Outpatient Dental Treatment Expenditure for Patients with Oromaxillofacial Cancer: A Cohort Study in Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Case Definition of Oromaxillofacial Cancer
2.3. Definition of Outcome
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
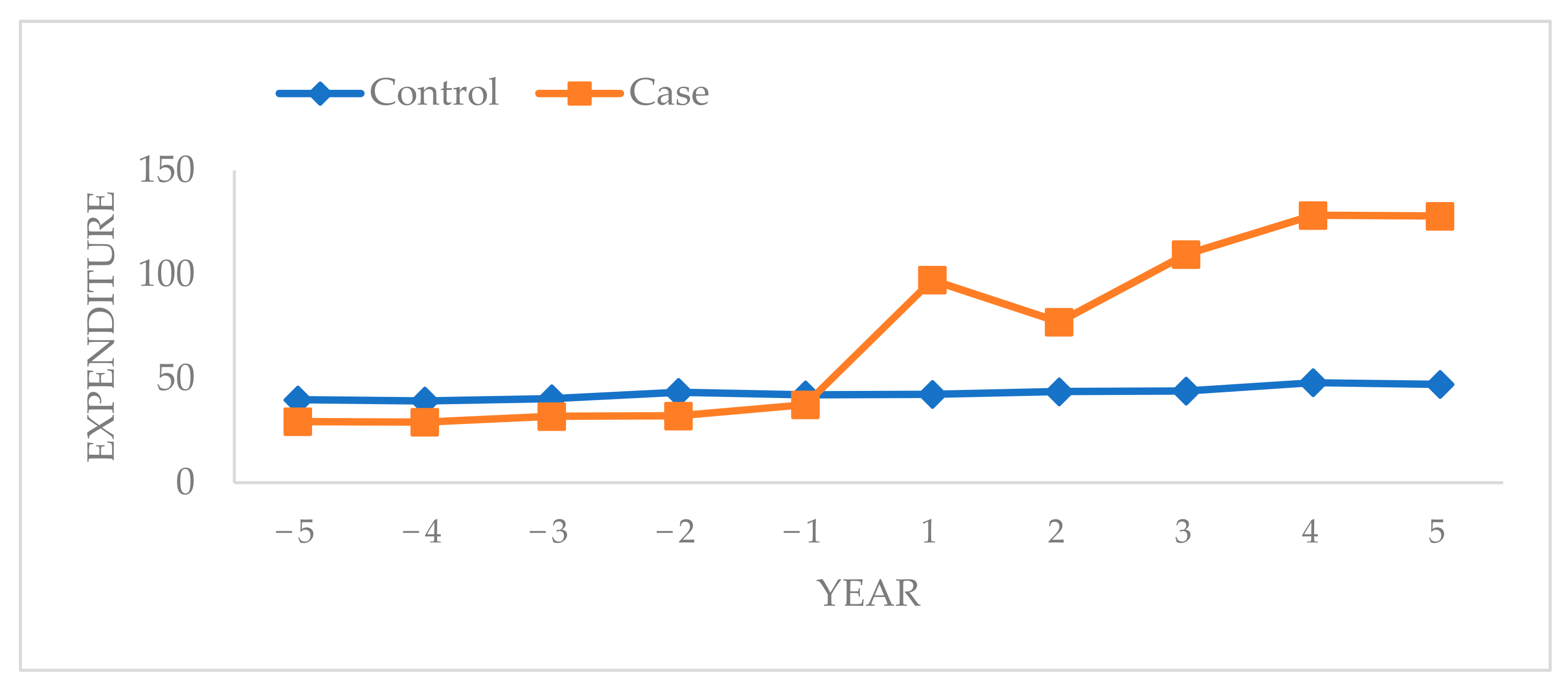
- The average annual expenditure was higher for male and female patients with oromaxillofacial cancer after diagnosis than before diagnosis.
- Among the patients diagnosed as having oromaxillofacial cancer, 88.67% were male, and most were aged 40–59 years with low income.
- The average annual expenditure began to increase 1 year before oromaxillofacial cancer diagnosis in the case group and was two times higher in the first year after diagnosis compared with that before diagnosis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saka-Herran, C.; Jane-Salas, E.; Mari-Roig, A.; Estrugo-Devesa, A.; Lopez-Lopez, J. Time-to-treatment in oral cancer: Causes and implications for survival. Cancers 2021, 13, 1321. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Chen, H.M.; Liao, K.H.; Ko, B.S.; Hsiao, F.Y. Economic burden of cancers in Taiwan: A direct and indirect cost estimate for 2007–2017. BMJ Open 2020, 10, e036341. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Hung, L.C.; Kung, P.T.; Lung, C.H.; Tsai, M.H.; Liu, S.A.; Chiu, L.T.; Huang, K.H.; Tsai, W.C. Assessment of the risk of oral cancer incidence in a high-risk population and establishment of a predictive model for oral cancer incidence using a population-based cohort in Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 665. [Google Scholar] [CrossRef] [PubMed]
- Irani, S. New insights into oral cancer-risk factors and prevention: A review of literature. Int. J. Prev. Med. 2020, 11, 202. [Google Scholar] [CrossRef]
- D’Addazio, G.; Artese, L.; Traini, T.; Rubini, C.; Caputi, S.; Sinjari, B. Immunohistochemical study of osteopontin in oral squamous cell carcinoma allied to fractal dimension. J. Biol. Regul. Homeost. Agents 2018, 32, 1033–1038. [Google Scholar]
- Sankaranarayanan, R.; Ramadas, K.; Amarasinghe, H.; Subramanian, S.; Johnson, N. Oral cancer: Prevention, early detection, and treatment. In Cancer: Disease Control Priorities, 3rd ed.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2015; Volume 3, p. 85. [Google Scholar]
- Milani, V.; Zara, A.; da Silva, E.N.; Cardoso, L.B.; Curado, M.P.; Ribeiro-Rotta, R.F. Direct healthcare costs of lip, oral cavity and oropharyngeal cancer in Brazil. PLoS ONE 2021, 16, e0246475. [Google Scholar] [CrossRef]
- Chiu, S.F.; Ho, C.H.; Chen, Y.C.; Wu, L.W.; Chen, Y.L.; Wu, J.H.; Wu, W.S.; Hung, H.K.; Chiang, W.F. Malignant transformation of oral potentially malignant disorders in Taiwan: An observational nationwide population database study. Medicine 2021, 100, e24934. [Google Scholar] [CrossRef]
- Raman, S.; Shafie, A.A.; Abraham, M.T.; Shim, C.K.; Maling, T.H.; Rajendran, S.; Cheong, S.C. Provider cost of treating oral potentially malignant disorders and oral cancer in Malaysian public hospitals. PLoS ONE 2021, 16, e0251760. [Google Scholar] [CrossRef]
- World Health Organization, International Agency for Research on Cancer. World Cancer Report; International Agency: Lyon, France, 2014. [Google Scholar]
- Sung, S.F.; Hsieh, C.Y.; Hu, Y.H. Two decades of research using Taiwan’s national health insurance claims data: Bibliometric and text mining analysis on pubmed. J. Med. Internet Res. 2020, 22, e18457. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.J.; Lo, W.C.; Yang, Y.W.; You, S.L.; Chen, C.J.; Lai, M.S. Incidence and survival of adult cancer patients in Taiwan, 2002–2012. J. Formos. Med. Assoc. 2016, 115, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Tagay, S.; Herpertz, S.; Langkafel, M.; Erim, Y.; Bockisch, A.; Senf, W.; Gorges, R. Health-related quality of life, depression and anxiety in thyroid cancer patients. Qual Life Res 2006, 15, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Bennette, C.S.; Richards, C.; Sullivan, S.D.; Ramsey, S.D. Steady increase in prices for oral anticancer drugs after market launch suggests a lack of competitive pressure. Health. Aff. 2016, 35, 805–812. [Google Scholar] [CrossRef]
- Subramanian, S.; Sankaranarayanan, R.; Bapat, B.; Somanathan, T.; Thomas, G.; Mathew, B.; Vinoda, J.; Ramadas, K. Cost-effectiveness of oral cancer screening: Results from a cluster randomized controlled trial in India. Bull. World Health Organ. 2009, 87, 200–206. [Google Scholar] [CrossRef]
- Amarasinghe, H.; Jayasinghe, R.D.; Dharmagunawardene, D.; Attygalla, M.; Scuffham, P.A.; Johnson, N.; Kularatna, S. Economic burden of managing oral cancer patients in Sri Lanka: A cross-sectional hospital -based costing study. BMJ Open 2019, 9, e027661. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, S.; Bilker, W.B.; Berlin, J.A.; Strom, B.L. Factors influencing the optimal control-to-case ratio in matched case-control studies. Am. J. Epidemiol. 1999, 149, 195–197. [Google Scholar] [CrossRef]
- Liu, C.Y.; Hung, Y.T.; Chuang, Y.L.; Chen, Y.J.; Weng, W.S.; Liu, J.S.; Liang, K.Y. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. Int. J. Environ. Res. Public Health 2006, 4, 1–22. [Google Scholar]
- Conway, D.I.; Purkayastha, M.; Chestnutt, I.G. The changing epidemiology of oral cancer: Definitions, trends, and risk factors. Br. Dent. J. 2018, 225, 867–873. [Google Scholar] [CrossRef]
- Cheong, S.C.; Vatanasapt, P.; Yi-Hsin, Y.; Zain, R.B.; Kerr, A.R.; Johnson, N.W. Oral cancer in southeast Asia. Transl. Res. Oral Oncol. 2017, 2. [Google Scholar] [CrossRef]
- Moore, S.; Johnson, N.; Pierce, A.; Wilson, D. The epidemiology of mouth cancer: A review of global incidence. Oral Dis. 2000, 6, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Cruz, G.D.; Ostroff, J.S.; Kumar, J.V.; Gajendra, S. Preventing and detecting oral cancer. Oral health care providers’ readiness to provide health behavior counseling and oral cancer examinations. J. Am. Dent. Assoc. 2005, 136, 594–601. [Google Scholar] [CrossRef]
- Han, S.; Chen, Y.; Ge, X.; Zhang, M.; Wang, J.; Zhao, Q.; He, J.; Wang, Z. Epidemiology and cost analysis for patients with oral cancer in a university hospital in China. BMC Public Health 2010, 10, 196. [Google Scholar] [CrossRef]
- Anwar, N.; Pervez, S.; Chundriger, Q.; Awan, S.; Moatter, T.; Ali, T.S. Oral cancer: Clinicopathological features and associated risk factors in a high risk population presenting to a major tertiary care center in Pakistan. PLoS ONE 2020, 15, e0236359. [Google Scholar] [CrossRef]
- Shih, Y.-C.T.; Xu, Y.; Liu, L.; Smieliauskas, F. Rising prices of targeted oral anticancer medications and associated financial burden on medicare beneficiaries. J. Clin. Oncol. 2017, 35, 2482–2489. [Google Scholar] [CrossRef]
- Pollaers, K.; Massingham, I.; Friedland, P.L.; Farah, C.S. The economic burden of oral squamous cell carcinoma in Australia. J. Oral Pathol. Med. 2019, 48, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.C.; Lin, H.W.; Yang, J.H.; Lin, P.Y.; Cheng, S.J.; Wu, Y.H.; Kuo, Y.S. Clinical outcomes of oral cancer patients who survive for more than 5 years in Taiwan. J. Formos. Med. Assoc. 2019, 118, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Meropol, N.J.; Schulman, K.A. Cost of cancer care: Issues and implications. J. Clin. Oncol. 2007, 25, 180–186. [Google Scholar] [CrossRef]
- Zavras, A.; Andreopoulos, N.; Katsikeris, N.; Zavras, N.; Cartsos, V.; Vamvakidis, A. Oral cancer treatment costs in Greece and the effect of advanced disease. BMC Public Health 2002, 2, 12. [Google Scholar] [CrossRef][Green Version]
- Decker, A.M.; Taichman, L.S.; D’Silva, N.J.; Taichman, R.S. Periodontal treatment in cancer patients: An interdisciplinary approach. Curr. Oral Health Rep. 2018, 5, 7–12. [Google Scholar] [CrossRef]
| Controls n = 38,655 | Cases n = 7731 | |
|---|---|---|
| Age | 54.92 ± 13.21 | 54.92 ± 13.21 |
| 0–19 | 110 (0.28) | 22 (0.28) |
| 20–39 | 4240 (10.97) | 848 (10.97) |
| 40–59 | 21,634 (55.97) | 4327 (55.97) |
| 60–79 | 11,319 (29.28) | 2264 (29.28) |
| ≥80 | 1352 (3.50) | 270 (3.49) |
| Sex | ||
| Male | 34,275 (88.67) | 6855 (88.67) |
| Female | 4380 (11.33) | 876 (11.33) |
| Urban level | ||
| Highest | 12,830 (33.19) | 2566 (33.19) |
| High | 9040 (23.39) | 1808 (23.39) |
| Middle | 5695 (14.73) | 1139 (14.73) |
| Low | 5600 (14.49) | 1120 (14.49) |
| Lowest | 5490 (14.20) | 1098 (14.20) |
| Income | ||
| <360 | 11,990 (31.02) | 2398 (31.02) |
| 360–720 | 17,645 (45.65) | 3529 (45.65) |
| >720–1080 | 3705 (9.58) | 741 (9.58) |
| >1080–1440 | 2140 (5.54) | 428 (5.54) |
| >1440–1800 | 1780 (4.6) | 356 (4.60) |
| >1800 | 1395 (3.61) | 279 (3.61) |
| Controls n = 38,655 | Cases n = 7731 | |
|---|---|---|
| Average expenditure | ||
| Before index day | ||
| 5 years | 39.81 | 29.35 |
| 4 years | 39.27 | 29.14 |
| 3 years | 40.41 | 31.86 |
| 2 years | 43.47 | 32.10 |
| 1 year | 42.24 | 37.52 |
| After index day | ||
| 1 year | 42.46 | 97.34 |
| 2 years | 43.76 | 77.23 |
| 3 years | 44.06 | 109.65 |
| 4 years | 48.08 | 128.43 |
| 5 years | 47.22 | 128.03 |
| Before | After | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 Years | 4 Years | 3 Years | 2 Years | 1 Year | 1 Year | 2 Years | 3 Years | 4 Years | 5 Years | |
| Age (years) | ||||||||||
| 0–19 | 56.56 | 49.39 | 19.21 | 142.40 | 156.75 | 146.61 | 181.37 | 285.39 | 164.29 | 216.23 |
| 20–39 | 50.93 | 43.15 | 49.77 | 61.16 | 61.52 | 234.04 | 104.04 | 124.67 | 129.38 | 146.66 |
| 40–59 | 50.86 | 54.54 | 58.16 | 57.44 | 71.67 | 197.78 | 126.05 | 140.68 | 157.35 | 140.72 |
| 60–79 | 88.77 | 86.08 | 96.82 | 91.63 | 102.71 | 198.34 | 93.73 | 121.80 | 110.36 | 89.83 |
| ≥80 | 161.48 | 110.27 | 123.99 | 114.60 | 122.56 | 99.90 | 69.90 | 39.50 | 22.29 | 21.57 |
| Sex | ||||||||||
| Male | 27.86 | 26.13 | 30.49 | 29.59 | 36.21 | 96.01 | 76.58 | 107.17 | 127.11 | 126.36 |
| Female | 41.04 | 52.63 | 42.55 | 51.81 | 47.73 | 107.70 | 81.70 | 125.50 | 136.42 | 137.82 |
| Treatment | ||||||||||
| Operative dentistry | 14.49 | 13.77 | 15.96 | 15.43 | 16.11 | 28.24 | 32.63 | 38.47 | 40.25 | 39.79 |
| Endodontics | 9.52 | 9.41 | 10.15 | 10.87 | 11.26 | 18.47 | 23.29 | 31.72 | 31.32 | 30.63 |
| Periodontics | 0.40 | 0.39 | 0.34 | 0.39 | 0.57 | 1.34 | 0.87 | 0.95 | 0.99 | 1.14 |
| Oral surgery | 4.95 | 5.56 | 5.40 | 5.41 | 9.58 | 49.28 | 20.44 | 38.51 | 55.87 | 56.47 |
| Age (Years) | Before | After | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 Years | 4 Years | 3 Years | 2 Years | 1 Year | 1 Year | 2 Years | 3 Years | 4 Years | 5 Years | |
| Operative dentistry | ||||||||||
| 0–19 | 40.25 | 31.88 | 19.21 | 128.56 | 105.59 | 30.19 | 158.06 | 158.87 | 153.95 | 141.46 |
| 20–39 | 38.20 | 23.79 | 28.96 | 33.12 | 36.33 | 77.28 | 52.30 | 51.97 | 51.87 | 52.61 |
| 40–59 | 24.70 | 26.72 | 29.76 | 28.96 | 29.22 | 55.15 | 52.44 | 51.00 | 48.12 | 43.55 |
| 60–79 | 36.60 | 36.00 | 45.15 | 37.63 | 40.74 | 56.33 | 34.62 | 32.36 | 27.98 | 22.75 |
| ≥80 | 77.89 | 52.42 | 49.07 | 45.51 | 56.34 | 49.03 | 29.52 | 10.56 | 10.95 | 0.60 |
| Endodontics | ||||||||||
| 0–19 | 16.31 | 14.87 | 0.00 | 12.60 | 43.00 | 0.00 | 23.31 | 87.23 | 10.33 | 68.53 |
| 20–39 | 8.90 | 12.90 | 14.74 | 22.66 | 14.43 | 36.39 | 20.83 | 30.26 | 20.70 | 32.75 |
| 40–59 | 16.81 | 17.21 | 18.69 | 18.71 | 22.09 | 38.55 | 39.91 | 39.64 | 38.55 | 34.21 |
| 60–79 | 33.07 | 29.58 | 31.36 | 33.11 | 32.92 | 40.86 | 30.89 | 41.81 | 34.01 | 21.14 |
| ≥80 | 47.62 | 34.89 | 38.48 | 30.91 | 23.21 | 16.17 | 18.62 | 4.92 | 3.24 | 5.85 |
| Periodontics | ||||||||||
| 0–19 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 20–39 | 0.00 | 0.00 | 0.00 | 0.00 | 0.43 | 2.61 | 2.02 | 0.85 | 0.29 | 1.37 |
| 40–59 | 0.98 | 0.56 | 0.83 | 0.98 | 1.39 | 3.23 | 1.00 | 1.34 | 1.44 | 1.41 |
| 60–79 | 1.11 | 2.01 | 0.93 | 0.97 | 1.00 | 1.77 | 1.49 | 1.00 | 0.71 | 0.41 |
| ≥80 | 0.00 | 0.37 | 0.00 | 0.00 | 4.02 | 2.32 | 2.76 | 0.00 | 0.74 | 0.00 |
| Oral surgery | ||||||||||
| 0–19 | 0.00 | 2.64 | 0.00 | 1.24 | 8.15 | 116.42 | 0.00 | 39.29 | 0.00 | 6.25 |
| 20–39 | 3.83 | 6.46 | 6.07 | 5.37 | 10.34 | 117.75 | 28.89 | 41.59 | 56.52 | 59.94 |
| 40–59 | 8.37 | 10.06 | 8.87 | 8.79 | 18.98 | 100.85 | 32.70 | 48.70 | 69.25 | 61.56 |
| 60–79 | 17.99 | 18.48 | 19.38 | 19.91 | 28.05 | 99.38 | 26.73 | 46.63 | 47.66 | 45.52 |
| ≥80 | 35.98 | 22.59 | 36.44 | 38.17 | 38.99 | 32.39 | 19.00 | 24.03 | 7.35 | 15.13 |
| USD $ | ||||||||||
| After vs. Before | Adjusted OR (95% CI) * | p-Value |
|---|---|---|
| 1 year | 2.23 (2.07–2.40) | <0.0001 |
| 2 years | 1.07 (0.99–1.16) | 0.0797 |
| 3 years | 1.19 (1.09–1.29) | <0.0001 |
| 4 years | 1.09 (1.01–1.18) | <0.0001 |
| 5 years | 1.22 (1.14–1.32) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikbal, M.; Shen, Y.-W.; Liang, W.-M.; Wu, T.-N.; Hsu, J.-T.; Fuh, L.-J. Outpatient Dental Treatment Expenditure for Patients with Oromaxillofacial Cancer: A Cohort Study in Taiwan. Int. J. Environ. Res. Public Health 2022, 19, 1066. https://doi.org/10.3390/ijerph19031066
Ikbal M, Shen Y-W, Liang W-M, Wu T-N, Hsu J-T, Fuh L-J. Outpatient Dental Treatment Expenditure for Patients with Oromaxillofacial Cancer: A Cohort Study in Taiwan. International Journal of Environmental Research and Public Health. 2022; 19(3):1066. https://doi.org/10.3390/ijerph19031066
Chicago/Turabian StyleIkbal, Muhammad, Yen-Wen Shen, Wen-Miin Liang, Trong-Neng Wu, Jui-Ting Hsu, and Lih-Jyh Fuh. 2022. "Outpatient Dental Treatment Expenditure for Patients with Oromaxillofacial Cancer: A Cohort Study in Taiwan" International Journal of Environmental Research and Public Health 19, no. 3: 1066. https://doi.org/10.3390/ijerph19031066
APA StyleIkbal, M., Shen, Y.-W., Liang, W.-M., Wu, T.-N., Hsu, J.-T., & Fuh, L.-J. (2022). Outpatient Dental Treatment Expenditure for Patients with Oromaxillofacial Cancer: A Cohort Study in Taiwan. International Journal of Environmental Research and Public Health, 19(3), 1066. https://doi.org/10.3390/ijerph19031066






