The Effect of Body Composition on Gait Variability Varies with Age: Interaction by Hierarchical Moderated Regression Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Body Composition
2.3. Gait
2.4. Statistical Analysis
3. Results
3.1. Independent Sample T-Test and Mann–Whitney Test
3.2. Hierarchical Moderated Regression Analysis
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brantley, J.A.; Luu, T.P.; Nakagome, S.; Zhu, F.; Contreras-Vidal, J.L. Full body mobile brain-body imaging data during unconstrained locomotion on stairs, ramps, and level ground. Sci. Data 2018, 5, 180133. [Google Scholar] [CrossRef]
- Monfort, S.M.; Pan, X.; Loprinzi, C.L.; Lustberg, M.B.; Chaudhari, A.M. Exploring the roles of central and peripheral nervous system function in gait stability: Preliminary insights from cancer survivors. Gait Posture 2019, 71, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Rosso, A.L.; Studenski, S.A.; Chen, W.G.; Aizenstein, H.J.; Alexander, N.B.; Bennett, D.A.; Black, S.E.; Camicioli, R.; Carlson, M.C.; Ferrucci, L. Aging, the central nervous system, and mobility. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 1379–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentine, R.J.; Misic, M.M.; Rosengren, K.S.; Woods, J.A.; Evans, E.M. Sex impacts the relation between body composition and physical function in older adults. Menopause 2009, 16, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, D.; Quinlan, L.R.; Browne, P.; Richardson, M.; Meskell, P.; ÓLaighin, G. A technological review of wearable cueing devices addressing freezing of gait in Parkinson’s disease. Sensors 2019, 19, 1277. [Google Scholar] [CrossRef] [Green Version]
- Ghai, S.; Ghai, I.; Effenberg, A.O. Effect of rhythmic auditory cueing on aging gait: A systematic review and meta-analysis. Aging Dis. 2018, 9, 901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, S.A.F.; Faintuch, J.; Valezi, A.C.; Sant’Anna, A.F.; Gama-Rodrigues, J.J.; de Batista Fonseca, I.C.; Souza, R.B.; Senhorini, R.C. Gait cinematic analysis in morbidly obese patients. Obes. Surg. 2005, 15, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Muehlbauer, T.; Granacher, U.; Borde, R.; Hortobagyi, T. Non-discriminant relationships between leg muscle strength, mass and gait performance in healthy young and old adults. Gerontology 2018, 64, 11–18. [Google Scholar] [CrossRef]
- Volpato, S.; Bianchi, L.; Lauretani, F.; Lauretani, F.; Bandinelli, S.; Guralnik, J.M.; Zuliani, G.; Ferrucci, L. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care 2012, 35, 1672–1679. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Shin, S. Effect of body composition on gait performance and variability of 20 year young adults: A preliminary study. J. Korean Soc. Study Phys. Educ. 2018, 23, 143–157. [Google Scholar] [CrossRef]
- Lai, P.P.; Leung, A.K.; Li, A.N.; Zhang, M. Three-dimensional gait analysis of obese adults. Clin. Biomech. 2008, 23, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lim, J.; Lee, S. Body fat-related differences in gait parameters and physical fitness level in weight-matched male adults. Clin. Biomech. 2021, 81, 105243. [Google Scholar] [CrossRef] [PubMed]
- Herssens, N.; Verbecque, E.; Hallemans, A.; Vereeck, L.; Van Rompaey, V.; Saeys, W. Do spatiotemporal parameters and gait variability differ across the lifespan of healthy adults? A systematic review. Gait Posture 2018, 64, 181–190. [Google Scholar] [CrossRef]
- Aboutorabi, A.; Arazpour, M.; Bahramizadeh, M.; Hutchins, S.W.; Fadayevatan, R. The effect of aging on gait parameters in able-bodied older subjects: A literature review. Aging Clin. Exp. Res. 2016, 28, 393–405. [Google Scholar] [CrossRef]
- Ko, S.-u.; Stenholm, S.; Ferrucci, L. Characteristic gait patterns in older adults with obesity—Results from the Baltimore Longitudinal Study of Aging. J. Biomech. 2010, 43, 1104–1110. [Google Scholar] [CrossRef] [Green Version]
- Michalakis, K.; Goulis, D.; Vazaiou, A.; Mintziori, G.; Polymeris, A.; Abrahamian-Michalakis, A. Obesity in the ageing man. Metabolism 2013, 62, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M. Gait variability: Methods, modeling and meaning. J. Neuroeng. Rehabil. 2005, 2, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socie, M.J.; Sosnoff, J.J. Gait variability and multiple sclerosis. Mult. Scler. Int. 2013, 2013, 645197. [Google Scholar] [CrossRef] [Green Version]
- Brach, J.S.; Berlin, J.E.; VanSwearingen, J.M.; Newman, A.B.; Studenski, S.A. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J. Neuroeng. Rehabil. 2005, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Brach, J.S.; Perera, S.; Studenski, S.; Katz, M.; Hall, C.; Verghese, J. Meaningful change in measures of gait variability in older adults. Gait Posture 2010, 31, 175–179. [Google Scholar] [CrossRef] [Green Version]
- Kyvelidou, A.; Kurz, M.J.; Ehlers, J.L.; Stergiou, N. Aging and partial body weight support affects gait variability. J. Neuroeng. Rehabil. 2008, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Callisaya, M.L.; Blizzard, L.; Schmidt, M.D.; McGinley, J.L.; Srikanth, V.K. Ageing and gait variability—A population-based study of older people. Age Ageing 2010, 39, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabiner, P.C.; Biswas, S.T.; Grabiner, M.D. Age-related changes in spatial and temporal gait variables. Arch. Phys. Med. Rehabil. 2001, 82, 31–35. [Google Scholar] [CrossRef]
- Maki, B.E. Gait changes in older adults: Predictors of falls or indicators of fear? J. Am. Geriatr. Soc. 1997, 45, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Rosano, C.; Brach, J.; Studenski, S.; Longstreth Jr, W.; Newman, A.B. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology 2007, 29, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brach, J.S.; Studenski, S.A.; Perera, S.; VanSwearingen, J.M.; Newman, A.B. Gait variability and the risk of incident mobility disability in community-dwelling older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Callisaya, M.L.; Blizzard, L.; McGinley, J.L.; Schmidt, M.D.; Srikanth, V.K. Sensorimotor factors affecting gait variability in older people—a population-based study. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2010, 65, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.Y.; Seo, S.G.; Kim, E.J.; Kim, S.J.; Lee, K.M.; Choi, I.H. Inter-segmental motions of the foot in healthy adults: Gender difference. J. Orthop. Sci. 2016, 21, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Yang Sun, P.; Ji-Won, K.; Yuri, K.; Moon-Seok, K. Effect of age and sex on gait characteristics in the Korean elderly people. Iran. J. Public Health 2018, 47, 666. [Google Scholar]
- Han, S.H.; Kim, C.O.; Kim, K.J.; Jeon, J.; Chang, H.; Kim, E.S.; Park, H. Quantitative analysis of the bilateral coordination and gait asymmetry using inertial measurement unit-based gait analysis. PLoS ONE 2019, 14, e0222913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Ancum, J.M.; Jonkman, N.H.; van Schoor, N.M.; Tressel, E.; Meskers, C.G.; Pijnappels, M.; Maier, A.B. Predictors of metabolic syndrome in community-dwelling older adults. PLoS ONE 2018, 13, e0206424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stathokostas, L.; McDonald, M.W.; Little, R.; Paterson, D.H. Flexibility of older adults aged 55–86 years and the influence of physical activity. J. Aging Res. 2013, 2013, 743843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Won, J.; Alfini, A.J.; Weiss, L.R.; Michelson, C.S.; Callow, D.D.; Ranadive, S.M.; Gentili, R.J.; Smith, J.C. Semantic memory activation after acute exercise in healthy older adults. J. Int. Neuropsychol. Soc. 2019, 25, 557–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stumme, J.; Jockwitz, C.; Hoffstaedter, F.; Amunts, K.; Caspers, S. Functional network reorganization in older adults: Graph-theoretical analyses of age, cognition and sex. NeuroImage 2020, 214, 116756. [Google Scholar] [CrossRef] [PubMed]
- de Zubicaray, G.I.; Rose, S.E.; McMahon, K.L. The structure and connectivity of semantic memory in the healthy older adult brain. Neuroimage 2011, 54, 1488–1494. [Google Scholar] [CrossRef]
- Anderson, L.J.; Erceg, D.N.; Schroeder, E.T. Utility of multifrequency bioelectrical impedance compared with dual-energy x-ray absorptiometry for assessment of total and regional body composition varies between men and women. Nutr. Res. 2012, 32, 479–485. [Google Scholar] [CrossRef]
- Buckinx, F.; Mouton, A.; Reginster, J.-Y.; Croisier, J.-L.; Dardenne, N.; Beaudart, C.; Nelis, J.; Lambert, E.; Appelboom, G.; Bruyère, O. Relationship between ambulatory physical activity assessed by activity trackers and physical frailty among nursing home residents. Gait Posture 2017, 54, 56–61. [Google Scholar] [CrossRef]
- Wang, L.; Hui, S.S.-C. Validity of four commercial bioelectrical impedance scales in measuring body fat among Chinese children and adolescents. BioMed Res. Int. 2015, 2015, 614858. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 1988. [Google Scholar]
- Schwartz, R.S.; Shuman, W.P.; Bradbury, V.L.; Cain, K.C.; Fellingham, G.W.; Beard, J.C.; Kahn, S.E.; Stratton, J.R.; Cerqueira, M.D.; Abrass, I.B. Body fat distribution in healthy young and older men. J. Gerontol. 1990, 45, M181–M185. [Google Scholar] [CrossRef]
- Castillo, C.; Carnicero, J.A.; de la Torre, M.Á.; Amor, S.; Guadalupe-Grau, A.; Rodríguez-Mañas, L.; García-García, F.J. Nonlinear relationship between waist to hip ratio, weight and strength in elders: Is gender the key? Biogerontology 2015, 16, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, M.; Mazzali, G.; Fantin, F.; Rossi, A.; Di Francesco, V. Sarcopenic obesity: A new category of obesity in the elderly. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 388–395. [Google Scholar] [CrossRef]
- Batista, F.S.; de Oliveira Gomes, G.A.; Neri, A.L.; Guariento, M.E.; Cintra, F.A.; da Luz Rosario de Sousa, M.; D’Elboux, M.J. Relationship between lower-limb muscle strength and frailty among elderly people. Sao Paulo Med. J. 2012, 130, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Menant, J.; Weber, F.; Lo, J.; Sturnieks, D.; Close, J.; Sachdev, P.; Brodaty, H.; Lord, S. Strength measures are better than muscle mass measures in predicting health-related outcomes in older people: Time to abandon the term sarcopenia? Osteoporos. Int. 2017, 28, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Shin, S. Analysis of the effect of obesity on gait performance and variability in old adults. Korean J. Phys. Educ. 2014, 53, 759–767. [Google Scholar]
- Mickle, K.J.; Steele, J.R. Obese older adults suffer foot pain and foot-related functional limitation. Gait Posture 2015, 42, 442–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messier, S.P.; Ettinger, W.H.; Doyle, T.E.; Morgan, T.; James, M.K.; O’Toole, M.L.; Burns, R. Obesity: Effects on gait in an osteoarthritic population. J. Appl. Biomech. 1996, 12, 161–172. [Google Scholar] [CrossRef]
- Gonzalez, M.; Gates, D.H.; Rosenblatt, N.J. The impact of obesity on gait stability in older adults. J. Biomech. 2020, 100, 109585. [Google Scholar] [CrossRef]
- Koster, A.; Ding, J.; Stenholm, S.; Caserotti, P.; Houston, D.K.; Nicklas, B.J.; You, T.; Lee, J.S.; Visser, M.; Newman, A.B. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2011, 66, 888–895. [Google Scholar] [CrossRef]
- de C Hamilton, A.F.; Jones, K.E.; Wolpert, D.M. The scaling of motor noise with muscle strength and motor unit number in humans. Exp. Brain Res. 2004, 157, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Sosnoff, J.J.; Newell, K.M. Are age-related increases in force variability due to decrements in strength? Exp. Brain Res. 2006, 174, 86–94. [Google Scholar] [CrossRef]
- Shin, S.; Valentine, R.J.; Evans, E.M.; Sosnoff, J.J. Lower extremity muscle quality and gait variability in older adults. Age Ageing 2012, 41, 595–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanaya, A.M.; Lindquist, K.; Harris, T.B.; Launer, L.; Rosano, C.; Satterfield, S.; Yaffe, K. Total and regional adiposity and cognitive change in older adults: The Health, Aging and Body Composition (ABC) study. Arch. Neurol. 2009, 66, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.A. The processing-speed theory of adult age differences in cognition. Psychol. Rev. 1996, 103, 403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, R.A.; Yarnall, A.J.; Duncan, G.W.; Breen, D.P.; Khoo, T.K.; Williams-Gray, C.H.; Barker, R.A.; Collerton, D.; Taylor, J.-P.; Burn, D.J. Cognitive decline and quality of life in incident Parkinson’s disease: The role of attention. Park. Relat. Disord. 2016, 27, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, K.L.; Sun, R.; Sosnoff, J.J. Cognition is associated with gait variability in individuals with multiple sclerosis. J. Neural Transm. 2017, 124, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, P.L.; Solomont, J.; Kowall, N.; Hausdorff, J.M. Influence of executive function on locomotor function: Divided attention increases gait variability in Alzheimer’s disease. J. Am. Geriatr. Soc. 2003, 51, 1633–1637. [Google Scholar] [CrossRef] [Green Version]
- Tashani, O.; Astita, R.; Sharp, D.; Johnson, M. Body mass index and distribution of body fat can influence sensory detection and pain sensitivity. Eur. J. Pain 2017, 21, 1186–1196. [Google Scholar] [CrossRef]
- Riskowski, J.; Mikesky, A.; Bahamonde, R.E.; Alvey III, T.; Burr, D.B. Proprioception, gait kinematics, and rate of loading during walking: Are they related? J. Musculoskelet. Neuronal Interact. 2005, 5, 379. [Google Scholar] [PubMed]
- Jayakody, O.; Breslin, M.; Srikanth, V.; Callisaya, M. Medical, sensorimotor and cognitive factors associated with gait variability: A longitudinal population-based study. Front. Aging Neurosci. 2018, 10, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | Young (n = 40) | Older (n = 40) | Total (n = 80) | p-Value |
|---|---|---|---|---|
| Age (years) | 22.25 ± 2.23 | 74.05 ± 6.86 | 48.15 ± 26.55 | <0.001 * |
| Height (cm) | 174.94 ± 5.05 | 168.35 ± 5.38 | 171.64 ± 6.15 | <0.001 * |
| Weight (kg) | 73.98 ± 10.53 | 69.23 ± 8.15 | 71.60 ± 9.66 | 0.027 * |
| BMI (kg/m2) | 23.35 ± 4.08 | 24.55 ± 2.76 | 23.95 ± 3.51 | NS |
| BFP (%) | 17.06 ± 6.32 | 26.28 ± 5.25 | 21.67 ± 7.41 | <0.001 * |
| SMM (kg) | 34.73 ± 3.62 | 28.02 ± 3.25 | 31.38 ± 4.80 | <0.001 * |
| Gait speed (m/s) | 1.27 ± 0.11 | 1.27 ± 0.17 | 1.27 ± 0.15 | NS |
| Stride time (s) | 1.06 ± 0.07 | 1.03 ± 0.05 | 1.05 ± 0.06 | NS |
| Stride length (m) | 1.34 ± 0.08 | 1.30 ± 0.17 | 1.32 ± 0.13 | NS |
| Stride time CV (%) | 2.18 ± 0.49 | 2.37 ± 0.67 | 2.27 ± 0.59 | NS |
| Stride length CV (%) | 3.48 ± 0.70 | 3.61 ± 1.17 | 3.54 ± 0.96 | NS |
| Predictors | Total (n = 80) | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| β | p | β | p | β | p | |
| Height (cm) | 0.231 | NS | 0.190 | NS | 0.114 | NS |
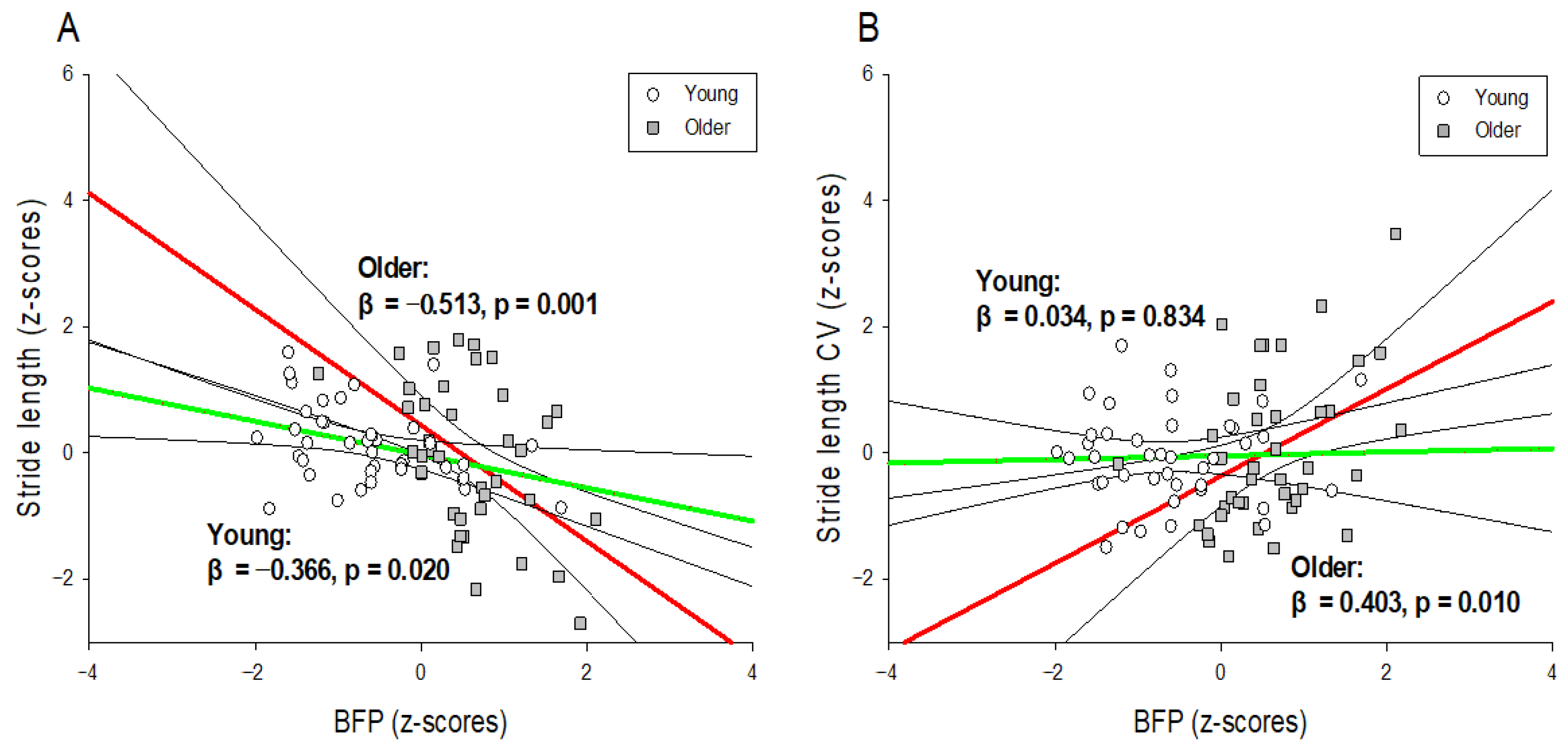
| BFP (%) | −0.397 | 0.001 *** | −0.485 | 0.001 *** | −0.563 | 0.001 *** |
| SMM (kg) | −0.222 | NS | −0.088 | NS | −0.093 | NS |
| Age (years) | 0.193 | NS | 0.216 | NS | ||
| BFP × Age | −0.277 | 0.010 ** | ||||
| SMM × Age | −0.037 | NS | ||||
| R2 block 1 = 0.185 | ΔR2 = 0.185 | |||||
| R2 block 2 = 0.197 | ΔR2 = 0.012 | |||||
| R2 block 3 = 0.268 | ΔR2 = 0.071 | |||||
| Predictors | Total (n = 80) | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| β | p | β | p | β | p | |
| Height (cm) | 0.149 | NS | 0.227 | NS | 0.305 | NS |
| BFP (%) | 0.171 | NS | 0.336 | 0.026 * | 0.416 | 0.007 ** |
| SMM (kg) | −0.284 | NS | −0.536 | 0.022 * | −0.531 | 0.019 * |
| Age (years) | −0.363 | NS | −0.386 | 0.036 * | ||
| BFP × Age | 0.290 | 0.010 ** | ||||
| SMM × Age | 0.032 | NS | ||||
| R2 block 1 = 0.081 | ΔR2 = 0.081 | |||||
| R2 block 2 = 0.125 | ΔR2 = 0.044 | |||||
| R2 block 3 = 0.202 | ΔR2 = 0.077 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Shin, S. The Effect of Body Composition on Gait Variability Varies with Age: Interaction by Hierarchical Moderated Regression Analysis. Int. J. Environ. Res. Public Health 2022, 19, 1171. https://doi.org/10.3390/ijerph19031171
Lee Y, Shin S. The Effect of Body Composition on Gait Variability Varies with Age: Interaction by Hierarchical Moderated Regression Analysis. International Journal of Environmental Research and Public Health. 2022; 19(3):1171. https://doi.org/10.3390/ijerph19031171
Chicago/Turabian StyleLee, Yungon, and Sunghoon Shin. 2022. "The Effect of Body Composition on Gait Variability Varies with Age: Interaction by Hierarchical Moderated Regression Analysis" International Journal of Environmental Research and Public Health 19, no. 3: 1171. https://doi.org/10.3390/ijerph19031171
APA StyleLee, Y., & Shin, S. (2022). The Effect of Body Composition on Gait Variability Varies with Age: Interaction by Hierarchical Moderated Regression Analysis. International Journal of Environmental Research and Public Health, 19(3), 1171. https://doi.org/10.3390/ijerph19031171






