A Community Outbreak of Legionnaires’ Disease with Two Strains of L. pneumophila Serogroup 1 Linked to an Aquatic Therapy Centre
Abstract
:1. Introduction
2. Methods
2.1. Epidemiological and Microbial Investigations
2.2. Environmental Investigation
2.3. Genomic Investigation
3. Results
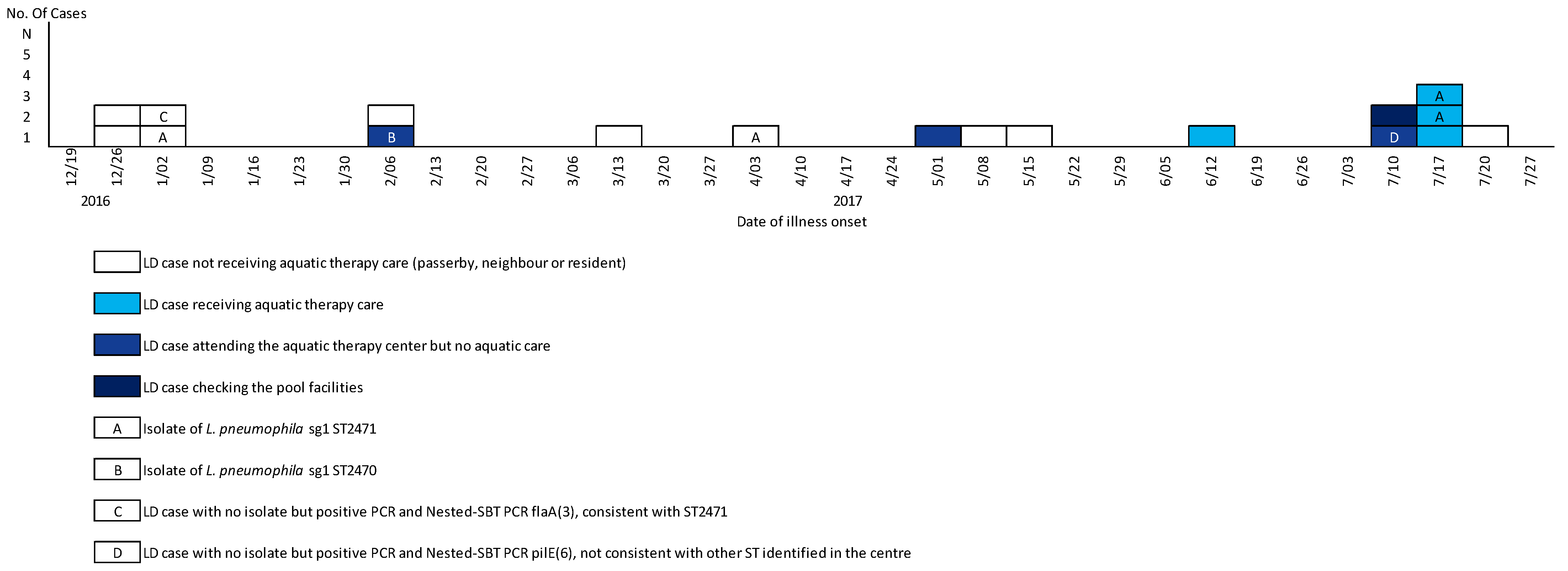
3.1. Epidemiological Results
3.2. Environmental and Microbial Investigation Results
3.2.1. Potential Sources Investigated
3.2.2. Aquatic Therapy Centre
3.3. Genotyping Results
3.3.1. Genotyping of Clinical Samples
3.3.2. Genotyping of Environmental Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cunha, B.A.; Burillo, A.; Bouza, E. Legionnaires’ disease. Lancet 2016, 387, 376–385. [Google Scholar] [CrossRef]
- Phin, N.; Parry-Ford, F.; Harrison, T.; Stagg, H.; Zhang, N.; Kumar, K.; Lortholary, O.; Zumla, P.S.A.; Abubakar, I. Epidemiology and clinical management of Legionnaires’ disease. Lancet Infect. Dis. 2014, 14, 1011–1021. [Google Scholar] [CrossRef]
- Orkis, L.T.; Harrison, L.H.; Mertz, K.J.; Brooks, M.M.; Bibby, K.J.; Stout, J.E. Environmental sources of community-acquired legionnaires’ disease: A review. Int. J. Hyg. Environ. Health 2018, 221, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Campese, C.; Jarraud, S.; Sommen, C.; Maine, C.; Che, D. Legionnaires’ disease in France: Sensitivity of the mandatory notification has improved over the last decade. Epidemiology Infect. 2013, 141, 2644–2649. [Google Scholar] [CrossRef] [PubMed]
- Haupt, T.E.; Heffernan, R.T.; Kazmierczak, J.J.; Nehls-Lowe, H.; Rheineck, B.; Powell, C.; Leonhardt, K.K.; Chitnis, A.S.; Davis, J.P. An Outbreak of Legionnaires Disease Associated with a Decorative Water Wall Fountain in a Hospital. Infect. Control Hosp. Epidemiol. 2012, 33, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ma’ayeh, S.Y.; Al-Hiyasat, A.S.; Hindiyeh, M.Y.; Khader, Y.S. Legionella pneumophila contamination of a dental unit water line system in a dental teaching centre. Int. J. Dent. Hyg. 2008, 6, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Mastro, T.D.; Fields, B.S.; Breiman, R.F.; Campbell, J.; Plikaytis, B.D.; Spika, J.S. Nosocomial Legionnaires’ Disease and Use of Medication Nebulizers. J. Infect. Dis. 1991, 163, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Campèse, C.; Descours, G.; Lepoutre, A.; Beraud, L.; Maine, C.; Che, D.; Jarraud, S. Legionnaires’ disease in France. Med. Mal. Infect. 2015, 45, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Alsibai, S.; Bilo de Bernardi, P.; Janin, C.; Che, D.; Investigation Team; Lee, J.V. Outbreak of legionellosis suspected to be related to a whirlpool spa display 2006, Lorquin, France. Euro Surveill. 2006, 11, 3063. [Google Scholar] [CrossRef] [PubMed]
- Valero, N.; De Simón, M.; Gallés, P.; Izquierdo, N.; Arimon, J.; González, R.; Manzanares-Laya, S.; Avellanes, I.; Gómez, A. Street Cleaning Trucks as Potential Sources of Legionella pneumophila. Emerg. Infect. Dis. 2017, 23, 1880–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, M.; Yakunin, E.; Valinsky, L.; Chalifa-Caspi, V.; Moran-Gilad, J. A bioinformatics tool for ensuring the backwards compatibility of Legionella pneumophila typing in the genomic era. Clin. Microbiol. Infect. 2017, 23, 306–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.; Machado, M.P.; Silva, D.N.; Rossi, M.; Moran-Gilad, J.; Santos, S.; Ramirez, M.; Carriço, J.A. chewBBACA: A complete suite for gene-by-gene schema creation and strain identification. Microb. Genom. 2018, 4, e000166. [Google Scholar] [CrossRef] [PubMed]
- Ginevra, C.; Lopez, M.; Forey, F.; Reyrolle, M.; Meugnier, H.; Vandenesch, F.; Etienne, J.; Jarraud, S.; Molmeret, M. Evaluation of a Nested-PCR-Derived Sequence-Based Typing Method Applied Directly to Respiratory Samples from Patients with Legionnaires’ Disease. J. Clin. Microbiol. 2009, 47, 981–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroki, T.; Amemura-Maekawa, J.; Hoya, H.; Furukawa, I.; Suzuki, M.; Masaoka, T.; Aikawa, K.; Hibi, K.; Morita, M.; Lee, K.; et al. Outbreak of Legionnaire’s Disease Caused by Legionella pneumophila Serogroups 1 and 13. Emerg. Infect. Dis. 2017, 23, 349–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Busó, L.; Eguiral, S.; Ecrespi, S.; Emoya, V.; Camaró, M.L.; Olmos, M.P.; Eadrián, F.; Emorera, V.; Egonzález-Morán, F.; Evanaclocha, H.; et al. Genomic Investigation of a Legionellosis Outbreak in a Persistently Colonized Hotel. Front. Microbiol. 2016, 6, 1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benkel, D.H.; McClure, E.M.; Woolard, D.; Rullan, J.V.; Miller, G.B.; Jenkins, S.R.; Hershey, J.H.; Benson, R.F.; Pruckler, J.M.; Brown, E.W.; et al. Outbreak of Legionnaires’ disease associated with a display whirlpool spa. Int. J. Epidemiol. 2000, 29, 1092–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, K.; Amemura-Maekawa, J.; Ohya, H.; Furukawa, I.; Suzuki, M.; Masaoka, T.; Aikawa, K.; Hibi, K.; Morita, M.; Lee, K.; et al. Outbreak of legionellosis associated with a spa pool, United Kingdom. Emerg. Infect Dis. 2017, 23, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Den Boer, J.W.; Yzerman, P.F.; Schellekens, J.; Lettinga, K.D.; Boshuizen, H.C.; Van Steenbergen, J.E.; Bosman, A.; Van den Hof, S.; Van Vliet, H.A.; Peeters, M.F.; et al. A large outbreak of Legionnaires’ disease at a flower show, the Netherlands, 1999. Emerg. Infect. Dis. 2002, 8, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Date of Illness | Status | Aquatic Therapy Care | L. pneumophila Urinary Antigen Testing | ST Subtyping | Pulsed-Field Gel ElectroPhoresis (PFGE) | Monoclonal Antibodies to Legionella Proteins (Mabs) | Nested-Polymerase Chain Reaction (PCR) |
|---|---|---|---|---|---|---|---|
| 2016-51 | passersby | no | (+) | - | - | - | - |
| 2016-51 | passersby | no | (+) | - | - | - | - |
| 2016-52 | passersby | no | (+) | 2471 | Sporadic ** | FRA/ALL | - |
| 2016-52 | neighbour | no | (+) | consistent 2471 | - | - | flaA(3) |
| 2017-05 | aquatic center patient | yes | (+) | 2470 | Louisa | FRA/ALL | - |
| 2017-05 | resident | no | (+) | - | - | - | - |
| 2017-10 | passerby | no | (+) | - | - | - | - |
| 2017-13 | working in the area | no | (+) | 2471 | Sporadic ** | FRA/ALL | - |
| 2017-18 | passerby | (+) | - | - | - | - | |
| 2017-17 | aquatic center patient | yes | (+) | - | - | - | negative |
| 2017-19 | passerby | no | (+) | - | - | - | ND |
| 2017-23 | aquatic center patient | no aquatic care * | (+) | - | - | - | ND |
| 2017-27 | aquatic center patient | yes | (+) | - | - | - | pilE(6) |
| 2017-27 | aquatic center visitor | facilities inspection | (+) | - | - | - | negative |
| 2017-28 | aquatic center patient | (+) | - | - | - | ND | |
| 2017-28 | aquatic center patient | no aquatic care * | (+) | 2471 | Sporadic ** | FRA/ALL | - |
| 2017-29 | resident | no | (+) | - | - | - | - |
| 2017-28 | aquatic center patient | no aquatic care * | (+) | 2471 | Sporadic ** | FRA/ALL | - |
| Local Laboratory Results | Typing of Strains by NCR Legionella | |||||
|---|---|---|---|---|---|---|
| Location | Quantification of Legionella in Environmental Samples | Date | L. pneumophila Serogroup | ST Subtyping | Date | |
| Car park | <10 cfu/L | April 2017 | Aquatic therapy center | |||
| Ornamental fountain | L. pneumophila undetectable | April 2017 | spa pool | 1 | 23 | July 2017 |
| Street-cleaning trucks (n = 10) | L. pneumophila undetectable | May 2017 | spa pool | 1 | 2470 | July 2017 |
| CTS (n = 3) | <10 cfu/L | May 2017 | spa pool | 1 | 2471 | July 2017 |
| spa pool | 1 | 2471 | July 2017 | |||
| Aquatic therapy center | spa pool | 1 | 2471 | July 2017 | ||
| Hot water production | L. pneumophila undetectable | February, May 2017 | spa pool | 1 | 2471 | July 2017 |
| Hot water point-of-use | L. pneumophila undetectable | February, May 2017 | Buffer pool | 1 | 23 | July 2017 |
| Cold water | L. pneumophila undetectable | May 2017 | Buffer pool | 1 | 23 | July 2017 |
| Condensation products * | L. pneumophila undetectable | July 2017 | Buffer pool | 1 | 23 | July 2017 |
| Spa pool | <10 cfu/L | February, May 2017 | Buffer pool | 1 | 1324 | July 2017 |
| Spa pool | non-quantifiable (interferent flora) | July 2017 | Buffer pool | 1 | 2471 | July 2017 |
| Buffer pool | nd | February, May 2017 | Buffer pool | 1 | 2471 | July 2017 |
| Buffer pool | non-quantifiable (interferent flora) | July 2017 | Buffer pool | 1 | 2471 | July 2017 |
| Shower | L. pneumophila: 160 cfu/L | May 2017 | Shower | 8 | 378 | May 2017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rousseau, C.; Ginevra, C.; Simac, L.; Fiard, N.; Vilhes, K.; Ranc, A.-G.; Jarraud, S.; Gornes, H.; Mouly, D.; Campese, C. A Community Outbreak of Legionnaires’ Disease with Two Strains of L. pneumophila Serogroup 1 Linked to an Aquatic Therapy Centre. Int. J. Environ. Res. Public Health 2022, 19, 1119. https://doi.org/10.3390/ijerph19031119
Rousseau C, Ginevra C, Simac L, Fiard N, Vilhes K, Ranc A-G, Jarraud S, Gornes H, Mouly D, Campese C. A Community Outbreak of Legionnaires’ Disease with Two Strains of L. pneumophila Serogroup 1 Linked to an Aquatic Therapy Centre. International Journal of Environmental Research and Public Health. 2022; 19(3):1119. https://doi.org/10.3390/ijerph19031119
Chicago/Turabian StyleRousseau, Cyril, Christophe Ginevra, Leslie Simac, Noel Fiard, Karine Vilhes, Anne-Gaëlle Ranc, Sophie Jarraud, Hervé Gornes, Damien Mouly, and Christine Campese. 2022. "A Community Outbreak of Legionnaires’ Disease with Two Strains of L. pneumophila Serogroup 1 Linked to an Aquatic Therapy Centre" International Journal of Environmental Research and Public Health 19, no. 3: 1119. https://doi.org/10.3390/ijerph19031119






