Breast Fluctuating Asymmetry in Women with Macromastia/Gigantomastia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Procedure
2.2. Measurements
2.3. Statistical Methods
3. Results
3.1. Reliability of Measurements
3.2. Breast Size and Volume Asymmetry
3.3. Breast Size and Nipple Position Asymmetry
3.4. Breast Asymmetry in Women with Macromastia vs. Women with “Normal” Breasts
3.5. Breast Size and FA and BMI
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manning, J.T.; Scutt, D.; Whitehouse, G.H.; Leinster, S.J. Breast asymmetry and phenotypic quality in women. Evol. Hum. Behav. 1997, 18, 223–236. [Google Scholar] [CrossRef]
- Thornhill, R.; Møller, A.P. Developmental stability, disease and medicine. Biol. Rev. Camb. Philos. Soc. 1997, 72, 497–548. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P.; Soler, M.; Thornhill, R. Breast asymmetry, sexual selection, and human reproductive success. Evol. Hum. Behav. 1995, 16, 207–219. [Google Scholar] [CrossRef]
- Dancey, A.; Khan, M.; Dawson, J.; Peart, F. Gigantomastia-a classification and review of the literature. J. Plast. Reconstr. Aesthetic Surg. 2008, 61, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Netscher, D.T.; Meade, R.A.; Goodman, C.M.; Brehm, B.J.; Friedman, J.D.; Thornby, J. Physical and psychosocial symptoms among 88 volunteer subjects compared with patients seeking plastic surgery procedures to the breast. Plast. Reconstr. Surg. 2000, 105, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.J.; Chester, D.L. Bilateral breast reduction surgery in elderly women--a retrospective review of outcomes. J. Plast. Reconstr. Aesthetic Surg. 2012, 65, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Antevski, B.; Jovkovski, O.; Filipovski, V.; Banev, S. Extreme gigantomastia in pregnancy: Case report-my experience with two cases in last 5 years. Arch. Gynecol. Obstet. 2011, 284, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Ezem, B.U.; Osuagwu, C.C.; Opara, K.A. Gestational gigantomastia with complete resolution in a Nigerian woman. BMJ Case Rep. 2011, 2011, bcr0120102632. [Google Scholar] [CrossRef] [PubMed]
- Swelstad, M.R.; Swelstad, B.B.; Rao, V.K.; Gutowski, K.A. Management of gestational gigantomastia. Plast. Reconstr. Surg. 2006, 118, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Kasielska-Trojan, A.; Danilewicz, M.; Sitek, A.; Antoszewski, B. Body size measurements, digit ratio (2D:4D) and oestrogen and progesterone receptors’ expressions in juvenile gigantomastia. J. Pediatr. Endocrinol. Metab. 2020, 33, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Singh, D. Adaptive significance of female physical attractiveness: Role of waist-to-hip ratio. J. Pers. Soc. Psychol. 1993, 65, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Singh, D. Body shape and women’s attractiveness: The critical role of waist-to-hip ratio. Hum. Nat. 1993, 4, 297–321. [Google Scholar] [CrossRef] [PubMed]
- Singh, D. Female health, attractiveness, and desirability for relationships: Role of breast asymmetry and waist-to-hip ratio. Ethol. Sociobiol. 1995, 16, 465–481. [Google Scholar] [CrossRef]
- Hudson, S.M.; Wilkinson, L.S.; De Stavola, B.L.; Dos-Santos-Silva, I. Left-right breast asymmetry and risk of screen-detected and interval cancers in a large population-based screening population. Br. J. Radiol. 2020, 93, 20200154. [Google Scholar] [CrossRef] [PubMed]
- Scutt, D.; Lancaster, G.A.; Manning, J.T. Breast asymmetry and predisposition to breast cancer. Breast Cancer Res. 2006, 8, R14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scutt, D.; Manning, J.T.; Whitehouse, G.H.; Leinster, S.J.; Massey, C.P. The relationship between breast asymmetry, breast size and the occurrence of breast cancer. Br. J. Radiol. 1997, 70, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Kasielska-Trojan, A.; Mikołajczyk, M.; Antoszewski, B. Breast Idea Volume Estimator (BIVE)-A new tool for breast volume estimation-presentation and validation for women. Plast. Reconstr. Surg. 2020, 146, 744e–748e. [Google Scholar] [CrossRef] [PubMed]
- Mikołajczyk, M.; Kasielska-Trojan, A.; Antoszewski, B. A New Tool for Breast Anthropometric Measurements: Presentation and Validation for Women and Men. Aesthetic Plast. Surg. 2019, 43, 1160–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusano, A.S.; Trichopoulos, D.; Terry, K.L.; Chen, W.Y.; Willett, W.C.; Michels, K.B. A prospective study of breast size and premenopausal breast cancer incidence. Int. J. Cancer 2006, 118, 2031–2034. [Google Scholar] [CrossRef] [PubMed]
- Folstad, I.; Karter, A.J. Parasites, bright males and the immunocompetence handicap. Am. Nat. 1992, 139, 603–622. [Google Scholar] [CrossRef]
- Imagawa, W.; Yang, J.; Guzman, R.; Nandi, S. Control of mammary gland growth and differentition. In The Physiology of Reproduction; Knobil, E., Neill, J.D., Greenwald, G.S., Markert, C.L., Pfaff, D.W., Eds.; Raven Press: New York, NY, USA, 1994; Volume 2. [Google Scholar]
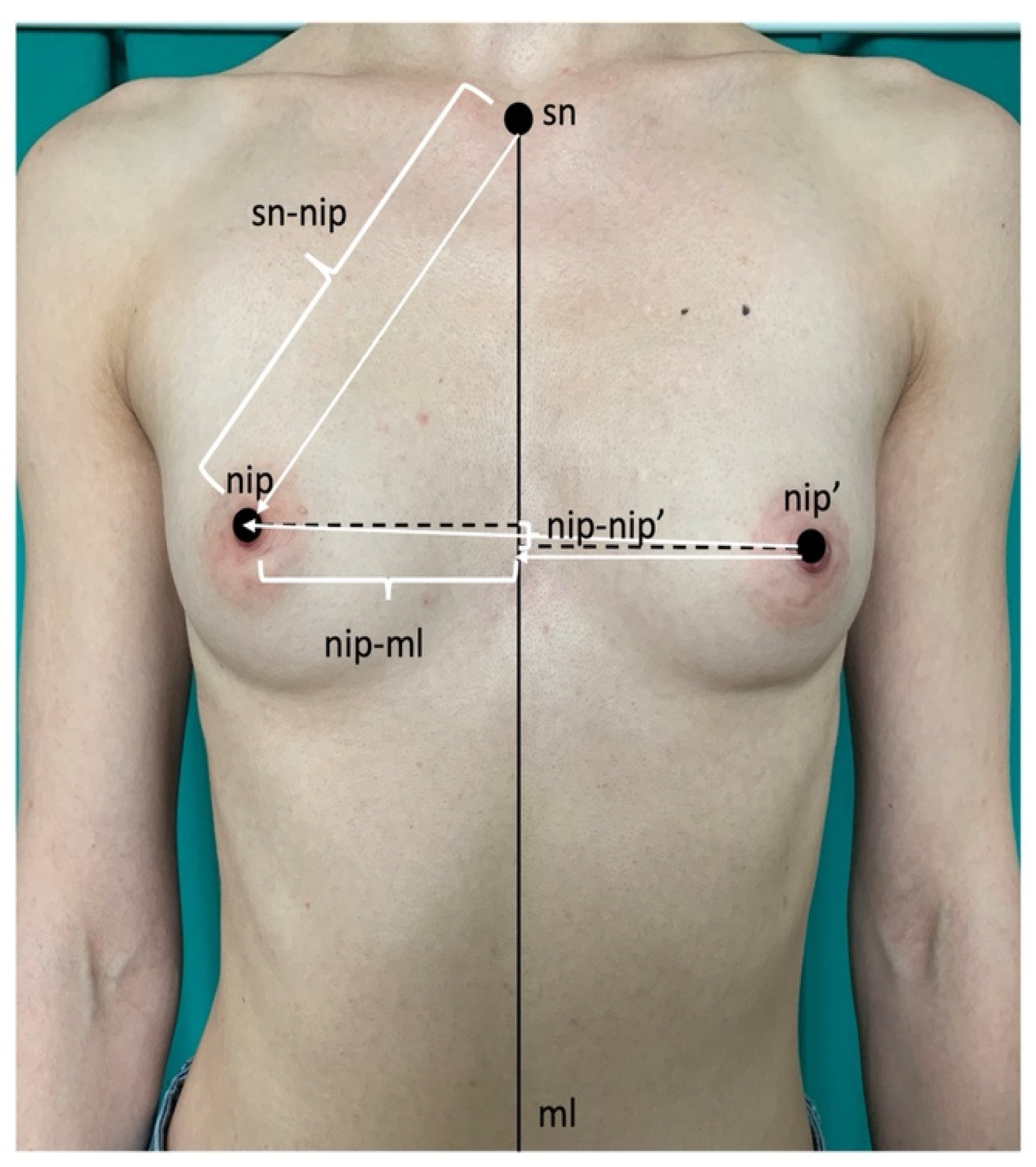

| All Women n = 65 | Normal Breasts n = 30 | Macromastia/ Gigantomastia n = 35 | t/Mann–Whitney | p | |
|---|---|---|---|---|---|
| Age [years] | 33.97 ± 12.08 | 27.53 ± 7.72 | 39.48 ± 12.49 | 4.01 * | <0.0001 |
| BMI [kg/m2] | 23.87 ± 2.72 | 22.45 ± 2.13 | 25.09 ± 2.60 | 4.43 | <0.0001 |
| Breast size—volume [cc] | 608.88 ± 377.46 | 283.08 ± 143.73 | 888.14 ± 276.82 | 11.28 | <0.0001 |
| All Women n = 65 | Normal Breasts n = 30 | Macromastia/Gigantomastia n = 35 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | F, p | x Age t, p | x Size 1 t, p | R2 | F, p | x Age t, p | x Size 1 t, p | R2 | F, p | x Age t, p | x Size 1 t, p | R2 |
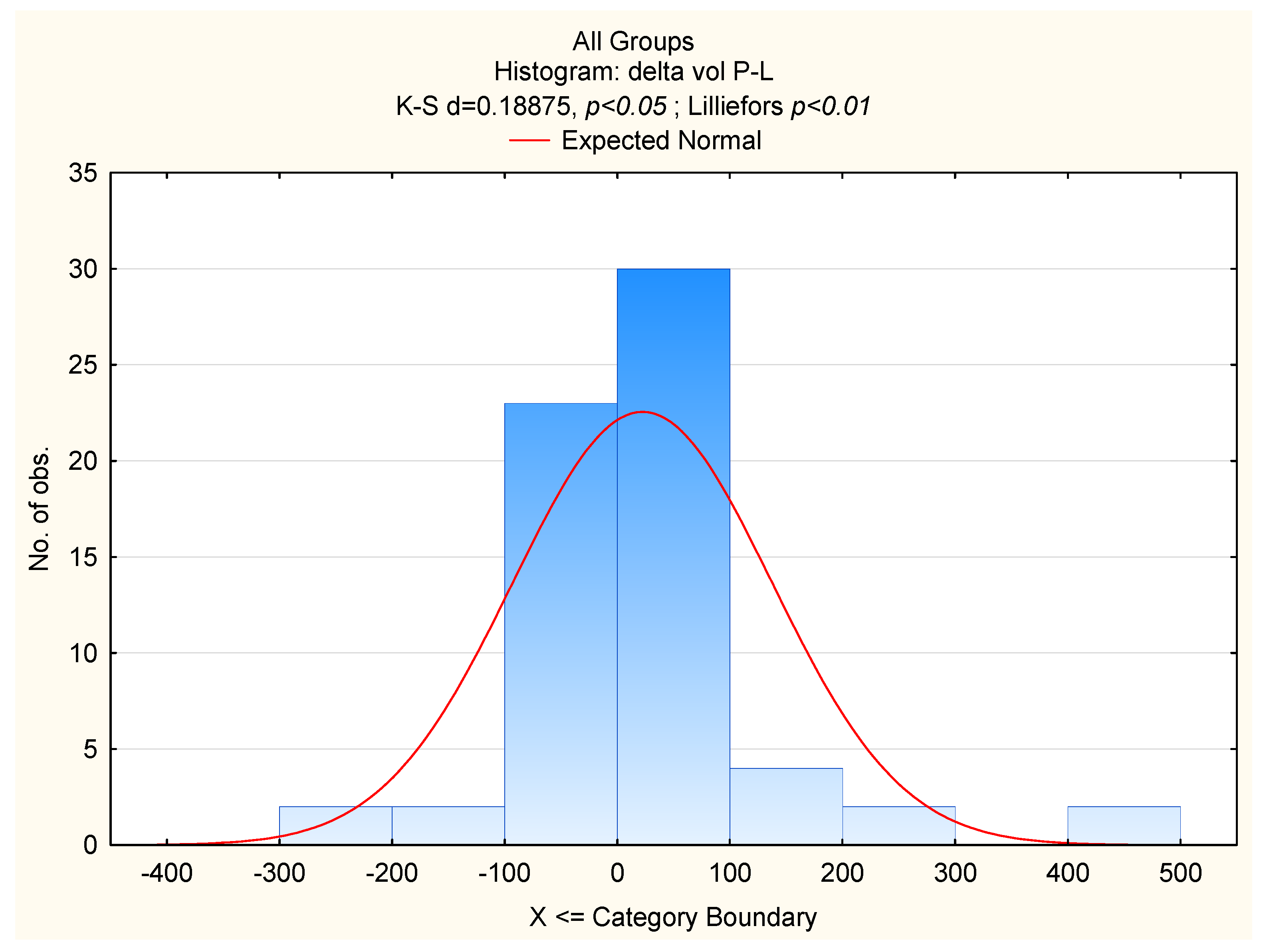
| delta V | 20.45, <0.001 | 0.13, 0.9 | 5.17, <0.001 | 0.4 | 3.43, 0.047 | −2.28, 0.03 | 1.52, 0.14 | 0.2 | 5.08, 0.012 | 1.08, 0.29 | 2.32, 0.027 | 0.24 |
| delta nip-nip’ | 9.08, <0.001 | −1.13, 0.26 | 4.02, <0.001 | 0.23 | 5.82, 0.021 | - | 2.41, 0.021 | 0.15 | 3.14, 0.087 | - | - | - |
| delta nip-ml * | 2.89, 0.06 | - | 2.42, 0.02 ** | 0.08 | 0.17, 0.84 | - | - | - | 0.16, 0.85 | - | - | - |
| delta sn-nip * | 12.56, <0.001 | 0.41, 0.68 | 3.86, 0.0003 | 0.29 | 0.54, 0.59 | - | - | - | 2.77, 0.08 | - | 2.31, 0.03 ** | 0.14 |
| FA | Normal Breasts n = 30 | Macromastia/ Gigantomastia n = 35 | Mann–Whitney Z | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Q1; Q3 | Mean | SD | Median | Q1; Q3 | |||
| delta V [cc] | 24.83 | 19.72 | 20.56 | 10.84; 31.77 | 108.4 | 113.44 | 71.55 | 24.91; 160.25 | 4.283 | <0.0001 |
| delta V/mean V [%] | 10.1 | 7.02 | 9.26 | 3.68; 14.68 | 11.36 | 9.53 | 9.26 | 3.50; 16.48 | 0.217 | 0.828 |
| delta nip-nip’ [cm] | 0.7 | 0.46 | 0.57 | 0.37; 1.02 | 1.79 | 1.54 | 1.61 | 0.42; 2.55 | 2.967 | 0.003 |
| delta sn-nip [cm] | 0.51 | 0.37 | 0.47 | 0.18; 0.86 | 1.55 | 1.40 | 1.17 | 0.40; 2.53 | 3.323 | 0.001 |
| delta nip-ml [cm] | 0.69 | 0.57 | 0.60 | 0.34; 0.93 | 1.08 | 0.70 | 1.01 | 0.54; 1.47 | 2.316 | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasielska-Trojan, A.; Zawadzki, T.; Antoszewski, B. Breast Fluctuating Asymmetry in Women with Macromastia/Gigantomastia. Int. J. Environ. Res. Public Health 2022, 19, 16895. https://doi.org/10.3390/ijerph192416895
Kasielska-Trojan A, Zawadzki T, Antoszewski B. Breast Fluctuating Asymmetry in Women with Macromastia/Gigantomastia. International Journal of Environmental Research and Public Health. 2022; 19(24):16895. https://doi.org/10.3390/ijerph192416895
Chicago/Turabian StyleKasielska-Trojan, Anna, Tomasz Zawadzki, and Bogusław Antoszewski. 2022. "Breast Fluctuating Asymmetry in Women with Macromastia/Gigantomastia" International Journal of Environmental Research and Public Health 19, no. 24: 16895. https://doi.org/10.3390/ijerph192416895





