Physical Activity Improves Cognition and Activities of Daily Living in Adults with Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
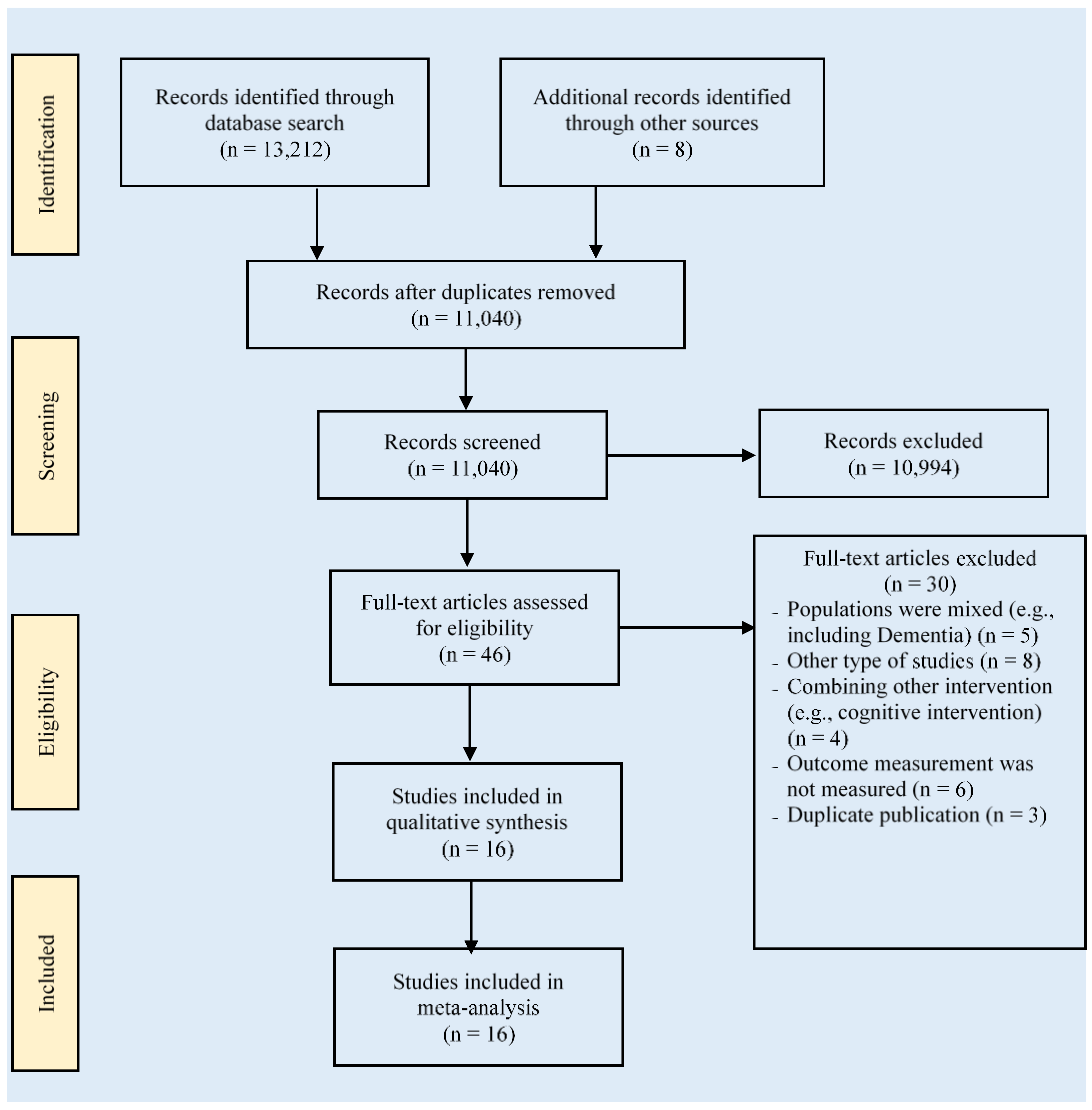
3.1. Search Results
3.2. Characteristics of Included Studies
3.3. Effects of PA Intervention on Global Cognition
3.4. Effects of PA Intervention on Activities of Daily Living
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2018, 14, 367–429. [Google Scholar]
- Ahn, N.; Kim, K. Effects of an elastic band resistance exercise program on lower extremity muscle strength and gait ability in patients with Alzheimer’s disease. J. Phys. Ther. Sci. 2015, 27, 1953–1955. [Google Scholar] [CrossRef] [Green Version]
- Wallace, L.; Walsh, S.; Brayne, C. The legacy of the 2013 G8 Dementia Summit: Successes, challenges, and potential ways forward. Lancet Health Longev. 2021, 2, e455–e457. [Google Scholar] [CrossRef]
- Gordon, B.R.; McDowell, C.P.; Lyons, M.; Herring, M.P. The Effects of Resistance Exercise Training on Anxiety: A Meta-Analysis and Meta-Regression Analysis of Randomized Controlled Trials. Sports Med. 2017, 47, 2521–2532. [Google Scholar] [CrossRef]
- Awick, E.A.; Ehlers, D.K.; Aguiñaga, S.; Daugherty, A.M.; Kramer, A.F.; McAuley, E. Effects of a randomized exercise trial on physical activity, psychological distress and quality of life in older adults. Gen. Hosp. Psychiatry 2017, 49, 44–50. [Google Scholar] [CrossRef]
- Sousa, N.; Mendes, R.; Abrantes, C.; Sampaio, J.; Oliveira, J. Effectiveness of combined exercise training to improve functional fitness in older adults: A randomized controlled trial. Geriatr. Gerontol. Int. 2014, 14, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Stillman, C.M.; Esteban-Cornejo, I.; Brown, B.; Bender, C.M.; Erickson, K.I. Effects of Exercise on Brain and Cognition Across Age Groups and Health States. Trends Neurosci. 2020, 43, 533–543. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Lee, Y.H.; Chan, J.C.; Chiou, P.Y.; Chou, X.Y.; Chiu, W.T.; Hung, C.T. Effects of exercise interventions on social and cognitive functioning of men with prostate cancer: A meta-analysis. Support. Care Cancer 2020, 28, 2043–2057. [Google Scholar] [CrossRef] [PubMed]
- Falck, R.S.; Davis, J.C.; Best, J.R.; Crockett, R.A.; Liu-Ambrose, T. Impact of exercise training on physical and cognitive function among older adults: A systematic review and meta-analysis. Neurobiol. Aging 2019, 79, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Lin, M.S.; Tzeng, I.S. Relationship Between Exercise and Alzheimer’s Disease: A Narrative Literature Review. Front. Neurosci. 2020, 14, 131. [Google Scholar] [CrossRef] [Green Version]
- Toots, A.; Littbrand, H.; Lindelöf, N.; Wiklund, R.; Holmberg, H.; Nordström, P.; Lundin-Olsson, L.; Gustafson, Y.; Rosendahl, E. Effects of a High-Intensity Functional Exercise Program on Dependence in Activities of Daily Living and Balance in Older Adults with Dementia. J. Am. Geriatr. Soc. 2016, 64, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Groot, C.; Hooghiemstra, A.M.; Raijmakers, P.G.H.M.; van Berckel, B.N.M.; Scheltens, P.; Scherder, E.J.A.; van der Flier, W.M.; Ossenkoppele, R. The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Res. Rev. 2016, 25, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Li, Y.; Li, J.; Zhou, C.; Li, F.; Yang, X. Physical activity can improve cognition in patients with Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Clin. Interv. Aging 2018, 13, 1593–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blondell, S.J.; Hammersley-Mather, R.; Veerman, J.L. Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies. BMC Public Health 2014, 14, 510. [Google Scholar] [CrossRef] [Green Version]
- Borges-Machado, F.; Silva, N.; Farinatti, P.; Poton, R.; Ribeiro, Ó.; Carvalho, J. Effectiveness of Multicomponent Exercise Interventions in Older Adults With Dementia: A Meta-Analysis. Gerontologist 2020, 61, e449–e462. [Google Scholar] [CrossRef]
- Demurtas, J.; Schoene, D.; Torbahn, G.; Marengoni, A.; Grande, G.; Zou, L.; Petrovic, M.; Maggi, S.; Cesari, M.; Lamb, S.; et al. Physical Activity and Exercise in Mild Cognitive Impairment and Dementia: An Umbrella Review of Intervention and Observational Studies. J. Am. Med. Dir. Assoc. 2020, 21, 1415–1422. [Google Scholar] [CrossRef]
- López-Ortiz, S.; Valenzuela, P.L.; Seisdedos, M.M.; Morales, J.S.; Vega, T.; Castillo-García, A.; Nisticò, R.; Mercuri, N.B.; Lista, S.; Lucia, A.; et al. Exercise interventions in Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Ageing Res. Rev. 2021, 72, 101479. [Google Scholar] [CrossRef]
- Chang, W.-T.; Kuo, Y.-J.; Huang, Y.-Y.; Tsai, M.-J.; Chen, Y.-P. Poor Activities of Daily Living Function Reflect Poor Quality of Life after Hip Fracture Surgery for Geriatric Patients. Soc. Health Behav. 2019, 2, 41. [Google Scholar]
- Medhi, G.; Sarma, J.; Pala, S.; Bhattacharya, H.; Bora, P.; Visi, V. Association between health related quality of life (HRQOL) and activity of daily living (ADL) among elderly in an urban setting of Assam, India. J. Fam. Med. Prim. Care 2019, 8, 1760. [Google Scholar]
- Dehghani, A.; Khoramkish, M.; Isfahani, S.S. Challenges in the daily living activities of patients with multiple sclerosis: A qualitative content analysis. Int. J. Community Based Nurs. Midwifery 2019, 7, 201–210. [Google Scholar]
- Kwak, Y.-S.; Um, S.-Y.; Son, T.-G.; Kim, D.-J. Effect of Regular Exercise on Senile Dementia Patients. Int. J. Sports Med. 2008, 29, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Sobol, N.A.; Hoffmann, K.; Frederiksen, K.S.; Vogel, A.; Vestergaard, K.; Brændgaard, H.; Gottrup, H.; Lolk, A.; Wermuth, L.; Jakobsen, S.; et al. Effect of aerobic exercise on physical performance in patients with Alzheimer’s disease. Alzheimer’s Dement. 2016, 12, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Kader, S.M.; Al-Jiffri, O.H. Aerobic exercise improves quality of life, psychological well-being and systemic inflammation in subjects with alzheimer’s disease. Afr. Health Sci. 2016, 16, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.M.; Huang, M.Z.; Liao, L.R.; Chung, R.C.; Kwok, T.C.; Pang, M.Y. Physical exercise improves strength, balance, mobility, and endurance in people with cognitive impairment and dementia: A systematic review. J. Physiother. 2018, 64, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Ojagbemi, A.; Akin-Ojagbemi, N. Exercise and Quality of Life in Dementia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Appl. Gerontol. 2019, 38, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Laver, K.; Dyer, S.; Whitehead, C.; Clemson, L.; Crotty, M. Interventions to delay functional decline in people with dementia: A systematic review of systematic reviews. BMJ Open 2016, 6, e010767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisani, S.; Mueller, C.; Huntley, J.; Aarsland, D.; Kempton, M.J. A meta-analysis of randomised controlled trials of physical activity in people with Alzheimer’s disease and mild cognitive impairment with a comparison to donepezil. Int. J. Geriatr. Psychiatry 2021, 36, 1471–1487. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.; Sherrington, C.; Herbert, R.; Moseley, A.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Choosing effect measures and computing estimates of effect. Cochrane Handb. Syst. Rev. Interv. 2019, 143–176. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Burgess, E.O.; Wu, J. The effects of Tai Chi exercise on cognitive function in older adults: A meta-analysis. J. Sport Health Sci. 2013, 2, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons Ltd.: Chichester, UK, 2011. [Google Scholar]
- Borenstein, M.; Higgins, J.P.T.; Hedges, L.V.; Rothstein, H.R. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res. Synth. Methods 2017, 8, 5–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Loprinzi, P.D.; Yang, L.; Liu, J.; Liu, S.; Zou, L. The Beneficial Effects of Traditional Chinese Exercises for Adults with Low Back Pain: A Meta-Analysis of Randomized Controlled Trials. Medicina 2019, 55, 118. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zou, L.; Chen, S.-T.; Bae, J.H.; Kim, D.Y.; Liu, X.; Song, W. Effects and Moderators of Exercise on Sarcopenic Components in Sarcopenic Elderly: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 649748. [Google Scholar] [CrossRef]
- Chang, C.; Wang, W.; Zhu, Y. Clinical effects of aerobic training in the treatment of Alzheimer’s disease. Chin. J. Rehabil. Med. 2015, 30, 1131–1134. [Google Scholar]
- De Oliveira Silva, F.; Ferreira, J.V.; Plácido, J.; Sant’Anna, P.; Araújo, J.; Marinho, V.; Laks, J.; Camaz Deslandes, A. Three months of multimodal training contributes to mobility and executive function in elderly individuals with mild cognitive impairment, but not in those with Alzheimer’s disease: A randomized controlled trial. Maturitas 2019, 126, 28–33. [Google Scholar] [CrossRef]
- De Souto Barreto, P.; Cesari, M.; Denormandie, P.; Armaingaud, D.; Vellas, B.; Rolland, Y. Exercise or Social Intervention for Nursing Home Residents with Dementia: A Pilot Randomized, Controlled Trial. J. Am. Geriatr. Soc. 2017, 65, E123–E129. [Google Scholar] [CrossRef]
- Fonte, C.; Smania, N.; Pedrinolla, A.; Munari, D.; Gandolfi, M.; Picelli, A.; Varalta, V.; Benetti, M.V.; Brugnera, A.; Federico, A.; et al. Comparison between physical and cognitive treatment in patients with MCI and Alzheimer’s disease. Aging 2019, 11, 3138–3155. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Sobol, N.A.; Frederiksen, K.S.; Beyer, N.; Vogel, A.; Vestergaard, K.; Brændgaard, H.; Gottrup, H.; Lolk, A.; Wermuth, L.; et al. Moderate-to-high intensity physical exercise in patients with Alzheimer’s disease: A randomized controlled trial. J. Alzheimer’s Dis. 2016, 50, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Holthoff, V.A.; Marschner, K.; Scharf, M.; Steding, J.; Meyer, S.; Koch, R.; Donix, M. Effects of physical activity training in patients with alzheimer’s dementia: Results of a pilot RCT study. PLoS ONE 2015, 10, e0121478. [Google Scholar] [CrossRef] [Green Version]
- Kemoun, G.; Thibaud, M.; Roumagne, N.; Carette, P.; Albinet, C.; Toussaint, L.; Paccalin, M.; Dugué, B. Effects of a physical training programme on cognitive function and walking efficiency in elderly persons with dementia. Dement. Geriatr. Cogn. Disord. 2010, 29, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, T.; Zhu, Y.; Wang, W.; Yin, M.; Zhang, W.; Zhang, T.; Wu, T. Aerobic exercise improves cognitive function in patients with Alzheimer’s disease. Chin. J. Rehabil. 2017, 32, 386–389. [Google Scholar]
- Mu, H.; Lv, J.; Hao, Z.; Li, W.; Li, M. Effect of aerobic exercise on abilities in daily life, cognitive function and psychological symptoms in patients with mild to moderate Alzheimer’s disease. Chin. J. Mult. Organ Dis. Elder. 2016, 15, 451–545. [Google Scholar]
- Pedroso, R.V.; Ayán, C.; Fraga, F.J.; Da Silva, T.M.V.; Cancela, J.M.; Santos-Galduròz, R.F. Effects of functional-task training on older adults with Alzheimer’s disease. J. Aging Phys. Act. 2018, 26, 97–105. [Google Scholar] [CrossRef]
- Venturelli, M.; Scarsini, R.; Schena, F. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am. J. Alzheimers Dis. Other Demen. 2011, 26, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, M.; Sollima, A.; Cè, E.; Limonta, E.; Bisconti, A.V.; Brasioli, A.; Muti, E.; Esposito, F. Effectiveness of Exercise- and Cognitive-Based Treatments on Salivary Cortisol Levels and Sundowning Syndrome Symptoms in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 53, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Vreugdenhil, A.; Cannell, J.; Davies, A.; Razay, G. A community-based exercise programme to improve functional ability in people with Alzheimer’s disease: A randomized controlled trial. Scand. J. Caring Sci. 2012, 26, 12–19. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Yang, S.; Chang, C.; Wang, Y.; Li, H.; Wang, Q.; Wang, W.; Wang, T.; Wu, T. The effect of aerobic exercise on cognitive function and activity of daily living in patients with Alzheimer’s disease. Chin. J. Rehabil. Med. 2014, 29, 1151–1155. [Google Scholar]
- Wang, Y.; Shen, F.; Zhu, Y.; Yang, S.; Li, H.; Wang, Q.; Wang, W.; Wu, T. Clinical Effects of Aerobic Exercises Training with Moderate and High Intensity in Alzheimer’s Disease Treatment. China J. Clin. Neurosci. 2014, 22, 504–509. [Google Scholar]
- Yang, S.-Y.; Shan, C.-L.; Qing, H.; Wang, W.; Zhu, Y.; Yin, M.-M.; Machado, S.; Yuan, T.-F.; Wu, T. The Effects of Aerobic Exercise on Cognitive Function of Alzheimer’s Disease Patients. CNS Neurol. Disord. Drug Targets 2015, 14, 1292–1297. [Google Scholar] [CrossRef]
- Qin, X.Y.; Zhang, S.P.; Cao, C.; Loh, Y.P.; Cheng, Y. Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: A systematic review and meta-analysis. JAMA Neurol. 2016, 73, 1316–1324. [Google Scholar] [CrossRef]
- Zhu, L.; Li, L.; Wang, L.; Jin, X.; Zhang, H. Physical Activity for Executive Function and Activities of Daily Living in AD Patients: A Systematic Review and Meta-Analysis. Front. Psychol. 2020, 11, 3227. [Google Scholar] [CrossRef]
- Cardona, M.I.; Afi, A.; Lakicevic, N.; Thyrian, J.R. Physical activity interventions and their effects on cognitive function in people with dementia: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 8753. [Google Scholar] [CrossRef]
- Raz, L.; Knoefel, J.; Bhaskar, K. The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow Metab. 2016, 36, 172–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, A.K.; Chou, A.; Bursley, B.; Smulofsky, J.; Jezequel, J. Systematic review of the effects of exercise on activities of daily living in people with alzheimers disease. Am. J. Occup. Ther. 2014, 68, 50–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.Y.; Pei, J.; Zhan, Y.J.; Cai, Y.W. Overview of Meta-Analyses of Five Non-pharmacological Interventions for Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 594432. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.X.; Liang, J.H.; Xu, Y.; Wang, Y.Q. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: A meta-analysis. BMC Geriatr. 2019, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Brigola, A.G.; Rossetti, E.S.; dos Santos, B.R.; Neri, A.L.; Zazzetta, M.S.; Inouye, K.; Pavarini, S.C.I. Relationship between cognition and frailty in elderly: A systematic review. Dement. Neuropsychol. 2015, 9, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Shimada, H.; Makizako, H.; Doi, T.; Park, H.; Tsutsumimoto, K.; Verghese, J.; Suzuki, T. Effects of Combined Physical and Cognitive Exercises on Cognition and Mobility in Patients With Mild Cognitive Impairment: A Randomized Clinical Trial. J. Am. Med. Dir. Assoc. 2018, 19, 584–591. [Google Scholar] [CrossRef]
- Herold, F.; Törpel, A.; Schega, L.; Müller, N.G. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements—A systematic review. Eur. Rev. Aging Phys. Act. 2019, 16, 10. [Google Scholar] [CrossRef]
- Potter, R.; Ellard, D.; Rees, K.; Thorogood, M. A systematic review of the effects of physical activity on physical functioning, quality of life and depression in older people with dementia. Int. J. Geriatr. Psychiatry 2011, 26, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Timmons, J.F.; Griffin, C.; Cogan, K.E.; Matthews, J.; Egan, B. Exercise Maintenance in Older Adults 1 Year After Completion of a Supervised Training Intervention. J. Am. Geriatr. Soc. 2020, 68, 163–169. [Google Scholar] [CrossRef] [PubMed]





| Study/Country | Severity of AD Age (Years) Total Sample Size Male (%) | Diagnostic Criteria | Time/Frequency/ Duration/Intensity | Intervention Protocol | Outcomes (Instrument) | |
|---|---|---|---|---|---|---|
| Experiment (Details) | Control | |||||
| Chang, 2015 [36] China | Mild to moderate 70.5 n = 57 38.6% | DSM-IV MMSE | 60–90 min/3 times/week 16 weeks 50–70% HRmax moderate | aerobic exercise (cycling, walking) | usual medical treatment | global cognition (MMSE) ADL |
| de Oliveira Silva, 2019 [37] Brazil | Mild 79.4 n = 27 40.7% | DSM-IV | 60 min/2 times/week 12 weeks 80% HRmax moderate | mixed exercises (treadmill, weight-lifting, balance exercises) | usual medical treatment | global cognition (MMSE) |
| de Souto Barreto, 2017 [38] France | Mod to severe 87.5 n = 91 15.4% | DSM-IV MMSE | 60 min/2 times/week 24 weeks moderate | mixed exercises (walking, weight-lifting, coordination, balance exercises) | social activity | global cognition (MMSE) ADL |
| Fonte, 2019 [39] Italy | Mild 79 n = 60 35% | NINCDS-ADRDA | 90 min/3 times/week 24 weeks 70% HRmax moderate | mixed exercises (cycling, walking, weight-lifting) | C1: cognitive treatment C2: usual medical treatment | global cognition (ADAS-Cog) ADL |
| Hoffmann, 2016 [40] Denmark | Mild 70.5 n = 200 56.5% | NINCDS-ADRDA | 60 min/3 times/week 16 weeks 70-80% HRmax moderate-high | mixed exercise (ergometer bicycle, cross-trainer, treadmill, strength training) | usual medical treatment | global cognition (MMSE) |
| Holthoff, 2015 [41] Germany | Mild or moderate 71.5 n = 30 50% | NINCDS-ADRDA | 30 min/3 times/week 12 weeks moderate | mixed exercises (movement trainer, resistance training) | usual medical treatment | global cognition (MMSE) ADL |
| Kemoun, 2010 [42] France | Moderate to severe 82 n = 31 26% | DSM-IV MMSE | 60 min/3 times/week 15 weeks moderate | mixed exercises (walking, equilibrium, stamina, ergocycle with the arms and the legs) | social activity | global cognition (ERFC) |
| Liu, 2017 [43] China | Mild 70.5 n = 48 43.8% | MRI MMSE | 40 min/3 times/week 12 weeks moderate | aerobic exercise (aerobic gymnastics: rowing movement, kicking movement) | usual medical treatment | global cognition (MMSE) |
| Mu, 2016 [44] China | Mild to moderate 73 n = 78 37.2% | NINCDS-ADRDA MMSE | 60 min/3 tims/week 16 weeks Moderate | aerobic exercise (brisk walking) | usual medical treatment | global cognition (MMSE) ADL (Barthel Index) |
| Pedroso, 2018 [45] Brazil | Mild to moderate 78.3 n = 57 24.6% | DSM-IV | 60 min/3 times/week 12 weeks 60–75% HRmax Moderate | mixed exercises (walking, stretching, jogging, climbing, and descending stairs) | C1: social activity C2: usual medical treatment | global cognition (MMSE) |
| Venturelli, 2011 [46] Italy | Severe 84 n = 24 0% | DSM-IV MMSE | >30 min/4 times/week 24 weeks moderate | aerobic exercise (walking) | social activity | global cognition (MMSE) |
| Venturelli, 2016 [47] Italy | Moderate to severe 84 n = 40 30% | DSM-IV MMSE | 60 min/5 times/week 3 months moderate | aerobic exercise (walking) | cognitive treatment | global cognition (MMSE) |
| Vreugdenhil, 2012 [48] Australia | Mild 74 n = 40 (16/24) 40% | NINCDS-ADRDA | >30 min/7 times/week 4 months | mixed exercises (brisk walking, upper and lower body strength, balance training) | usual medical treatment | global cognition (MMSE) ADL |
| Wang, W 2014 [49] China | Mild to moderate 70.5 n = 54 38.9% | NINCDS-ADRDA | 40 min/3tims/week 6 months Moderate 70% HRmax | aerobic exercise (cycling) | social activity | global cognition (MMSE) ADL |
| Wang, Y 2014 [50] China | Mild to moderate 70.7 n = 39 33.3% | NINCDS-ADRDA | 30 min/3 times/week 12 weeks 50% HR reserve | aerobic exercise (cycling) | usual medical treatment | global cognition (MMSE) ADL |
| Yang, 2015 [51] China | Mild to moderate 72 n = 50 34% | NINCDS-ADRDA MMSE | 40 min/3 times/week 12 weeks 70% HRmax | aerobic exercise (cycling) | health education | global cognition (MMSE) |
| Study | Score | Methodological Quality | PEDro Item Number | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |||
| Chang, 2015 [36] | 5 | Fair | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||
| de Oliveira Silva, 2019 [37] | 7 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| de Souto Barreto, 2017 [38] | 6 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| Fonte, 2019 [39] | 8 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| Hoffmann, 2016 [40] | 8 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| Holthoff, 2015 [41] | 5 | Fair | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||
| Kemoun, 2010 [42] | 6 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| Liu, 2017 [43] | 6 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| Mu, 2016 [44] | 7 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Pedroso, 2018 [45] | 5 | Fair | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||
| Venturelli, 2011 [46] | 5 | Fair | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||
| Venturelli, 2016 [47] | 6 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| Vreugdenhil, 2012 [48] | 6 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| Wang, W 2014 [49] | 7 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Wang, Y 2014 [50] | 7 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Yang, 2015 [51] | 6 | Good | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| Moderator | Level | Number of Trials | Sub-Analysis | Between-Group Homogeneity | ||||
|---|---|---|---|---|---|---|---|---|
| SMD | 95% CI | I2 % | Q-Value | df(Q) | p-Value | |||
| Type | Aerobic exercise | 8 | 0.60 | 0.32 to 0.88 | 43.6% | 4.69 | 1 | 0.03 |
| Mixed exercises | 10 | 0.24 | 0.06 to 0.41 | 0% | ||||
| Duration | ≤12 weeks | 8 | 0.39 | 0.15 to 0.63 | 0% | 0.12 | 1 | 0.73 |
| ≥13 weeks | 10 | 0.45 | 0.20 to 0.69 | 50.3% | ||||
| Low (≤2) | 2 | 0.20 | −0.17 to 0.58 | 0% | 1.48 | 2 | 0.48 | |
| Frequency | Medium (3–4) | 14 | 0.46 | 0.25 to 0.67 | 42.7% | |||
| High (5–7) | 2 | 0.31 | −0.13 to 0.76 | 0% | ||||
| Session time | ≤45 min | 7 | 0.66 | 0.33 to 0.99 | 45.4% | 4.41 | 1 | 0.04 |
| >45 min | 11 | 0.27 | 0.11 to 0.42 | 0% | ||||
| AD stage | Mild AD | 7 | 0.20 | −0.003 to 0.39 | 0% | 6.26 | 2 | 0.04 |
| Mild to moderate AD | 7 | 0.54 | 0.32 to 0.76 | 0% | ||||
| Moderate to severe AD | 4 | 0.75 | 0.03 to 1.47 | 79% | ||||
| Control type | Health education | 1 | 0.63 | 0.06 to 1.20 | 0% | 2.72 | 3 | 0.44 |
| Cognitive treatment | 2 | 0.15 | −0.33 to 0.63 | 0% | ||||
| Social activity | 5 | 0.66 | 0.11 to 1.21 | 72.7% | ||||
| Usual medical treatment | 11 | 0.35 | 0.19 to 0.52 | 0% | ||||
| Study quality | Good | 13 | 0.36 | 0.21 to 0.51 | 66% | 0.24 | 1 | 0.62 |
| Fair | 5 | 0.51 | 0.05 to 1.06 | 2% | ||||
| Moderator | Number of Trials | β | 95% CI | Q-Value | df(Q) | p-Value | ||
| Session time | 18 | −0.0105 | −0.0197 to −0.0013 | 4.98 | 1 | 0.03 | ||
| Duration (weeks) | 18 | −0.0163 | −0.0523 to 0.0196 | 0.79 | 1 | 0.37 | ||
| Frequency (per week) | 18 | 0.0430 | −0.1166 to 0.2026 | 0.28 | 1 | 0.60 | ||
| Total training time (during experiment) | 18 | −0.0001 | −0.0002 to 0.0001 | 1.38 | 1 | 0.24 | ||
| Age | 18 | −0.0031 | −0.03333 to 0.0271 | 0.042 | 1 | 0.84 | ||
| Male percentage | 18 | −0.0022 | −0.0145 to 0.0101 | 0.12 | 1 | 0.73 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Chen, S.; Liu, X.; Zhang, Y.; Zhao, M.; Li, W. Physical Activity Improves Cognition and Activities of Daily Living in Adults with Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 1216. https://doi.org/10.3390/ijerph19031216
Zhou S, Chen S, Liu X, Zhang Y, Zhao M, Li W. Physical Activity Improves Cognition and Activities of Daily Living in Adults with Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. International Journal of Environmental Research and Public Health. 2022; 19(3):1216. https://doi.org/10.3390/ijerph19031216
Chicago/Turabian StyleZhou, Shengwen, Sitong Chen, Xiaolei Liu, Yanjie Zhang, Mengxian Zhao, and Wenjiao Li. 2022. "Physical Activity Improves Cognition and Activities of Daily Living in Adults with Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" International Journal of Environmental Research and Public Health 19, no. 3: 1216. https://doi.org/10.3390/ijerph19031216
APA StyleZhou, S., Chen, S., Liu, X., Zhang, Y., Zhao, M., & Li, W. (2022). Physical Activity Improves Cognition and Activities of Daily Living in Adults with Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. International Journal of Environmental Research and Public Health, 19(3), 1216. https://doi.org/10.3390/ijerph19031216







