Vithanage, M.; Rajapaksha, A.U.; Tang, X.Y.; Thiele-Bruhn, S.; Kim, K.H.; Lee, S.E.; Ok, Y.S. Sorption and transport of sulfamethazine in agricultural soils amended with invasive-plant-derived biochar. J. Environ. Manag. 2014, 141, 95–103. [Google Scholar] [CrossRef]
- Pinna, M.V.; Castaldi, P.; Deiana, P.; Pusino, A.; Garau, G. Sorption behavior of sulfamethazine on unamended and manure-amended soils and short-term impact on soil microbial community. Ecotox. Environ. Saf. 2012, 84, 234–242. [Google Scholar] [CrossRef]
- Ren, M.; Tang, X.Y.; Geng, C.N.; Guan, Z.; Liu, C.; Xian, Q.S. Effects of biochar on adsorption-desorption and migration of antibiotics in slope farmland of purple soil. Soils 2020, 52, 978–986. [Google Scholar]
- Wang, N.; Guo, X.Y.; Xu, J.; Hao, L.J.; Kong, D.Y.; Gao, S.X. Sorption and transport of five sulfonamide antibiotics in agricultural soil and soil-manure systems. J. Environ. Sci. Health B 2015, 50, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, C. Research on the Features of Sorption and Desorption for Fluoroquinolones and Sulfonamides on Purple Soil. Master’s Thesis, Chongqing University, Chongqing, China, 2018. [Google Scholar]
- Zhou, Z.Q.; Liu, C.; Yang, H.W.; Xian, Q.S.; Tang, X.Y. Effects of biochar application on sorption-desorption process and leaching behaviour of sulfonamide antibiotics. Soils 2018, 50, 353–360. [Google Scholar]
- Doretto, K.M.; Rath, S. Sorption of sulfadiazine on Brazilian soils. Chemosphere 2013, 90, 2027–2034. [Google Scholar] [CrossRef] [Green Version]
- Kasteel, R.; Mboh, C.M.; Unold, M.; Groeneweg, j.; Vanderborght, J.; Vereecken, H. Transformation and sorption of the veterinary antibiotic sulfadiazine in two soils a short-term batch study. Environ. Sci. Technol. 2010, 44, 4651–4657. [Google Scholar] [CrossRef]
- Kong, J.J.; Pei, Z.G.; Wen, P.; Shan, X.Q.; Chen, Z.L. Adsorption of sulfadiazine and sulfathiazole by soils. Environ. Chem. 2008, 27, 736–741. [Google Scholar]
- Li, S.H.; Liu, C.; Tang, X.Y.; Yang, H.W. Leaching characteristics of dissolved organic matter in chicken manure and its effect on antibiotic migration in orchard. Trans. Chin. Soc. Agric. Eng. 2020, 36, 37–46. [Google Scholar]
- Lou, F.L. Effects of Dissolved Organic Matter form Pig Manure on Sorption and Migration of Antibiotics in Purple Soil. Master’s Thesis, Southwest Jiaotong University, Chengdu, China, 2019. [Google Scholar]
- Shao, Z.Z.; Li, Q.; Xu, S.H. Effect of silica colloids on adsorption and migration of sulfadiazine in soil relative to ionic intensity. Acta Petrol. Sin. 2018, 55, 411–421. [Google Scholar]
- Shao, Z.Z. Adsorptin, Migration and Numercical Simulation of Sulfadiazine in Soil under Silica Colloids. Master’s Thesis, Qingdao University, Qingdao, China, 2018. [Google Scholar]
- Sukul, P.; Lamshoft, M.; Zuhlke, S.; Spiteller, M. Sorption and desorption of sulfadiazine in soil and soil-manure systems. Chemosphere 2008, 73, 1344–1350. [Google Scholar] [CrossRef]
- Xu, Z.; Lv, S.; Hu, S.; Chao, L.; Rong, F.; Wang, X.; Dong, M.; Liu, K.; Li, M.; Liu, A. Effect of soil solution properties and Cu2+ co-existence on the adsorption of sulfadiazine onto paddy soil. Int. J. Environ. Res. Public Health 2021, 18, 13383. [Google Scholar] [CrossRef]
- Zhang, C.L.; Wang, Y.; Wen, C.B.; Wang, F.A. Study on the adsorption for sulfadiazine in the different type soils. J. Agric. Mech. Res. 2007, 9, 143–146. [Google Scholar]
- Kocarek, M.; Kodesova, R.; Vondrackova, L.; Golovko, O.; Fer, M.; Klement, A.; Nikodem, A.; Jaksik, O.; Grabic, R. Simultaneous sorption of four ionizable pharmaceuticals in different horizons of three soil types. Environ. Pollut. 2016, 218, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Schmidtova, Z.; Kodesova, R.; Grabicova, K.; Kocarek, M.; Fer, M.; Svecova, H.; Klement, A.; Nikodem, A.; Grabic, R. Competitive and synergic sorption of carbamazepine, citalopram, clindamycin, fexofenadine, irbesartan and sulfamethoxazole in seven soils. J. Contam. Hydrol. 2020, 234, 103680. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Sarmah, A.K.; Manley-Harris, M. Co-contaminants and factors affecting the sorption behaviour of two sulfonamides in pasture soils. Environ. Pollut. 2013, 180, 165–172. [Google Scholar] [CrossRef]
- Srinivasan, P.; Sarmah, A.K. Characterisation of agricultural waste-derived biochars and their sorption potential for sulfamethoxazole in pasture soil: A spectroscopic investigation. Sci. Total Environ. 2015, 502, 471–480. [Google Scholar] [CrossRef]
- Wang, X.S. The Characteristic Study of Different Type of Soils Absorption/Desorption for Sulfamethoxazole. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2017. [Google Scholar]
- Wang, J.Q. Adsorption-Desorption and Vertical Migration of Sulfamethoxazole in a Soil under the Impact of Microplastics. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2021. [Google Scholar]
- Wu, Y.; Chen, D.H.; Huang, M.H. Sorption and pH effect on selected antibiotics in soils. Adv. Mater. Res. 2011, 356–360, 35–38. [Google Scholar] [CrossRef]
- Bao, Y.Y.; Zhou, Q.X.; Wang, Y.; Xie, X.J. Effect of soil organic matter on adsorption and desorption of oxytetracycline in soils. China Environ. Sci. 2009, 29, 651–655. [Google Scholar]
- Bao, Y.Y.; Zhou, Q.X.; Zhang, H. Influences of cation species on adsorption and desorption of oxytetracycline in two typical soils of China. Environ. Sci. 2009, 30, 551–556. [Google Scholar]
- Bao, Y.Y.; Zhou, Q.X.; Wang, Y.; Yu, Q.; Xie, X.J. Adsorption and desorption of three tetracycline antibiotics in cinnamon soils of China. China Environ. Sci. 2010, 30, 1383–1388. [Google Scholar]
- Cao, Z.L.; Yu, H.M.; Ge, C.J.; Luo, J.W.; Wang, P.; Zhao, Y.Y.; Li, J.T. Effects of dissolved organic matter on adsorption-desorption behavior of oxytetracycline in soil system. Chin. J. Trop. Crop. 2018, 39, 825–831. [Google Scholar]
- Chen, W.W.; Chen, T.; Yang, P.; Guo, P.; Zhang, W.Q.; Zhang, X.Y. Adsorption behavior of oxytetracycline on black soil under the influence of coexisting Cu2+. Jiangsu Environ. Sci. 2017, 45, 240–244. [Google Scholar]
- Conde-Cid, M.; Ferreira-Coelho, G.; Núñez-Delgado, A.; Fernández-Calviño, D.; Arias-Estévez, M.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J. Competitive adsorption of tetracycline, oxytetracycline and chlortetracycline on soils with different pH value and organic matter content. Environ. Res. 2019, 178, 108669. [Google Scholar] [CrossRef] [PubMed]
- He, H. Effects of Biochar on Sorption and Transportation of Florfenicol in a Purple Soil. Master’s Thesis, Southwest Jiaotong University, Chengdu, China, 2019. [Google Scholar]
- Li, G.A.; Chen, Z.H.; Liu, Z.F.; Li, Y. Adsorption analysis of oxytetracycline on fluvo-aquic soils in Beijing. Geoscience 2015, 29, 377–382. [Google Scholar]
- Li, X.; Wang, D.S.; Zhang, T. Adsorption-desorption behavior of oxytetracycline (OTC) and chlortetracycline (CTC) adsorption-desorption. J. Earth Environ. 2015, 6, 317–322. [Google Scholar]
- Li, X. The Study on Environmental Behavior of Tetracycline Antibiotics in Different Texture Soils. Master’s Thesis, Liaoning Technical University, Fuxin, China, 2015. [Google Scholar]
- Li, Y.; Pan, T.; Miao, D.; Chen, Z.; Tao, Y. Sorption–desorption of typical tetracyclines on different soils: Environment hazards analysis with partition coefficients and hysteresis index. Environ. Eng. Sci. 2015, 32, 865–871. [Google Scholar] [CrossRef]
- Li, J.; Yu, S.G.; Shen, L.E.; Cui, M.; Wang, Y.L. Influence of microplastics on sorption behaviors of oxytetracycline onto soils: A preliminary study. Environ. Chem. 2021, 40, 3133–3143. [Google Scholar]
- Liu, B.; Bao, Y.Y.; Zhou, Q.X.; Zhang, C.D. Effect of N, P fertilizers on adsorption of oxytetracycline to cinnamon soil. China Environ. Sci. 2014, 34, 2057–2062. [Google Scholar]
- Mette, R.; Spliid, N.H. Sorption and mobility of metronidazole, olaquindox, oxytetracycline and tylosin in soil. Chemosphere 2000, 40, 715–722. [Google Scholar]
- Ming, L. Studies of the Effect of DOM on the Adsorption of Cu and OTC in Combined System. Master’s Thesis, Jilin University, Changchun, China, 2014. [Google Scholar]
- Peng, F.J.; Ying, G.G.; Zhou, L.J.; Liu, Y.S.; Pan, C.G.; Liang, Q.Y. Adsorption and desorption of oxytetracycline on typical soils and soil-adsorbed oxytetracycline’s bioavailability. Geochimica 2015, 44, 71–78. [Google Scholar]
- Qi, H.M. Sorption of Oxytetracycline to Wushan Soil, Red Soil and Their Main Components. Master’s Thesis, Dalian University of Technology, Dalian, China, 2008. [Google Scholar]
- Shi, L.P.; Jiang, Y.F.; Guang, A.L.; Yuan, L.M.; Liu, L.L.; Zhan, H.Y. Effect of natural organic matter on adsorption behavior of oxytetracycline onto sierozem soils in northwest China. Res. Environ. Sci. 2019, 32, 1584–1593. [Google Scholar]
- Wang, D.S.; Zhang, T.; Chao, Y. Influence of different strength and species of cation on adsorption of oxytetracycline in meadow soils. Ecol. Environ. Sci. 2014, 23, 870–875. [Google Scholar]
- Wang, D.S.; Li, X. Adsorption and desorption features of tetracycline antibiotics in different texture soils. J. Saf. Environ. 2017, 17, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.Y.; Wan, Y.; Zhou, Q.X.; Li, W.M.; Liu, Y.X. Competitive adsorption and desorption of oxytetracycline and cadmium with different input loadings on cinnamon soil. J. Soils Sediments 2012, 13, 364–374. [Google Scholar] [CrossRef]
- Yan, L.; Pan, D.Q.; Jiang, X.X.; Ji, X.L.; Yang, H.H.; Li, S.Q.; Yu, M. Adsorption behaviour of tetracycline antibiotics in black soil and albic soil. J. Northeast Agric. Univ. 2017, 48, 54–62. [Google Scholar]
- Yao, Y. Adsorption of OTC on Soils. Master’s Thesis, Liaoning Technical University, Fuxin, China, 2013. [Google Scholar]
- Yi, L.L.; Jiao, W.T.; Chen, W.P. Adsorption characteristics of three types of antibiotics in the soil profiles. Environ. Chem. 2013, 32, 2357–2363. [Google Scholar]
- Zhang, M.K.; Wang, L.P.; Zheng, S.A. Adsorption and transport characteristics of two exterior-source antibiotics in some agricultural soils. Acta Ecol. Sin. 2008, 28, 761–766. [Google Scholar]
- Zhu, W.G.; Duan, Y.Y.; Meng, G.J.; Guo, R.C.; Li, X.H. Adsorption-desorption of tetracycline and oxytetracycline in Cu contaminated soil. J. Henan Univ. 2020, 50, 11–18. [Google Scholar]
- Alvarez-Esmoris, C.; Conde-Cid, M.; Fernandez-Sanjurjo, M.J.; Nunez-Delgado, A.; Alvarez-Rodriguez, E.; Arias-Estevez, M. Environmental relevance of adsorption of doxycycline, enrofloxacin, and sulfamethoxypyridazine before and after the removal of organic matter from soils. J. Environ. Manag. 2021, 287, 112354. [Google Scholar] [CrossRef]
- Bao, Y.; Zhou, Q.; Wang, Y. Adsorption characteristics of tetracycline by two soils: Assessing role of soil organic matter. Aust. J. Soil Res. 2009, 47, 286–295. [Google Scholar] [CrossRef]
- Bao, Y.J.; Ding, H.S.; Bao, Y.Y. Effects of temperature on the adsorption and desorption of tetracycline in soils. Adv. Mater. Res. 2013, 726–731, 344–347. [Google Scholar] [CrossRef]
- Jiao, S.J.; Sum, Z.H.; Zheng, S.R.; Yi, D.Q.; Pu, H.Q.; Chen, L.Y. Sorption and desorption of tetracycline on Wushantu soil. J. Agro-Environ. Sci. 2008, 27, 1732–1736. [Google Scholar]
- Wan, Y.; Bao, Y.Y.; Zhou, Q.X. Simultaneous adsorption and desorption of cadmium and tetracycline on cinnamon soil. Chemosphere 2010, 80, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Bao, Y.Y.; Zhou, Q.X. Adsorption and desorption of tetracycline and effect of cadmium on these in two typical soils of China. J. Agro-Environ. Sci. 2010, 29, 85–90. [Google Scholar]
- Wu, T.X.; Zhou, M.; Guo, H.D.; Duan, H.; Chen, H. Adsorption of tetracycline on loess soils. Acta Sci. Circum. 2008, 28, 2311–2314. [Google Scholar]
- Zhang, G.X.; Liu, X.T.; Sun, K.; Zhao, Y.; Lin, C.Y. Sorption of tetracycline to sediments and soils: Assessing the roles of pH, the presence of cadmium and properties of sediments and soils. Front. Environ. Sci. Eng. China 2010, 4, 421–429. [Google Scholar] [CrossRef]
- Ding, Y.H.; Hu, Y.Z.; Chen, J.Q. Research on the adsorption and desorption behavior of chlorotetracycline in silty clay loam. J. Anhui Agric. Sci. 2011, 39, 8979–8982. [Google Scholar]
- Jiang, Y.F.; Liang, X.R.; Yuan, L.M.; Nan, Z.J.; Deng, X.R.; Wu, Y.Q.; Ma, F.F.; Diao, J.R. Effect of livestock manure on chlortetracycline sorption behaviour and mechanism in agricultural soil in Northwest China. Chem. Eng. J. 2021, 415, 129020. [Google Scholar] [CrossRef]
- Liu, X.C.; Dong, Y.H. Adsorption-desorption of chlortetracycline in various cultivated soils in China. Acta Pedologica Sinica 2009, 46, 861–868. [Google Scholar]
- Liu, X.C.; Dong, Y.H.; Liu, H.J. Competitive absorption of chlortetracycline by cation in typical soils of China. Acta Pedol. Sin. 2010, 47, 781–785. [Google Scholar]
- Liu, F.; Liu, H.J.; Yang, S.J.; Dong, Y.H.; Zhang, Z.L.; Sun, W.T.; Liu, X.C. Adsorption and desorption behavior of chlortetracycline in brown soils of different farming conditions. J. Anhui Agric. Sci. 2011, 39, 16053–16055, 16065. [Google Scholar]
- Nan, Z.J.; Jiang, Y.F.; Mao, H.H.; Liang, X.R.; Deng, X.R. Effect of corn stalk biochar on the adsorption of aureomycin from sierozem. Environ. Sci. 2021, 42, 5896–5904. [Google Scholar]
- Wan, Y.; Bao, Y.Y.; Zhou, Q.X. Effect of soil organic matter and cadmium (II) on adsorption and desorption of chlortetracycline in soil. Environ. Sci. 2010, 31, 3050–3055. [Google Scholar]
- Yang, Y.B. Study on Adsorption Characteristics of Chlorotetracycline in Different Soil in Jilin Province. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2016. [Google Scholar]
- Zhang, Y.L.; Lin, S.S.; Dai, C.M.; Shi, L.; Zhou, X.F. Sorption-desorption and transport of trimethoprim and sulfonamide antibiotics in agricultural soil: Effect of soil type, dissolved organic matter, and pH. Environ. Sci. Pollut. Res. 2014, 21, 5827–5835. [Google Scholar] [CrossRef] [PubMed]


| Antibiotic | Physical-Chemical Properties | Chemical Structure | Species Distribution f | |
|---|---|---|---|---|
| SCP | Molecular formula | C10H9ClN4O2S |  | 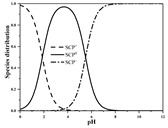 |
| Solubility (mg L−1) | 7000.00 c | |||
| Mw (g mol−1) | 284.72 | |||
| logKow | –0.80 a | |||
| pKa1 | 1.87 a | |||
| pKa2 | 5.45 a | |||
| SMT | Molecular formula | C12H14N4O2S | 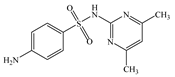 | 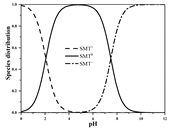 |
| Solubility (mg L−1) | 1500.00 a | |||
| Mw (g mol−1) | 278.34 | |||
| logKow | 0.14 a | |||
| pKa1 | 2.07 a | |||
| pKa2 | 7.49 a | |||
| SDZ | Molecular formula | C10H10N4O2S |  | 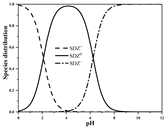 |
| Solubility (mg L−1) | 77.00 a | |||
| Mw (g mol−1) | 250.30 | |||
| logKow | −1.05 a | |||
| pKa1 | 2.10 a | |||
| pKa2 | 6.28 a | |||
| SMX | Molecular formula | C10H11N3O3S | 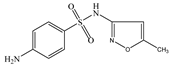 | 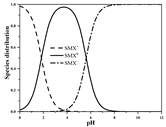 |
| Solubility (mg L−1) | 370.00 b | |||
| Mw (g mol−1) | 253.28 | |||
| logKow | 0.89 b | |||
| pKa1 | 1.83 b | |||
| pKa2 | 5.62 b | |||
| OTC | Molecular formula | C22H24N2O9 | 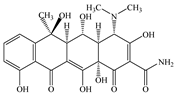 | 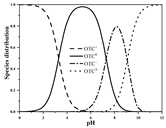 |
| Solubility (mg L−1) | 1000.00 c | |||
| Mw (g mol−1) | 460.40 | |||
| logKow | –0.12 c | |||
| pKa1 | 3.30 c | |||
| pKa2 | 7.30 c | |||
| pKa3 | 9.10 c | |||
| TC | Molecular formula | C22H24N2O8 |  | 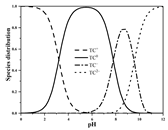 |
| Solubility (mg L−1) | 231.00 d | |||
| Mw (g mol−1) | 444.43 | |||
| logKow | −1.37 d | |||
| pKa1 | 3.20 d | |||
| pKa2 | 7.80 d | |||
| pKa3 | 9.60 d | |||
| CTC | Molecular formula | C22H23ClN2O8 | 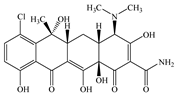 | 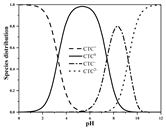 |
| Solubility (mg L−1) | 4120.00 e | |||
| Mw (g mol−1) | 479.00 | |||
| logKow | 2.07 e | |||
| pKa1 | 3.30 e | |||
| pKa2 | 7.44 e | |||
| pKa3 | 9.27 e | |||
| Antibiotic | Parameter | Statistics | ||||
|---|---|---|---|---|---|---|
| Max | Min | Mean | Median | Nobs * | ||
| SCP | Kf (mg1−1/n L1/n kg−1) | 15.33 | 0.60 | 5.25 | 4.60 | 83 |
| n | 2.98 | 0.90 | 1.18 | 1.20 | 83 | |
| Kd (L kg−1) | 23.10 | 0.30 | 4.42 | 3.25 | 98 | |
| SMT | Kf (mg1−1/n L1/n kg−1) | 16.00 | 0.13 | 3.35 | 2.67 | 142 |
| n | 4.00 | 0.41 | 1.20 | 1.20 | 142 | |
| Kd (L kg−1) | 14.20 | 0.11 | 2.45 | 1.62 | 153 | |
| SDZ | Kf (mg1−1/n L1/n kg−1) | 4.86 | 0.45 | 1.65 | 1.45 | 77 |
| n | 2.17 | 0.28 | 1.00 | 1.00 | 77 | |
| Kd (L kg−1) | 12.70 | 0.09 | 2.05 | 1.40 | 104 | |
| SMX | Kf (mg1−1/n L1/n kg−1) | 12.60 | 0.133 | 2.76 | 2.00 | 59 |
| n | 2.38 | 0.48 | 1.28 | 1.20 | 59 | |
| Kd (L kg−1) | 28.50 | 0.02 | 2.49 | 1.40 | 72 | |
| OTC | Kf (mg1−1/n L1/n kg−1) | 5110.00 | 74.00 | 1901.52 | 1814.00 | 133 |
| n | 6.25 | 0.30 | 2.09 | 1.85 | 126 | |
| Kd (L kg−1) | 2191.00 | 16.76 | 692.59 | 601.17 | 121 | |
| TC | Kf (mg1−1/n L1/n kg−1) | 6928.09 | 0.28 | 1707.47 | 1640.61 | 107 |
| n | 6.85 | 0.39 | 2.10 | 2.13 | 107 | |
| Kd (L kg−1) | 1940.89 | 10.06 | 481.53 | 362.30 | 90 | |
| CTC | Kf (mg1−1/n L1/n kg−1) | 8176.99 | 302.00 | 3220.16 | 3131.56 | 93 |
| n | 4.00 | 0.43 | 2.20 | 2.33 | 93 | |
| Kd (L kg−1) | 4473.20 | 147.08 | 1596.77 | 1219.21 | 88 | |
| Antibiotic | Soil Property | Kf | n | Kd |
|---|---|---|---|---|
| SCP | pH | 0.071 | 0.248 * | 0.080 |
| OC | 0.772 ** | –0.120 | 0.631 ** | |
| CEC | 0.338 ** | 0.215 * | 0.200 * | |
| Sand | –0.207 * | –0.122 | –0.170 | |
| Silt | 0.075 | 0.081 | –0.080 | |
| Clay | 0.310 ** | 0.107 | 0.372 ** | |
| SMT | pH | –0.009 | 0.056 | 0.011 |
| OC | 0.754 ** | –0.172 * | 0.717 ** | |
| CEC | 0.409 ** | 0.035 | 0.269 ** | |
| Sand | –0.137 | –0.211 * | 0.040 | |
| Silt | 0.124 | 0.179 * | –0.062 | |
| Clay | 0.101 | 0.163 * | 0.014 | |
| SDZ | pH | 0.068 | 0.160 | 0.267 ** |
| OC | 0.364 ** | –0.151 | 0.686 ** | |
| CEC | –0.119 | 0.088 | 0.353 ** | |
| Sand | –0.439 ** | –0.263 * | –0.415 ** | |
| Silt | 0.287 * | 0.195 | 0.203 * | |
| Clay | 0.380 ** | 0.195 | 0.442 ** | |
| SMX | pH | –0.381 ** | 0.082 | –0.389 ** |
| OC | 0.738 ** | –0.180 | 0.626 ** | |
| CEC | 0.435 ** | 0.026 | 0.270 * | |
| Sand | 0.052 | 0.109 | –0.008 | |
| Silt | –0.214 | –0.028 | –0.063 | |
| Clay | 0.235 | –0.150 | 0.119 | |
| OTC | pH | –0.749 ** | –0.612 ** | –0.268 ** |
| OC | 0.587 ** | 0.216 * | 0.670 ** | |
| CEC | –0.488 ** | –0.490 ** | 0.146 | |
| Sand | 0.350 ** | 0.374 ** | –0.030 | |
| Silt | –0.313 ** | –0.293 ** | 0.011 | |
| Clay | –0.174 * | –0.252 ** | 0.048 | |
| TC | pH | –0.570 ** | –0.727 ** | 0.283 * |
| OC | 0.585 ** | 0.135 | 0.151 | |
| CEC | –0.093 | –0.603 ** | 0.480 ** | |
| Sand | 0.186 * | 0.474 ** | –0.704 ** | |
| Silt | –0.175 | –0.463 ** | 0.457 ** | |
| Clay | –0.104 | –0.265 ** | 0.669 ** | |
| CTC | pH | –0.457 ** | –0.737 ** | –0.149 |
| OC | 0.684 ** | 0.302 ** | 0.419 ** | |
| CEC | –0.240 * | –0.672 ** | 0.073 | |
| Sand | 0.127 | 0.576 ** | –0.061 | |
| Silt | –0.081 | –0.417 ** | –0.049 | |
| Clay | –0.104 | –0.426 ** | 0.226 * |
| Antibiotic | Pedotransfer Function | r2 | RMSE (RMSE/SD) | NSE | Nobs 1 |
|---|---|---|---|---|---|
| SCP | 0.616 ** 2 | 2.01(61.1%) | 0.63 | 68 | |
| 0.689 ** | 2.34 (55.1%) | 0.70 | 80 | ||
| SMT | 0.565 ** | 1.24 (65.6%) | 0.57 | 107 | |
| 0.510 ** | 1.58 (69.6%) | 0.52 | 114 | ||
| SDZ | 0.380 ** | 0.39 (77.2%) | 0.40 | 53 | |
| 0.507 ** | 0.73 (69.4%) | 0.52 | 83 | ||
| SMX | 0.666 ** | 1.07 (56.2%) | 0.69 | 49 | |
| 0.525 ** | 0.88 (67.2%) | 0.54 | 57 | ||
| OTC | 0.606 ** | 851.94 (62.2%) | 0.61 | 104 | |
| 0.444 ** | 328.77 (74.2%) | 0.45 | 94 | ||
| TC | 0.509 ** | 612.94 (68.8%) | 0.53 | 84 | |
| 0.607 ** | 179.22 (61.3%) | 0.62 | 67 | ||
| CTC | 0.524 ** | 1340.46 (68.0%) | 0.54 | 73 | |
| 0.371 ** | 811.11 (78.2%) | 0.39 | 72 |
| Antibiotic | Pedotransfer Function | R2 |
|---|---|---|
| SCP | 0.619 ** | |
| 0.695 ** | ||
| SDZ | 0.579 ** | |
| SMX | 0.552 ** | |
| OTC | 0.647 ** | |
| TC | 0.545 ** | |
| 0.633 ** | ||
| CTC | 0.549 ** |
| Antibiotic | Pedotransfer Function | r2 | Origin | Nobs 1 | RMSE (RMSE/SD) | NSE | Reference |
|---|---|---|---|---|---|---|---|
| SCP | 0.829 ** 2 | Galicia (Spain) | 50 | 1.35 (62.4%) | 0.61 | [32] | |
| SMT | 0.92 ** | Iowa (USA) | 5 | 1.62 (63.0%) | 0.60 | [56] | |
| SDZ | 0.675 ** | Galicia (Spain) | 50 | 1.81 (149.6%) | −1.20 | [31] | |
| OTC | 0.349 ** | Galicia (Spain) | 63 | 2052.63 (221.87%) | −3.90 | [13] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Tang, X.; Qi, M.; Cheng, J. New Models for Estimating the Sorption of Sulfonamide and Tetracycline Antibiotics in Soils. Int. J. Environ. Res. Public Health 2022, 19, 16771. https://doi.org/10.3390/ijerph192416771
Hu J, Tang X, Qi M, Cheng J. New Models for Estimating the Sorption of Sulfonamide and Tetracycline Antibiotics in Soils. International Journal of Environmental Research and Public Health. 2022; 19(24):16771. https://doi.org/10.3390/ijerph192416771
Chicago/Turabian StyleHu, Jinsheng, Xiangyu Tang, Minghui Qi, and Jianhua Cheng. 2022. "New Models for Estimating the Sorption of Sulfonamide and Tetracycline Antibiotics in Soils" International Journal of Environmental Research and Public Health 19, no. 24: 16771. https://doi.org/10.3390/ijerph192416771





