S100B Maternal Blood Levels in Gestational Diabetes Mellitus Are Birthweight, Gender and Delivery Mode Dependent
Abstract
:1. Introduction
2. Materials and Methods
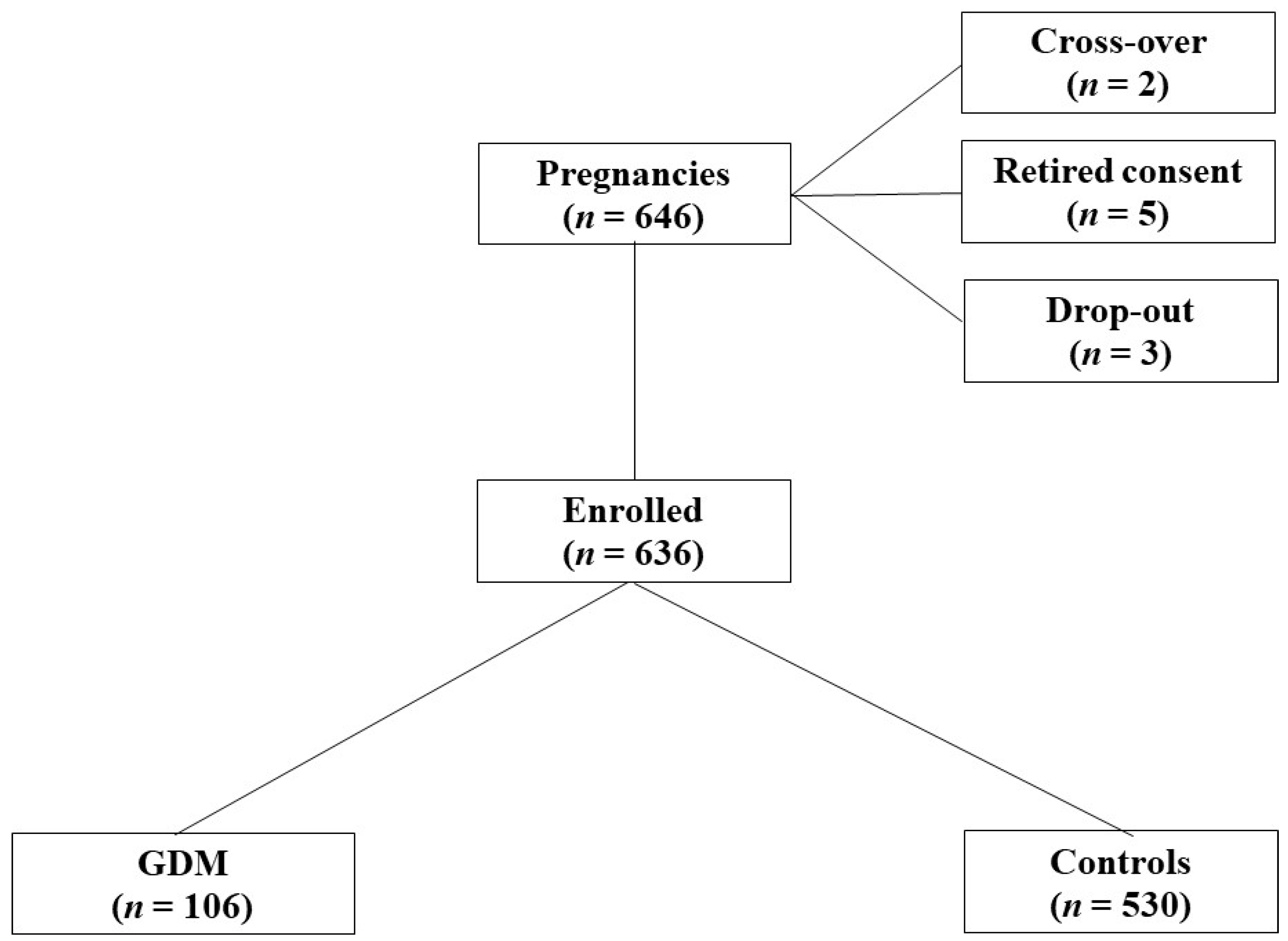
2.1. Population
2.2. S100B Measurement
2.3. Neurological Examination
2.4. Monitoring Parameters
3. Statistical Analysis
4. Results
4.1. S100B Protein Measurements
4.1.1. S100B and Gender
4.1.2. S100B and Delivery Mode
4.1.3. S100B and Neurological Outcome
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Zhang, C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: A global perspective. Curr. Diabetes Rep. 2016, 16, 1–11. [Google Scholar] [CrossRef]
- HAPO Study Cooperative Research Group; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef] [Green Version]
- Bourbon, J.R.; Farrell, P.M. Fetal lung development in the diabetic pregnancy. Pediatr. Res. 1985, 19, 253–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeMarini, S.; Mimouni, F.; Tsang, R.C.; Khoury, J.; Hertzberg, V. Impact of metabolic control of diabetes during pregnancy on neonatal hypocalcemia. Obstet. Gynecol. 1994, 83, 918–922. [Google Scholar] [CrossRef]
- Van den Heuvel, J.F.M.; Groenhof, T.K.; Veerbeek, J.H.; Van Solinge, W.W.; Lely, A.T.; Franx, A.; Bekker, M.N. eHealth as the Next-Generation perinatal care: An overview of the literature. J. Med. Internet Res. 2018, 20, e202. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.A.; Silver, R.M. Perinatal asphyxia from the obstetric standpoint. Clin. Perinatol. 2016, 43, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Michetti, F.; Corvino, V.; Geloso, M.C.; Lattanzi, W.; Bernardini, C.; Serpero, L.; Gazzolo, D. The S100B protein in biological fluids: More than a lifelong biomarker of brain distress. J. Neurochem. 2012, 120, 644–659. [Google Scholar] [CrossRef]
- Bersani, I.; Pluchinotta, F.; Dotta, A.; Savarese, I.; Campi, F.; Auriti, C.; Chuklantseva, N.; Piersigilli, F.; Gazzolo, F.; Varrica, A.; et al. Early predictors of perinatal brain damage: The role of neurobiomarkers. Clin. Chem. Lab. Med. 2020, 58, 471–486. [Google Scholar] [CrossRef] [Green Version]
- Gazzolo, D.; Bruschettini, M.; Corvino, V.; Oliva, R.; Sarli, R.; Lituania, M.; Bruschettini, P.; Michetti, F. S100B protein concentrations in amniotic fluid correlate with gestational age and with cerebral ultrasound scanning results in healthy fetuses. Clin. Chem. 2001, 47, 954–956. [Google Scholar] [CrossRef] [Green Version]
- Gazzolo, D.; Vinesi, P.; Marinoni, E.; Di Iorio, R.; Marras, M.; Lituania, M.; Bruschettini, P.; Michetti, F. S100B protein concentrations in cord blood: Correlations with gestational age in term and preterm deliveries. Clin. Chem. 2000, 46, 998–1000. [Google Scholar] [CrossRef]
- Gazzolo, D.; Bruschettini, M.; Lituania, M.; Serra, G.; Gandullia, E.; Michetti, F. S100B protein in urine is correlated with gestational age in healthy preterm and term newborns. Clin. Chem. Lab. Med. 2001, 47, 1132–1133. [Google Scholar] [CrossRef] [Green Version]
- Gazzolo, D.; Lituania, M.; Bruschettini, M.; Ciotti, S.; Sacchi, R.; Serra, G.; Calevo, M.G.; Corvino, V.; Buonocore, G.; Michetti, F. S100B protein levels in saliva: Correlation with gestational age in normal term and preterm newborns. Clin. Biochem. 2005, 38, 229–233. [Google Scholar] [CrossRef]
- Gazzolo, D.; Grutzfeld, D.; Michetti, F.; Toesca, A.; Lituania, M.; Bruschettini, M.; Dobrzanska, A.; Bruschettini, P. Increased S100B in cerebrospinal fluid of infants with bacterial meningitis: Relationship to brain damage and routine cerebrospinal fluid findings. Clin. Chem. 2004, 50, 941–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gazzolo, D.; Di Iorio, R.; Marinoni, E.; Masetti, P.; Serra, G.; Giovannini, L.; Michetti, F. S100B protein is increased in asphyxiated term infants developing intraventricular hemorrhage. Crit. Care Med. 2002, 30, 1356–1360. [Google Scholar] [CrossRef] [PubMed]
- Florio, P.; Michetti, F.; Bruschettini, M.; Lituani, M.; Bruschettini, P.; Severi, F.M.; Petraglia, F.; Gazzolo, D. Amniotic fluid S100B protein in mid-gestation and intrauterine fetal death. Lancet 2004, 364, 270–272. [Google Scholar] [CrossRef]
- Gazzolo, D.; Marinoni, E.; Di Iorio, R.; Bruschettini, M.; Kornacka, M.; Lituania, M.; Majewska, U.; Serra, G.; Michetti, F. Measurement of urinary S100B protein concentrations for the early identification of brain damage in asphyxiated full-term infants. Arch. Pediatr. Adolesc. Med. 2003, 157, 1163–1168. [Google Scholar] [CrossRef]
- Gazzolo, D.; Bruschettini, M.; Lituania, M.; Serra, G.; Bonacci, W.; Michetti, F. Increased urinary S100B protein as an early indicator of intraventricular hemorrhage in preterm infants: Correlation with the grade of hemorrhage. Clin. Chem. Lab. Med. 2001, 47, 1836–1838. [Google Scholar] [CrossRef]
- Gazzolo, D.; Marinoni, E.; Di Iorio, R.; Bruschettini, M.; Kornacka, M.; Lituania, M.; Majewska, U.; Serra, G.; Michetti, F. Urinary S100B protein measurements: A tool for the early identification of hypoxic-ischemic encephalopathy in asphyxiated full-term infants. Crit. Care Med. 2004, 32, 131–136. [Google Scholar] [CrossRef]
- Gazzolo, D.; Frigiola, A.; Bashir, M.; Iskander, I.; Mufeed, H.; Aboulgar, H.; Venturini, P.; Marras, M.; Serra, G.; Frulio, R.; et al. Diagnostic accuracy of S100B urinary testing at birth in full-term asphyxiated newborns to predict neonatal death. PLoS ONE 2009, 4, e4298. [Google Scholar] [CrossRef]
- Gazzolo, D.; Pluchinotta, F.R.; Bashir, M.; Aboulgar, H.; Said, H.M.; Iman, I.; Ivani, G.; Conio, A.; Tina, L.G.; Nigro, F.; et al. Neurological abnormalities in full-term asphyxiated newborns and salivary S100B testing: The “Cooperative Multitask against Brain Injury of Neonates” (CoMBINe) International Study. PLoS ONE 2015, 10, e0115194. [Google Scholar] [CrossRef]
- Gazzolo, D.; Marinoni, E.; Di Iorio, R.; Lituania, M.; Marras, M.; Bruschettini, M.; Bruschettini, P.; Frulio, R.; Michetti, F.; Petraglia, F.; et al. High maternal blood S100B concentrations in pregnancies complicated by intrauterine growth restriction and intraventricular hemorrhage. Clin. Chem. 2006, 52, 819–826. [Google Scholar] [CrossRef]
- Sannia, A.; Zimmermann, L.J.; Gavilanes, A.W.; Vles, H.J.; Serpero, L.D.; Frulio, R.; Michetti, F.; Gazzolo, D. S100B Protein maternal and fetal bloodstreams gradient in healthy and small for gestational age pregnancies. Clin. Chim. Acta 2011, 412, 1337–1340. [Google Scholar] [CrossRef]
- Serpero, L.D.; Bianchi, V.; Pluchinotta, F.; Conforti, E.; Baryshnikova, E.; Guaschino, R.; Cassinari, M.; Trifoglio, O.; Calevo, M.G.; Gazzolo, D. S100B maternal blood levels are gestational age- and gender-dependent in healthy pregnancies. Clin. Chem. Lab. Med. 2017, 55, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Thoms, A. Ultrasound measurement of the fetal head to abdomen circumference ratio in the assessment of growth retardation. Br. J. Obstet. Gynecol. 2000, 182, 650–654. [Google Scholar] [CrossRef]
- Villar, J.; Ismail, L.C.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Chiefari, E.; Arcidiacono, B.; Foti, D.; Brunetti, A. Gestational diabetes mellitus: An updated overview. J. Endocrinol. Investig. 2017, 40, 899–909. [Google Scholar] [CrossRef]
- Prechtl, H.F.R. Assessment methods for the newborn infant: A critical evaluation. In Psychobiology of the Human Newborn, 3rd ed.; Stratton, D., Ed.; Wiley: Chichester, UK, 1982; pp. 21–52. [Google Scholar]
- Bellissima, V.; Visser, G.; Ververs, T.; Bel, F.; Termote, J.; Heide, M.; Gazzolo, D. Antenatal maternal antidepressants drugs affect S100B concentrations in Fetal-Maternal biological fluids. CNS Neurol. Disord. Drug Targets 2015, 14, 49–54. [Google Scholar] [CrossRef]
- Bellissima, V.; Visser, G.H.; Ververs, T.; Pluchinotta, F.; Varrica, A.; Baryshnikova, E.; Tina, L.G.; Nigro, F.; Gavilanes, A.W.; Godos, J.; et al. Antenatal maternal antidepressants drugs treatment affects S100B levels in maternal-fetal biological fluids in a dose dependent manner. Clin. Chim. Acta 2020, 501, 20–26. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Ferreira, A.; Van Eldik, L.J. S100β Induces neuronal cell death through Nitric Oxide release from Astrocytes. J. Neurochem. 1997, 69, 2294–2301. [Google Scholar] [CrossRef]
- Schulpis, K.H.; Margeli, A.; Akalestos, A.; Vlachos, G.; Partsinevelos, G.A.; Papastamataki, M.; Antsaklis, A.; Papassotiriou, I. Effects of mode of delivery on maternal–neonatal plasma antioxidant status and on protein S100B serum concentrations. Scand. J. Clin. Lab. Investig. 2006, 66, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Garnier, Y.; Frigiola, A.; Volti, G.L.; Florio, P.; Frulio, R.; Berger, R.; Alm, S.; Von Duering, M.U.; Coumans, A.B.; Reis, F.M.; et al. Increased Maternal/Fetal blood S100B levels following systemic endotoxin administration and periventricular white matter injury in preterm fetal sheep. Reprod. Sci. 2009, 16, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Giussani, D.A.; Thakor, A.S.; Frulio, R.; Gazzolo, D. Acute Hypoxia Increases S100β protein in association with blood flow redistribution away from peripheral circulations in fetal sheep. Pediatr. Res. 2005, 58, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gazzolo, D.; Michetti, F.; Bruschettini, M.; Marchese, N.; Lituania, M.; Mangraviti, S.; Pedrazzi, E.; Bruschettini, P. Pediatric Concentrations of S100B protein in blood: Age- and Sex-related changes. Clin. Chem. 2003, 49, 967–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hüppi, P.; Warfield, S.; Kikinis, R.; Barnes, P.D.; Zientara, G.P.; Jolesz, F.A.; Tsuji, M.K.; Volpe, J.J. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann. Neurol. 1998, 43, 224–235. [Google Scholar] [CrossRef]
- Guihard-Costa, A.-M.; Larroche, J.-C. Differential growth between the fetal brain and its infratentorial part. Early Hum. Dev. 1990, 23, 27–40. [Google Scholar] [CrossRef]
- Haynes, R.L.; Borenstein, N.S.; Desilva, T.M.; Folkerth, R.D.; Liu, L.G.; Volpe, J.J.; Kinney, H.C. Axonal development in the cerebral white matter of the human fetus and infant. J. Comp. Neurol. 2005, 484, 156–167. [Google Scholar] [CrossRef]
- Back, S.A.; Luo, N.L.; Borenstein, N.S.; Levine, J.M.; Volpe, J.J.; Kinney, H.C. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J. Neurosci. 2001, 21, 1302–1312. [Google Scholar] [CrossRef]
- Tina, L.G.; Frigiola, A.; Abella, R.; Artale, B.; Puleo, G.; D’Angelo, S.; Musmarra, C.; Tagliabue, P.; Volti, G.L.; Florio, P.; et al. Near infrared spectroscopy in healthy preterm and term newborns: Correlation with gestational age and standard monitoring parameters. Curr. Neurovasc. Res. 2009, 6, 148–154. [Google Scholar] [CrossRef]
- Steiner, J.; Bernstein, H.-G.; Schiltz, K.; Haase, T.; Meyer-Lotz, G.; Dobrowolny, H.; Müller, U.J.; Martins-De-Souza, D.; Borucki, K.; Schroeter, M.L.; et al. Decrease of serum S100B during an oral glucose tolerance test correlates inversely with the insulin response. Psychoneuroendocrinology 2014, 39, 33–38. [Google Scholar] [CrossRef]



| Parameters | GDM (n = 106) | Controls (n = 530) | p |
|---|---|---|---|
| Maternal age, (years) | 24.7 ± 3.9 | 25.6 ± 4.4 | NS |
| Delivery mode, n/total | |||
| Caesarean section | 38/106 | 117/530 | NS |
| Vaginal | 58/106 | 413/530 | NS |
| Gestational age (weeks) | 38 ± 2 | 39 ± 1 * | <0.001 |
| Birth weight (g) | 3019 ± 167 | 2902 ± 190 * | <0.001 |
| Gender male/female | 57/59 | 261/269 | 0.006 |
| Apgar score > 7, n/total | |||
| at 1 min | 104/106 | 530/530 | NS |
| at 5 min | 106/106 | 530/530 | NS |
| pH > 7.20, n/total | 106/106 | 530/530 | NS |
| pCO2, mmHg | 41.2 ± 2.7 | 44.2 ± 2.1 | <0.001 |
| pO2, mmHg | 43.2 ± 0.6 | 41.4 ± 1.8 | <0.001 |
| BE | 3.2 ± 0.7 | 2.2 ± 0.9 | <0.001 |
| RBC count, 1012/L | 4.1 ± 0.1 | 4.2 ± 0.2 | <0.001 |
| Hb, g/L | 143 ± 3 | 142 ± 2 | <0.001 |
| Ht (%) | 42.4 ± 0.5 | 41.5 ± 0.4 | <0.001 |
| Plasma glucose, mmol/L | 2.2 ± 0.2 | 4.2 ± 0.3 * | <0.001 |
| Na+, mmol/L | 23 ± 0.7 | 23 ± 0.7 | NS |
| Ca++, mmol/L | 0.2 ± 0.03 | 0.4 ± 0.02 * | <0.001 |
| K+, mmol/L | 0.7 ± 0.06 | 0.8 ± 0.07 | <0.001 |
| Neurological examination | |||
| Normal/ Suspect/Abnormal | 70/36/0 | 530/0/0 * | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abella, L.; D’Adamo, E.; Strozzi, M.; Sanchez-de-Toledo, J.; Perez-Cruz, M.; Gómez, O.; Abella, E.; Cassinari, M.; Guaschino, R.; Mazzucco, L.; et al. S100B Maternal Blood Levels in Gestational Diabetes Mellitus Are Birthweight, Gender and Delivery Mode Dependent. Int. J. Environ. Res. Public Health 2022, 19, 1028. https://doi.org/10.3390/ijerph19031028
Abella L, D’Adamo E, Strozzi M, Sanchez-de-Toledo J, Perez-Cruz M, Gómez O, Abella E, Cassinari M, Guaschino R, Mazzucco L, et al. S100B Maternal Blood Levels in Gestational Diabetes Mellitus Are Birthweight, Gender and Delivery Mode Dependent. International Journal of Environmental Research and Public Health. 2022; 19(3):1028. https://doi.org/10.3390/ijerph19031028
Chicago/Turabian StyleAbella, Laura, Ebe D’Adamo, Mariachiara Strozzi, Joan Sanchez-de-Toledo, Miriam Perez-Cruz, Olga Gómez, Ernesto Abella, Maurizio Cassinari, Roberto Guaschino, Laura Mazzucco, and et al. 2022. "S100B Maternal Blood Levels in Gestational Diabetes Mellitus Are Birthweight, Gender and Delivery Mode Dependent" International Journal of Environmental Research and Public Health 19, no. 3: 1028. https://doi.org/10.3390/ijerph19031028
APA StyleAbella, L., D’Adamo, E., Strozzi, M., Sanchez-de-Toledo, J., Perez-Cruz, M., Gómez, O., Abella, E., Cassinari, M., Guaschino, R., Mazzucco, L., Maconi, A., Testa, S., Zanelli, C., Perrotta, M., Roberta, P., Renata, N. C., Gasparroni, G., Vitacolonna, E., Chiarelli, F., & Gazzolo, D. (2022). S100B Maternal Blood Levels in Gestational Diabetes Mellitus Are Birthweight, Gender and Delivery Mode Dependent. International Journal of Environmental Research and Public Health, 19(3), 1028. https://doi.org/10.3390/ijerph19031028







