Fast Pyrolysis of Cellulose and the Effect of a Catalyst on Product Distribution
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Materials
2.2. Catalyst Characterization: Nitrogen (N2) Adsorption
2.3. Sample Preparation
2.4. Fast Pyrolysis by Py-GC/MS
3. Results and Discussion
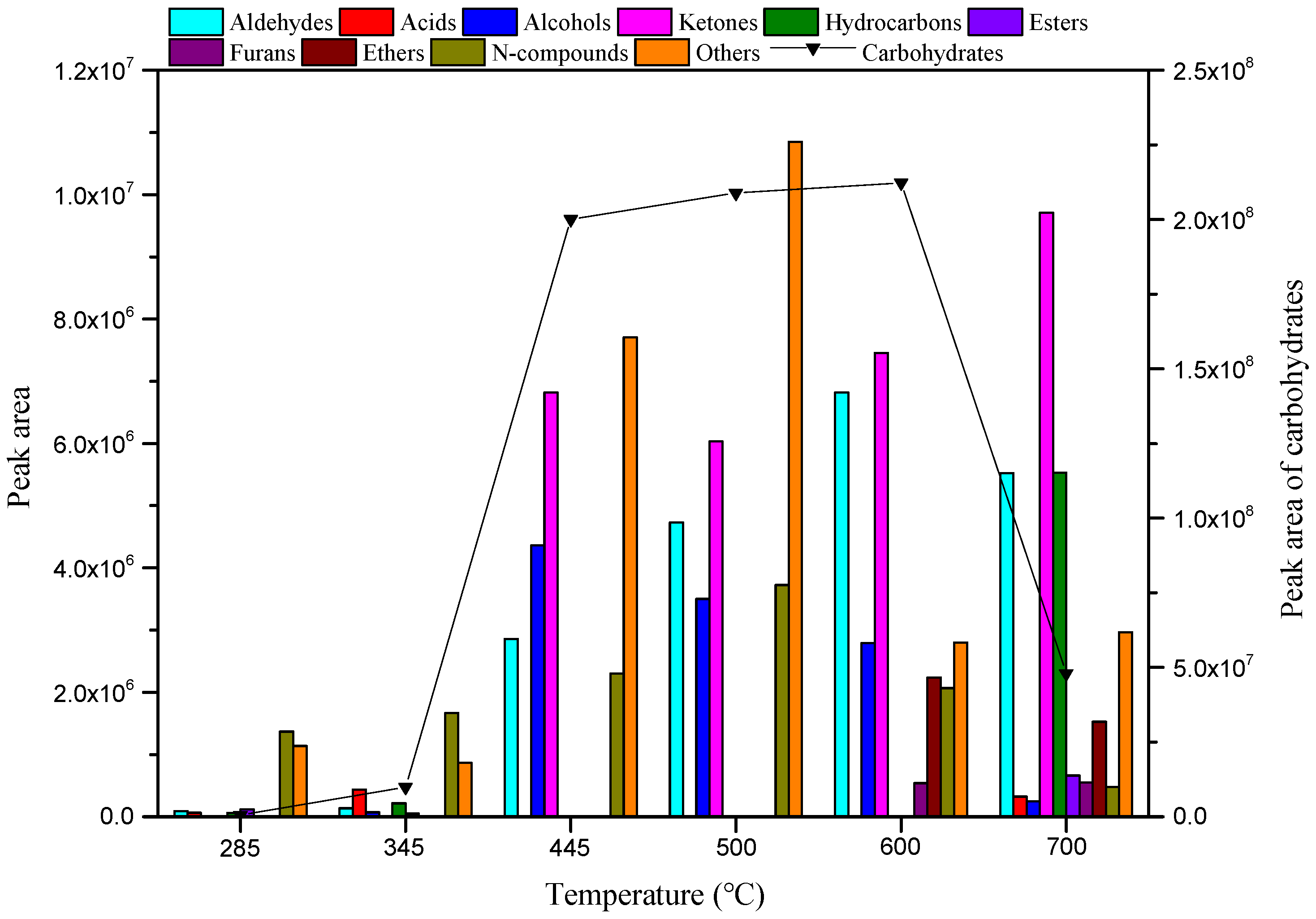
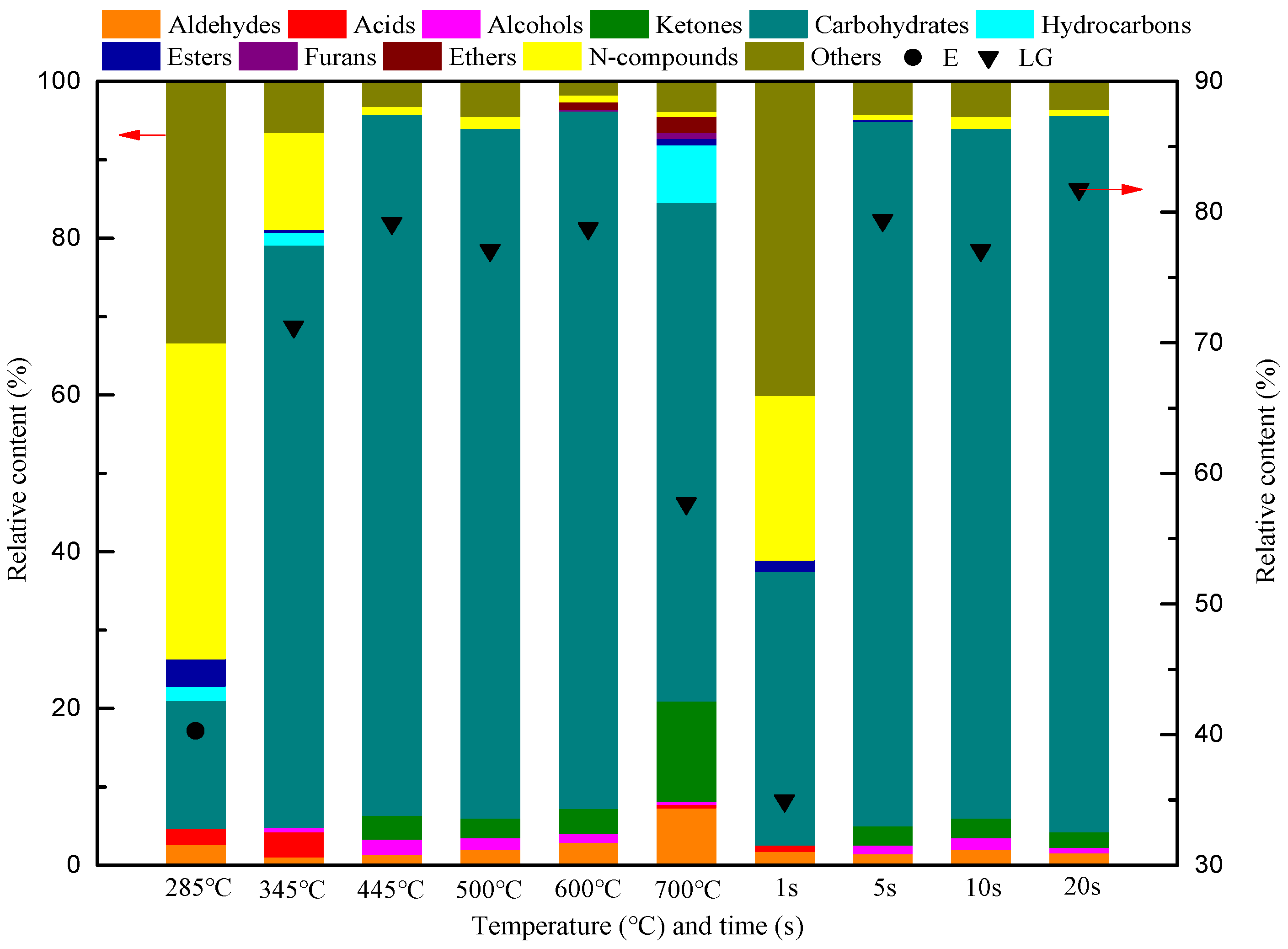
3.1. Effect of Reaction Temperature and Time on the Distribution of MC Pyrolysis Products
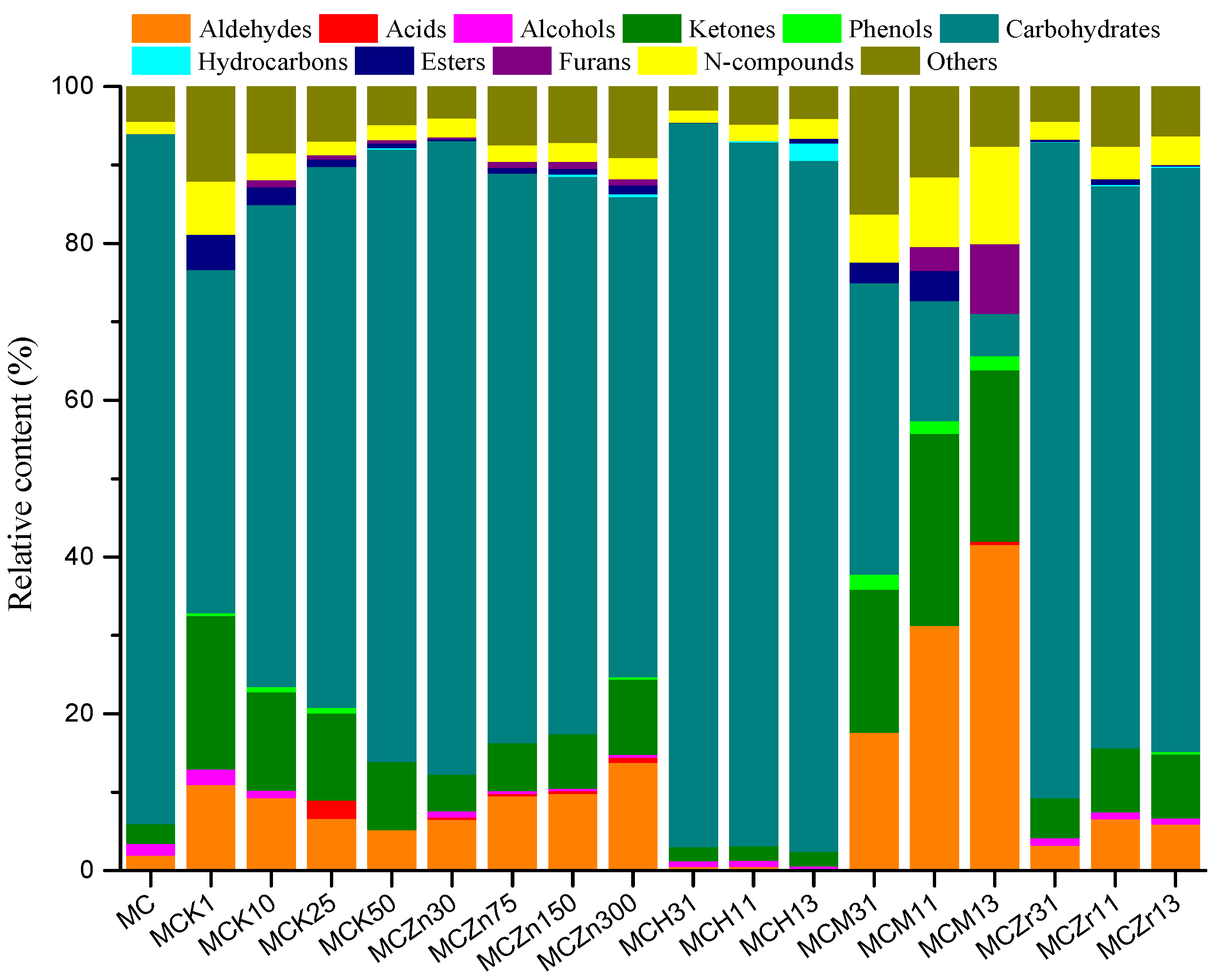
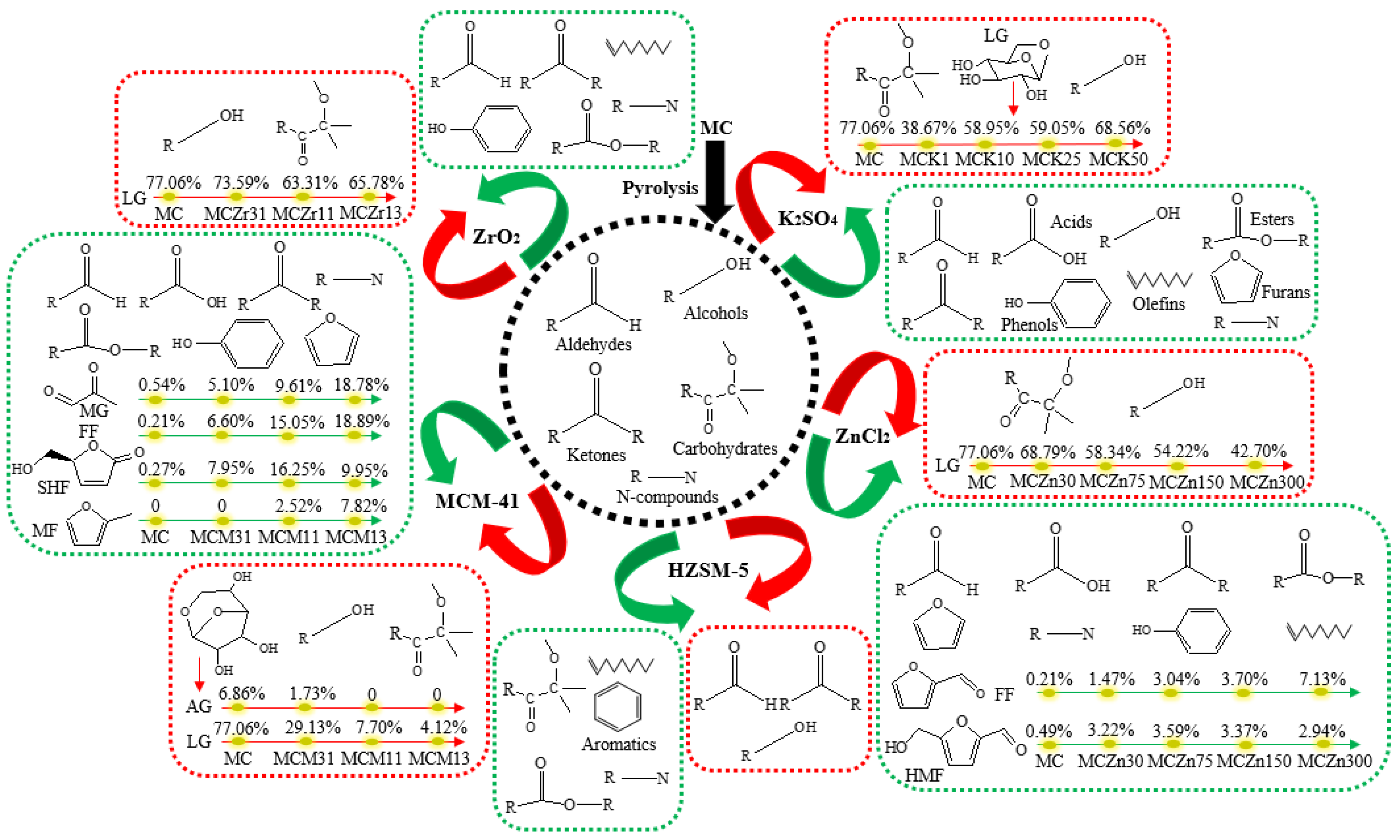
3.2. Effect of Catalyst Type and Amount on the Pyrolysis Product Distribution of MC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sun, T.; Li, Z.; Zhang, Z.; Wang, Z.; Yang, S.; Yang, Y.; Wang, X.; Liu, S.; Zhang, Q.; Lei, T. Fast Corn Stalk Pyrolysis and the Influence of Catalysts on Product Distribution. Bioresour. Technol. 2020, 301, 122739. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liao, Y.; Guo, Z.; Cao, Y.; Ma, X. Products Distribution and Generation Pathway of Cellulose Pyrolysis. J. Clean. Prod. 2019, 232, 1309–1320. [Google Scholar] [CrossRef]
- Wang, W.; Shi, Y.; Cui, Y.; Li, X. Catalytic Fast Pyrolysis of Cellulose for Increasing Contents of Furans and Aromatics in Biofuel Production. J. Anal. Appl. Pyrolysis 2018, 131, 93–100. [Google Scholar] [CrossRef]
- Yang, H.; Lei, S.; Xu, K.; Fang, Y.; Chen, X.; Chen, Y.; Wang, X.; Chen, H. Catalytic Pyrolysis of Cellulose with Sulfonated Carbon Catalyst to Produce Levoglucosenone. Fuel Process. Technol. 2022, 234, 107323. [Google Scholar] [CrossRef]
- Wang, Y.; Pääkkönen, T.; Miikki, K.; Maina, N.; Nieminen, K.; Zitting, A.; Penttilä, P.; Tao, H.; Kontturi, E. Degradation of Cellulose Polymorphs into Glucose by HCl Gas with Simultaneous Suppression of Oxidative Discoloration. Carbohyd. Polym. 2022, 302, 120388. [Google Scholar] [CrossRef]
- Yang, X.; Fu, Z.; Han, D.; Zhao, Y.; Li, R.; Wu, Y. Unveiling the Pyrolysis Mechanisms of Cellulose: Experimental and Theoretical Studies. Renew. Energy 2020, 147, 1120–1130. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, Y.; Yang, H.; Xin, S.; Zhang, X.; Wang, X.; Chen, H. Effect of Volatiles Interaction During Pyrolysis of Cellulose, Hemicellulose, and Lignin at Different Temperatures. Fuel 2019, 248, 1–7. [Google Scholar] [CrossRef]
- Deng, C.; Liaw, S.B.; Gao, X.; Wu, H. Differences in Soot Produced from Rapid Pyrolysis of Xylan, Cellulose and Lignin under Pulverized-Fuel Conditions. Fuel 2020, 265, 116991. [Google Scholar] [CrossRef]
- Saragai, S.; Kudo, S.; Sperry, J.; Ashik, U.P.M.; Asano, S.; Hayashi, J.-I. Catalytic Deep Eutectic Solvent for Levoglucosenone Production by Pyrolysis of Cellulose. Bioresour. Technol. 2022, 344, 126323. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y.; Li, S.; Lu, Y.; Wang, J.; Zhu, K.; Chen, J.; Zheng, Y.; Zheng, Z. Catalytic Fast Pyrolysis of Cellulose over Ce0.8Zr0.2-XAlxO2 Catalysts to Produce Aromatic Hydrocarbons: Analytical Py-GC × GC/MS. Fuel Process. Technol. 2020, 205, 106438. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, X.; Wang, Y.; Liu, C.; Ma, H.; Zhou, C.; Qi, F.; Yang, J. Online Photoionization Mass Spectrometric Evaluation of Catalytic Co-Pyrolysis of Cellulose and Polyethylene over Hzsm-5. Bioresour. Technol. 2019, 275, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Lei, T.; Li, Z.; Zhang, Z.; Yang, S.; Xin, X.; Zhang, M.; He, X.; Zhang, Q.; Zhang, L. Catalytic Co-Pyrolysis of Corn Stalk and Polypropylene over Zn-Al Modified Mcm-41 Catalysts for Aromatic Hydrocarbon-Rich Oil Production. Ind. Crop. Prod. 2021, 171, 113843. [Google Scholar] [CrossRef]
- Liu, R.; Rahman, M.M.; Li, C.; Chai, M.; Sarker, M.; Wang, Y.; Cai, J. Catalytic Pyrolysis of Microcrystalline Cellulose Extracted from Rice Straw for High Yield of Hydrocarbon over Alkali Modified Zsm-5. Fuel 2021, 285, 119038. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Zhong, Z.; Ding, K.; Deng, A.; Min, M.; Chen, P.; Ruan, R. Catalytic Fast Co-pyrolysis of Bamboo Residual and Waste Lubricating Oil over an Ex-situ Dual Catalytic Beds of MgO and Hzsm-5: Analytical PY-GC/MS Study. Energy Convers. Manag. 2017, 139, 222–231. [Google Scholar] [CrossRef]
- Xu, J.; Liao, Y.; Lin, Y.; Ma, X.; Yu, Z. Study on Catalytic Pyrolysis of Eucalyptus to Produce Aromatic Hydrocarbons by Zn-Fe Co-Modified Hzsm-5 Catalysts. J. Anal. Appl. Pyrolysis 2019, 139, 96–103. [Google Scholar] [CrossRef]
- Bai, X.; Li, J.; Jia, C.; Shao, J.; Yang, Q.; Chen, Y.; Yang, H.; Wang, X.; Chen, H. Preparation of Furfural by Catalytic Pyrolysis of Cellulose Based on Nano Na/Fe-Solid Acid. Fuel 2019, 258, 116089. [Google Scholar] [CrossRef]
- Leng, E.; Guo, Y.; Yin, Y.; Yu, Y.; Gong, X.; Chen, J.; Xue, Y.E.J. In Situ Evolution of Functional Groups in Char During Cellulose Pyrolysis under the Catalysis of KCl and CaCl2. Fuel 2022, 309, 122227. [Google Scholar] [CrossRef]
- Leng, E.; Costa, M.; Gong, X.; Zheng, A.; Liu, S.; Xu, M. Effects of KCl and CaCl2 on the Evolution of Anhydro Sugars in Reaction Intermediates During Cellulose Fast Pyrolysis. Fuel 2019, 251, 307–315. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Hu, B.; Li, Y.; Li, K.; Lu, Q. Selective Preparation of 1-Hydroxy-3,6-Dioxabicyclo[3.2.1]Octan-2-One by Fast Pyrolysis of Cellulose Catalyzed with Metal-Loaded Nitrided Hzsm-5. Bioresour. Technol. 2020, 309, 123370. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Jae, J.; Kim, B.-S.; Hong, Y.; Jung, S.-C.; Park, Y.-K. Catalytic Co-Pyrolysis of Torrefied Yellow Poplar and High-Density Polyethylene Using Microporous HZSM-5 and Mesoporous Al-MCM-41 Catalysts. Energy Convers. Manag. 2017, 149, 966–973. [Google Scholar] [CrossRef]
- Chi, Y.; Xue, J.; Zhuo, J.; Zhang, D.; Liu, M.; Yao, Q. Catalytic Co-Pyrolysis of Cellulose and Polypropylene over All-Silica Mesoporous Catalyst Mcm-41 and Al-Mcm-41. Sci. Total Environ. 2018, 633, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, K.; Hu, B.; Zhang, Z.-X.; Zhang, G.; Feng, S.-Y.; Wang, T.-P.; Lu, Q. Catalytic Fast Pyrolysis of Cellulose for Selective Production of 1-Hydroxy-3,6-Dioxabicyclo[3.2.1]Octan-2-One Using Nickel-Tin Layered Double Oxides. Ind. Crop. Prod. 2021, 162, 113269. [Google Scholar] [CrossRef]
- Khan, I.; Zada, N.; Khan, I.; Sadiq, M.; Saeed, K. Enhancement of photocatalytic potential and recoverability of Fe3O4 nanoparticles by decorating over monoclinic zirconia. J. Environ. Health Sci. Eng. 2020, 18, 1473–1489. [Google Scholar] [CrossRef]
- Fu, X.; Wang, X.; Li, Y.; Xin, Y.; Li, S. Enhancing and Upgrading Bio-Oil During Catalytic Pyrolysis of Cellulose: The Synergistic Effect of Potassium Cation and Different Anions Impregnation. Fuel Process. Technol. 2019, 193, 338–347. [Google Scholar] [CrossRef]
- Jiang, L.; Luo, J.; Xu, F.; Qian, L.; Wang, Y.; Li, H.; Fang, Z. High Yield Production of Levoglucosan Via Catalytic Pyrolysis of Cellulose at Low Temperature. Fuel 2022, 323, 124369. [Google Scholar] [CrossRef]
- Bu, Q.; Cao, M.; Wang, M.; Zhang, X.; Mao, H. The Effect of Torrefaction and Zsm-5 Catalyst for Hydrocarbon Rich Bio-Oil Production from Co-Pyrolysis of Cellulose and Low Density Polyethylene Via Microwave-Assisted Heating. Sci. Total Environ. 2021, 754, 142174. [Google Scholar] [CrossRef]
- Hu, B.; Cheng, A.-S.; Li, Y.; Huang, Y.-B.; Liu, J.; Zhang, B.; Li, K.; Zhao, L.; Wang, T.-P.; Lu, Q. A Sustainable Strategy for the Production of 1,4:3,6-Dianhydro-A-D-Glucopyranose through Oxalic Acid-Assisted Fast Pyrolysis of Cellulose. Chem. Eng. J. 2022, 436, 135200. [Google Scholar] [CrossRef]
- Orrego-Restrepo, E.; Ordonez-Loza, J.; Chejne, F. Novel Methodology for Evaluation of Cellulose Pyrolysis Kinetics Implementing Infrared Spectroscopy in Situ. J. Anal. Appl. Pyrolysis 2022, 166, 105589. [Google Scholar] [CrossRef]
- Dai, G.; Wang, K.; Wang, G.; Wang, S. Initial Pyrolysis Mechanism of Cellulose Revealed by in-Situ Drift Analysis and Theoretical Calculation. Combust. Flame 2019, 208, 273–280. [Google Scholar] [CrossRef]
- Hu, B.; Lu, Q.; Wu, Y.-T.; Xie, W.-L.; Cui, M.-S.; Liu, J.; Dong, C.-Q.; Yang, Y.-P. Insight into the Formation Mechanism of Levoglucosenone in Phosphoric Acid-Catalyzed Fast Pyrolysis of Cellulose. J. Energy Chem. 2020, 43, 78–89. [Google Scholar] [CrossRef]
- Huang, X.; Yamasaki, K.; Kudo, S.; Sperry, J.; Hayashi, J.-I. Influence of Ionic Liquid Type on Porous Carbon Formation During the Ionothermal Pyrolysis of Cellulose. J. Anal. Appl. Pyrolysis 2020, 145, 104728. [Google Scholar] [CrossRef]
- Jiang, L.-Q.; Lin, Q.; Lin, Y.; Xu, F.-X.; Zhang, X.; Zhao, Z.-L.; Li, H.-B. Impact of Ball-Milling and Ionic Liquid Pretreatments on Pyrolysis Kinetics and Behaviors of Crystalline Cellulose. Bioresour. Technol. 2020, 305, 123044. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, C.; Shan, R.; Zhang, J.; Zhu, L.; Chen, Y. Municipal Sludge Derived Solid Acids for Levoglucosenone Production Via Cellulose Fast Pyrolysis. J. Anal. Appl. Pyrolysis 2022, 167, 105663. [Google Scholar] [CrossRef]
- Wang, P.; Shen, Y. Catalytic Pyrolysis of Cellulose and Chitin with Calcined Dolomite–Pyrolysis Kinetics and Products Analysis. Fuel 2022, 312, 122875. [Google Scholar] [CrossRef]
- Lu, Q.; Hu, B.; Zhang, Z.-X.; Wu, Y.-T.; Cui, M.-S.; Liu, D.-J.; Dong, C.-Q.; Yang, Y.-P. Mechanism of Cellulose Fast Pyrolysis: The Role of Characteristic Chain Ends and Dehydrated Units. Combust. Flame 2018, 198, 267–277. [Google Scholar] [CrossRef]



| Sample | Proximate Analysis (wt.%) | Ultimate Analysis (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Moisture | Volatile | Fixed Carbon | Ash | C | H | O | N | S | |
| MC | 6.58 | 87.32 | 5.58 | 0.52 | 44.76 | 6.01 | 48.15 | 0.51 | 0.05 |
| Sample | Si/Al | Crystallinity (%) | Crystal Size (μm) | SBET (m2/g) | Pore Volume (mL/g) | Avg. Pore Diameter (nm) | Adsorption n-Hexane (mg/g) | Na2O (% m/m) | LOI (% m/m) |
|---|---|---|---|---|---|---|---|---|---|
| HZSM-5 | 46 | 95 | 0.5–1 | 350 | 0.110 | 0.253 | - | 0.08 | ≤10 |
| MCM-41 | 25 | 91 | - | 750 | 0.728 | 3.531 | 90 | 0.79 | 13 |
| Sample | Abbreviation | The K+ or Zn2+ Content |
|---|---|---|
| MC:HZSM-5 = 3:1 | MCH31 | - |
| MC:HZSM-5 = 1:1 | MCH11 | - |
| MC:HZSM-5 = 1:3 | MCH13 | - |
| MC:MCM-41 = 3:1 | MCM31 | - |
| MC:MCM-41 = 1:1 | MCM11 | - |
| MC:MCM-41 = 1:3 | MCM13 | - |
| MC:ZrO2 = 3:1 | MCZr31 | - |
| MC:ZrO2 = 1:1 | MCZr11 | - |
| MC:ZrO2 = 1:3 | MCZr13 | - |
| MC + K2SO4 1 g/L | MCK1 | 0.17% |
| MC + K2SO4 10 g/L | MCK10 | 0.39% |
| MC + K2SO4 25 g/L | MCK25 | 0.65% |
| MC + K2SO4 50 g/L | MCK50 | 1.05% |
| MC + ZnCl2 30 mg | MCZn30 | 0.48% |
| MC + ZnCl2 75 mg | MCZn75 | 0.78% |
| MC + ZnCl2 150 mg | MCZn150 | 1.07% |
| MC + ZnCl2 300 mg | MCZn300 | 1.25% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.; Zhang, L.; Yang, Y.; Li, Y.; Ren, S.; Dong, L.; Lei, T. Fast Pyrolysis of Cellulose and the Effect of a Catalyst on Product Distribution. Int. J. Environ. Res. Public Health 2022, 19, 16837. https://doi.org/10.3390/ijerph192416837
Sun T, Zhang L, Yang Y, Li Y, Ren S, Dong L, Lei T. Fast Pyrolysis of Cellulose and the Effect of a Catalyst on Product Distribution. International Journal of Environmental Research and Public Health. 2022; 19(24):16837. https://doi.org/10.3390/ijerph192416837
Chicago/Turabian StyleSun, Tanglei, Lu Zhang, Yantao Yang, Yanling Li, Suxia Ren, Lili Dong, and Tingzhou Lei. 2022. "Fast Pyrolysis of Cellulose and the Effect of a Catalyst on Product Distribution" International Journal of Environmental Research and Public Health 19, no. 24: 16837. https://doi.org/10.3390/ijerph192416837
APA StyleSun, T., Zhang, L., Yang, Y., Li, Y., Ren, S., Dong, L., & Lei, T. (2022). Fast Pyrolysis of Cellulose and the Effect of a Catalyst on Product Distribution. International Journal of Environmental Research and Public Health, 19(24), 16837. https://doi.org/10.3390/ijerph192416837





