Cerebellar Agenesis and Bilateral Polimicrogyria Associated with Rare Variants of CUB and Sushi Multiple Domains 1 Gene (CSMD1): A Longitudinal Neuropsychological and Neuroradiological Case Study
Abstract
1. Introduction
Clinical Case
2. Materials and Methods
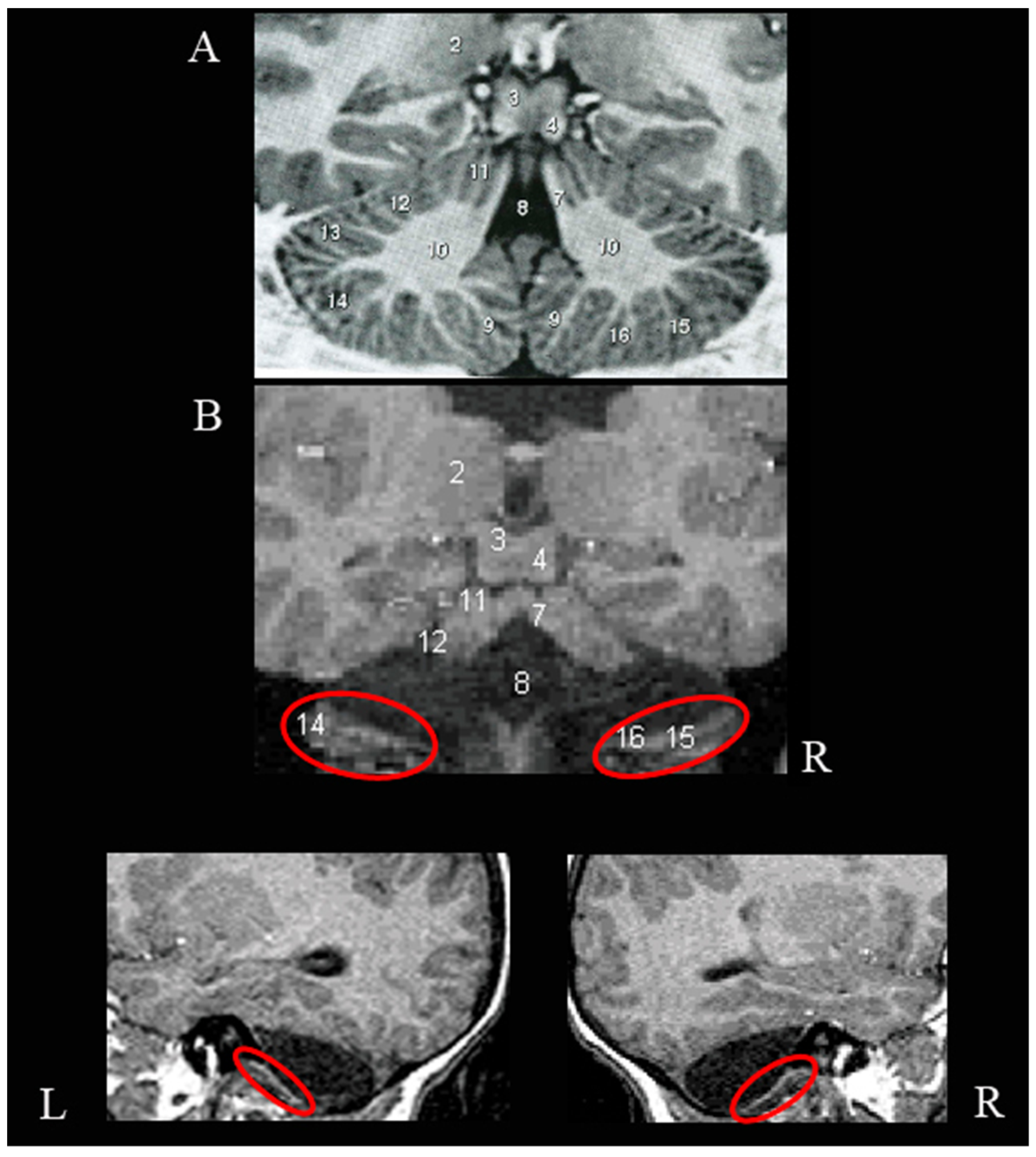
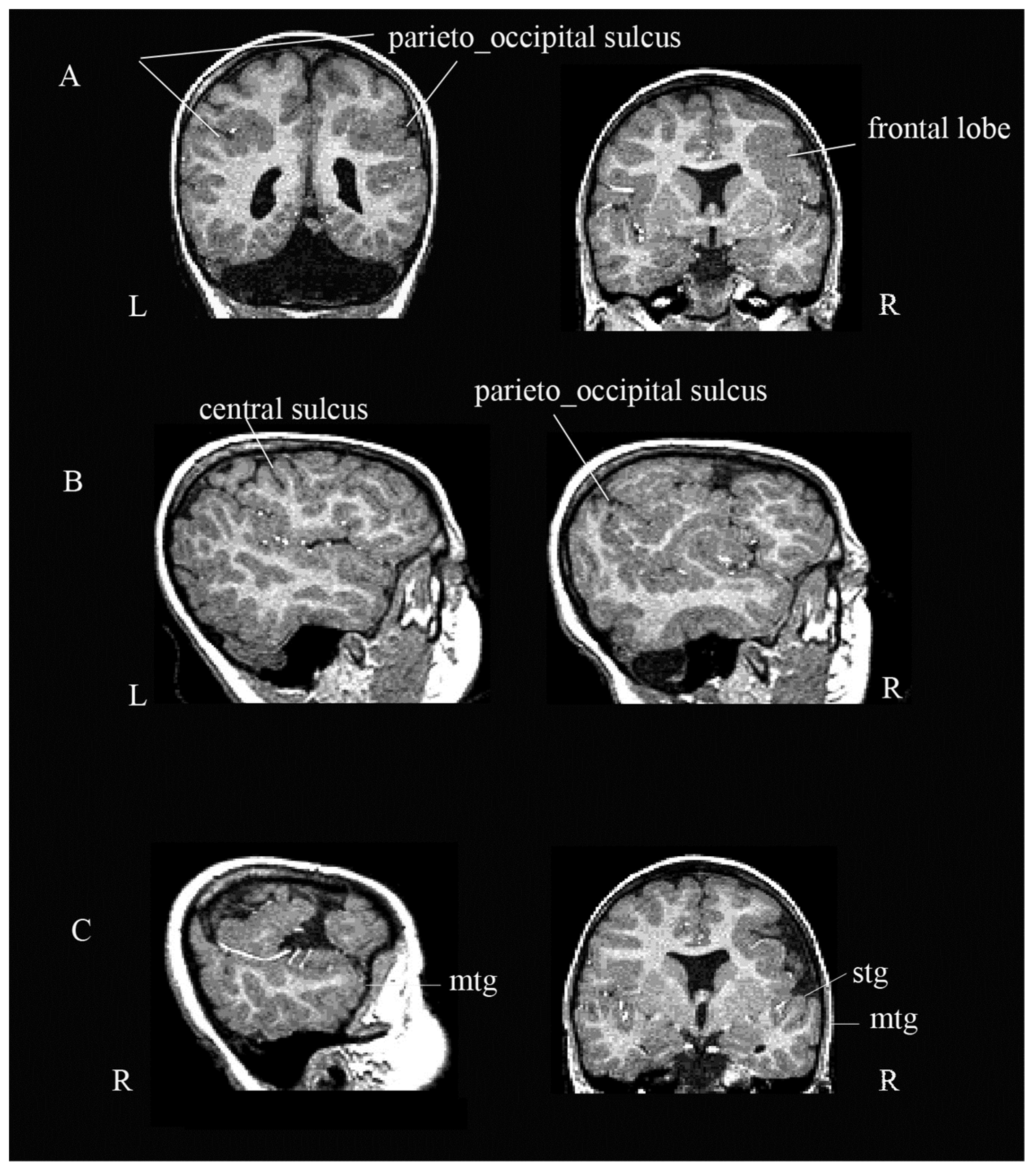
2.1. Neuroradiological Examination
2.2. Genetic Analysis
2.2.1. Exome Sequencing
2.2.2. Whole Genome Array-CGH
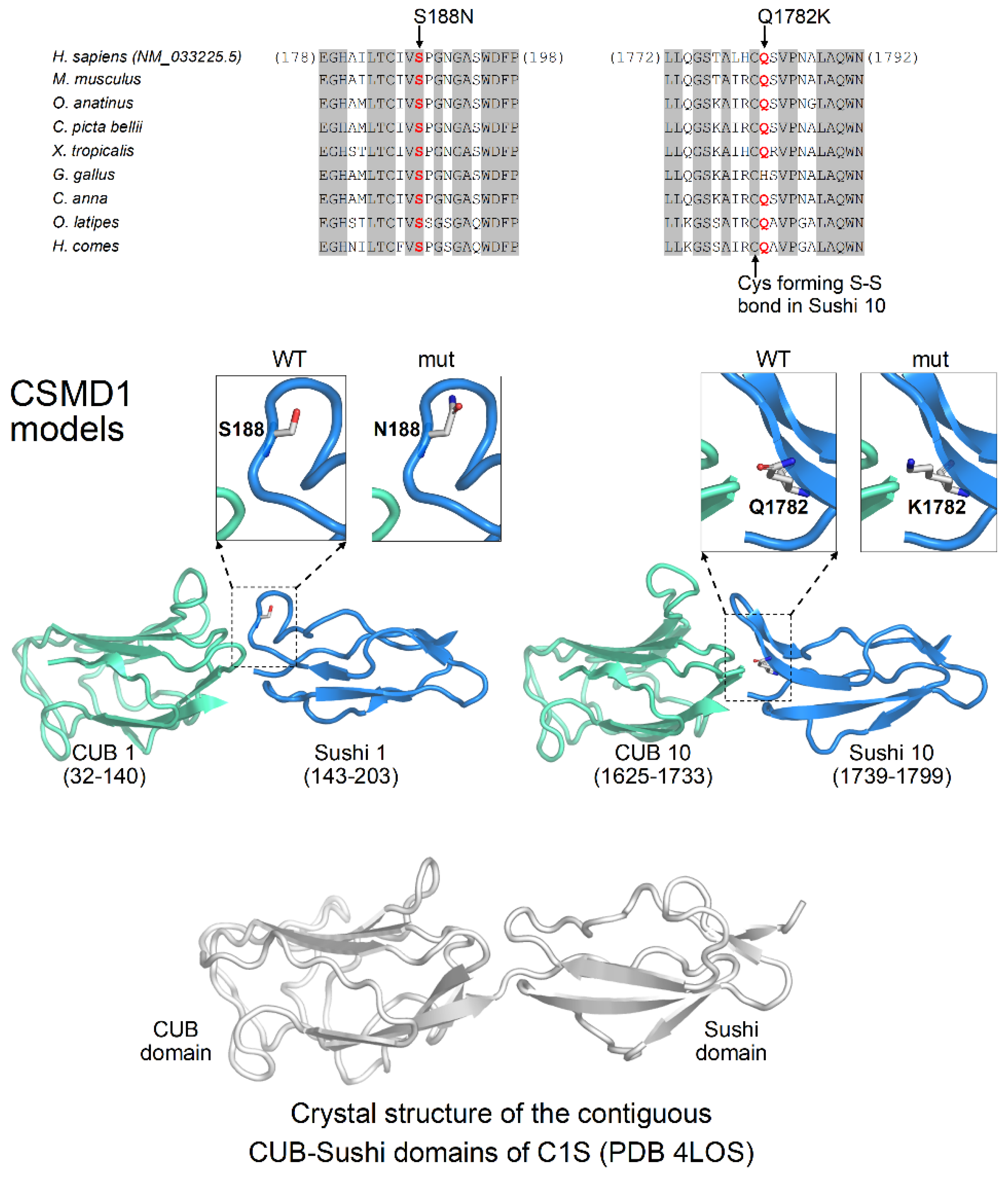
2.2.3. Homology Modeling
2.3. Neuropsychological and Behavioral Examination
3. Results
3.1. Neuroradiological Examination
3.2. Genetic Analysis
Homology Modeling
3.3. Neuropsychological and Behavioral Examination
3.3.1. Intellectual Level
3.3.2. Neuropsychological Tasks
4. Discussion
4.1. Neuropsychological Outcome
4.2. Behavioral, Psychopathological and Adaptive Outcome
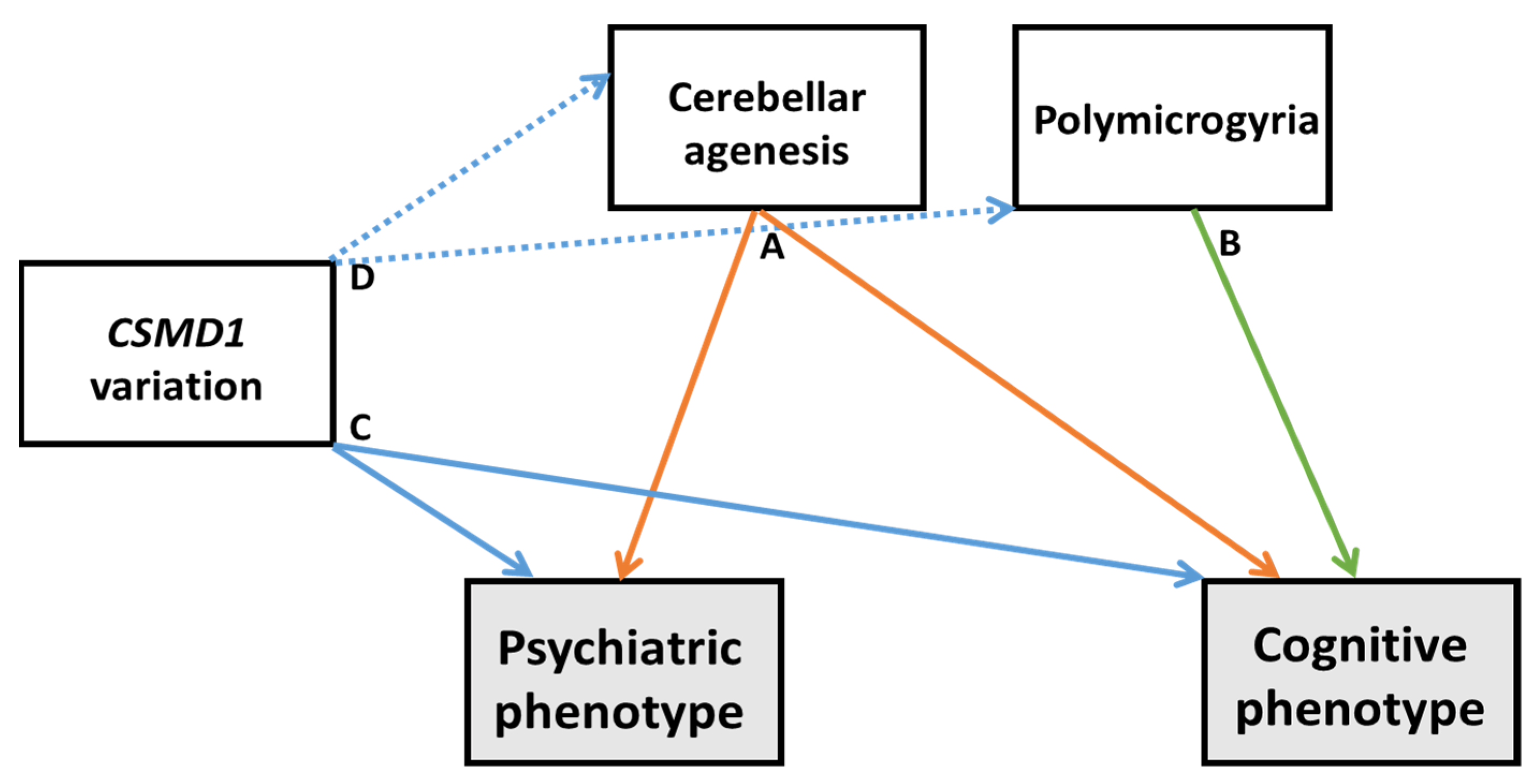
4.3. Etiological Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leto, K.; Arancillo, M.; Becker, E.B.E.; Buffo, A.; Chiang, C.; Ding, B.; Dobyns, W.; Dusart, I.; Haldipur, P.; Hatten, M.E.; et al. Consensus Paper: Cerebellar Development. Cerebellum 2016, 15, 789–828. [Google Scholar] [CrossRef] [PubMed]
- Lerman-Sagie, T.; Prayer, D.; Stöcklein, S.; Malinger, G. Fetal Cerebellar Disorders. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 155, pp. 3–23. ISBN 978-0-444-64189-2. [Google Scholar]
- Serrano, M. Epigenetic Cerebellar Diseases. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 155, pp. 227–244. ISBN 978-0-444-64189-2. [Google Scholar]
- Stewart, R.M. Cerebellar Agenesis. J. Ment. Sci. 1956, 102, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.J.M.; Coleman, L.T.; Mitchell, L.A.; Smith, L.J.; Harvey, A.S.; Scheffer, I.E.; Storey, E.; Nowotny, M.J.; Sloane, R.A.; Lubitz, L. Near-Total Absence of the Cerebellum. Neuropediatrics 2001, 32, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Titomanlio, L.; Romano, A.; Del Giudice, E. Cerebellar agenesis. Neurology 2005, 64, E21. [Google Scholar] [CrossRef]
- Poretti, A.; Prayer, D.; Boltshauser, E. Morphological spectrum of prenatal cerebellar disruptions. Eur. J. Paediatr. Neurol. 2009, 13, 397–407. [Google Scholar] [CrossRef]
- Meola, A.; Fernandez-Miranda, J.C. Peduncles Without Cerebellum: The Cerebellar Agenesis. Eur. Neurol. 2015, 74, 162. [Google Scholar] [CrossRef]
- Bosemani, T.; Poretti, A. Cerebellar disruptions and neurodevelopmental disabilities. Semin. Fetal Neonatal Med. 2016, 21, 339–348. [Google Scholar] [CrossRef]
- Sellick, G.S.; Barker, K.T.; Stolte-Dijkstra, I.; Fleischmann, C.; Coleman, R.J.; Garrett, C.; Gloyn, A.; Edghill, E.L.; Hattersley, A.T.; Wellauer, P.K.; et al. Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat. Genet. 2004, 36, 1301–1305. [Google Scholar] [CrossRef]
- Nowak, D.A.; Timmann, D.; Hermsdörfer, J. Dexterity in cerebellar agenesis. Neuropsychologia 2007, 45, 696–703. [Google Scholar] [CrossRef]
- Glickstein, M. Cerebellar agenesis. Brain 1994, 117, 1209–1212. [Google Scholar] [CrossRef]
- Timmann, D.; Dimitrova, A.; Hein-Kropp, C.; Wilhelm, H.; Dörfler, A. Cerebellar Agenesis: Clinical, Neuropsychological and MR Findings. Neurocase 2003, 9, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Manto, M.; Mariën, P. Schmahmann’s syndrome—Identification of the third cornerstone of clinical ataxiology. Cerebellum Ataxias 2015, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D. The cerebellum and cognition. Neurosci. Lett. 2019, 688, 62–75. [Google Scholar] [CrossRef]
- Boyd, J.D. A Case of Neocerebellar Hypoplasia. J. Anat. 1940, 74, 557. [Google Scholar]
- Sener, R.N.; Jinkins, J.R. Subtotal agenesis of the cerebellum in an adult: MRI Demonstration. Neuroradiology 1993, 35, 286–287. [Google Scholar] [CrossRef]
- Sener, R. Cerebellar agenesis versus vanishing cerebellum in Chiari II malformation. Comput. Med. Imaging Graph. 1995, 19, 491–494. [Google Scholar] [CrossRef]
- Velioglu, S.K.; Kuzeyli, K.; Ozmenoglu, M. Cerebellar agenesis: A case report with clinical and MR imaging findings and a review of the literature. Eur. J. Neurol. 1998, 5, 503–506. [Google Scholar] [CrossRef]
- Ashraf, O.; Jabeen, S.; Khan, A.; Shaheen, F. Primary cerebellar agenesis presenting as isolated cognitive impairment. J. Pediatr. Neurosci. 2016, 11, 150–152. [Google Scholar] [CrossRef]
- Yu, F.; Jiang, Q.-J.; Sun, X.-Y.; Zhang, R.-W. A new case of complete primary cerebellar agenesis: Clinical and imaging findings in a living patient. Brain 2015, 138, e353. [Google Scholar] [CrossRef][Green Version]
- Wu, B.; Yao, J.; Wu, G.-Y.; Li, X.; Gao, W.-J.; Zhang, R.-W.; Sui, J.-F. Absence of associative motor learning and impaired time perception in a rare case of complete cerebellar agenesis. Neuropsychologia 2018, 117, 551–557. [Google Scholar] [CrossRef]
- Mitoma, H.; Manto, M. The physiological basis of therapies for cerebellar ataxias. Ther. Adv. Neurol. Disord. 2016, 9, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Chheda, M.G.; Sherman, J.C.; Schmahmann, J.D. Neurologic, Psychiatric, and Cognitive Manifestations in Cerebellar Agenesis. Neurology 2002, 58, A356. [Google Scholar]
- Ronconi, L.; Casartelli, L.; Carna, S.; Molteni, M.; Arrigoni, F.; Borgatti, R. When one is Enough: Impaired Multisensory Integration in Cerebellar Agenesis. Cereb. Cortex 2016, 27, bhw049. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Dimitrova, A.; Hein-Kropp, C.; Wilhelm, H.; Gizewski, E.; Timmann, D. Cerebellar agenesis II: Motor and language functions. Neurocase 2005, 11, 103–113. [Google Scholar] [CrossRef]
- Mormina, E.; Briguglio, M.; Morabito, R.; Arrigo, A.; Marino, S.; Di Rosa, G.; Micalizzi, A.; Valente, E.M.; Salpietro, V.; Vinci, S.L.; et al. A rare case of cerebellar agenesis: A probabilistic Constrained Spherical Deconvolution tractographic study. Brain Imaging Behav. 2015, 10, 158–167. [Google Scholar] [CrossRef]
- Gelal, F.M.; Kalaycı, T.; Celebisoy, M.; Karakas, L.; Akkurt, H.E.; Koc, F. Clinical and MRI findings of cerebellar agenesis in two living adult patients. Ann. Indian Acad. Neurol. 2016, 19, 255–257. [Google Scholar] [CrossRef]
- Patel, S.; Barkovich, A.J. Analysis and Classification of Cerebellar Malformations. AJNR Am. J. Neuroradiol. 2002, 23, 1074–1087. [Google Scholar]
- Cushion, T.; Dobyns, W.; Mullins, J.; Stoodley, N.; Chung, S.-K.; Fry, A.E.; Hehr, U.; Gunny, R.; Aylsworth, A.S.; Prabhakar, P.; et al. Overlapping cortical malformations and mutations in TUBB2B and TUBA1A. Brain 2013, 136, 536–548. [Google Scholar] [CrossRef]
- Parisi, M.A.; Dobyns, W. Human malformations of the midbrain and hindbrain: Review and proposed classification scheme. Mol. Genet. Metab. 2003, 80, 36–53. [Google Scholar] [CrossRef]
- Sajan, S.A.; Fernandez, L.; Nieh, S.E.; Rider, E.; Bukshpun, P.; Wakahiro, M.; Christian, S.L.; Rivière, J.-B.; Sullivan, C.T.; Sudi, J.; et al. Both Rare and De Novo Copy Number Variants Are Prevalent in Agenesis of the Corpus Callosum but Not in Cerebellar Hypoplasia or Polymicrogyria. PLoS Genet. 2013, 9, e1003823. [Google Scholar] [CrossRef]
- Oegema, R.; Cushion, T.; Phelps, I.G.; Chung, S.-K.; Dempsey, J.C.; Collins, S.; Mullins, J.; Dudding, T.; Gill, H.; Green, A.; et al. Recognizable cerebellar dysplasia associated with mutations in multiple tubulin genes. Hum. Mol. Genet. 2015, 24, 5313–5325. [Google Scholar] [CrossRef] [PubMed]
- Breuss, M.W.; Nguyen, T.; Srivatsan, A.; Leca, I.; Tian, G.; Fritz, T.; Hansen, A.H.; Musaev, D.; McEvoy-Venneri, J.; James, K.N.; et al. Uner Tan syndrome caused by a homozygous TUBB2B mutation affecting microtubule stability. Hum. Mol. Genet. 2016, 26, 258–269. [Google Scholar] [CrossRef]
- Henderson, S.E.; Sugden, D.; Barnett, A.L. Harcourt Assessment Movement Assessment Battery for Children-2; Harcourt Assessment: London, UK, 2007; ISBN 978-0-7491-0167-1. [Google Scholar]
- Šali, A.; Blundell, T.L. Comparative Protein Modelling by Satisfaction of Spatial Restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.; Garthwaite, P.H. Investigation of the single case in neuropsychology: Confidence limits on the abnormality of test scores and test score differences. Neuropsychologia 2002, 40, 1196–1208. [Google Scholar] [CrossRef]
- Roid, G.H.; Miller, L.J. Leiter-R: Leiter International Performance Scale-Revised; Giunti. Organizzazioni Speciali: Firenze, Italy, 2015; ISBN 978-88-09-40229-4. [Google Scholar]
- Tavano, A.; Fabbro, F.; Borgatti, R. Language and Social Communication in Children with Cerebellar Dysgenesis. Folia Phoniatr. Logop. 2007, 59, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, L.E.; Brookshire, R.H.; MacLennan, D.L.; Schumacher, J.G.; Porrazzo, S.A. Revised administration and scoring procedures for the Boston Naming test and norms for non-brain-damaged adults. Aphasiology 1989, 3, 569–580. [Google Scholar] [CrossRef]
- Riva, D.; Nichelli, F.; Devoti, M. Developmental Aspects of Verbal Fluency and Confrontation Naming in Children. Brain Lang. 2000, 71, 267–284. [Google Scholar] [CrossRef]
- Cianchetti, C.; Sannio Fancello, G. Test di Valutazione del Linguaggio Livello Prescolare; Organizzazioni Speciali: Firenze, Italy, 1997. [Google Scholar]
- Dunn, L.M.; Dunn, L.M.; Stella, G. Peabody: Test di Vocabolario Recettivo = Peabody Picture Vocabulary Test, PPVT; Omega: Torino, Italy, 2000; ISBN 978-88-7241-326-5. [Google Scholar]
- Rustioni Metz Lancaster, D. Associazione la Nostra Famiglia PVCL: Prove di Valutazione della Comprensione Linguistica: Manuale + Schede di Registrazione; Giunti, O.S., Ed.; Organizzazioni Speciali: Firenze, Italy, 2008; ISBN 978-88-09-40309-3. [Google Scholar]
- Marotta, L.; Ronchetti, C. CMF: Valutazione delle Competenze Metafonologiche; Erickson: Trento, Italy, 2008; ISBN 978-88-6137-311-2. [Google Scholar]
- Vicari, S. PROMEA: Prove di Memoria e Apprendimento per L’età Evolutiva: Manuale; Giunti, O.S., Ed.; Organizzazioni Speciali: Firenze, Italy, 2007; ISBN 978-88-09-40299-7. [Google Scholar]
- Nissen, M.J.; Bullemer, P. Attentional requirements of learning: Evidence from performance measures. Cogn. Psychol. 1987, 19, 1–32. [Google Scholar] [CrossRef]
- Vicari, S.; Finzi, A.; Menghini, D.; Marotta, L.; Baldi, S.; Petrosini, L. Do children with developmental dyslexia have an implicit learning deficit? J. Neurol. Neurosurg. Psychiatry 2005, 76, 1392–1397. [Google Scholar] [CrossRef]
- Biancardi, A.; Stoppa, E. Il Test delle Campanelle Modificato: Una Proposta per lo Studio Dell’attenzione in Età Evolutiva. Psichiatr. Dell’Infanz. Dell’Adolesc. 1997, 64, 73–84. [Google Scholar]
- Sannio Fancello, G.; Vio, C.; Cianchetti, C. Torre di Londra: Test di Valutazione delle Funzioni Esecutive (Pianificazione e Problem Solving); Test e Strumenti di Valutazione Psicologica e Educativa; Erickson: Trento, Italy, 2006; ISBN 978-88-7946-941-8. [Google Scholar]
- Menghini, D.; Armando, M.; Calcagni, M.; Napolitano, C.; Pasqualetti, P.; Sergeant, J.A.; Pani, P.; Vicari, S. The influence of Generalized Anxiety Disorder on Executive Functions in children with ADHD. Eur. Arch. Psychiatry Clin. Neurosci. 2018, 268, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Beery, K.E. VMI Developmental Test of Visuo-Motor Integration; Organizzazioni Speciali: Firenze, Italy, 2000. [Google Scholar]
- Hammill, D.D.; Pearson, N.A.; Voress, J.K. Ianes, Rio Test TPV: Test di Percezione Visiva e Integrazione Visuo-Motoria; Centro studi Erickson: Trento, Italy, 1994; ISBN 978-88-7946-114-6. [Google Scholar]
- Cornoldi, C.; Colpo, G. Nuove Prove di Lettura MT per la Scuola Media Inferiore, Manuale; Organizzazioni Speciali: Firenze, Italy, 1995. [Google Scholar]
- Stella, G.; Apolito, A. Lo Screening Precoce Nella Scuola Elementare: Può Una Prova di 16 Parole Prevedere i Disturbi Specifici di Apprendimento? Dislessia 2014, 1, 111–118. [Google Scholar]
- Cornoldi, C.; Lucangeli, D.; Bellina, M. AC-MT: Test di Valutazione Delle Abilità di Calcolo—Gruppo MT; Erikson: Trento, Italy, 2002; ISBN 978-88-7946-477-2. [Google Scholar]
- Sparrow, S.S.; Cicchetti, D.V.; Balla, D.A.; Pedrabissi, L.; Balboni, G. Vineland Adaptive Behavior Scales: Intervista, Forma Completa: Manuale; Organizzazioni Speciali: Firenze, Italy, 2003; ISBN 978-88-09-40231-7. [Google Scholar]
- Balboni, G.; Sparrow, S.S. Vineland-II: Vineland Adaptive Behavior Scales; Giunti, O.S., Ed.; Psychometrics: Firenze, Italy, 2017; ISBN 978-88-09-99473-7. [Google Scholar]
- Achenbach, T.M.; Rescorla, L.A. Manual for the ASEBA School-Age Form & Profiles; University of Vermont, Research Center for Children, Youth, and Families: Burlington, VT, USA, 2001. [Google Scholar]
- Kaufman, J.; Birmaher, B.; Brent, D.; Rao, U.; Flynn, C.; Moreci, P.; Williamson, D.; Ryan, N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial Reliability and Validity Data. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, M.-E.; Limperopoulos, C. Neurodevelopmental outcomes in children with cerebellar malformations: A systematic review. Dev. Med. Child Neurol. 2009, 51, 256–267. [Google Scholar] [CrossRef]
- Bolduc, M.-E.; Du Plessis, A.J.; Sullivan, N.; Khwaja, O.S.; Zhang, X.; Barnes, K.; Robertson, R.; Limperopoulos, C. Spectrum of neurodevelopmental disabilities in children with cerebellar malformations. Dev. Med. Child Neurol. 2011, 53, 409–416. [Google Scholar] [CrossRef]
- Etan, U. Two families with quadrupedalism, mental retardation, no speech, and infantile hypotonia (Uner Tan Syndrome Type-II); a novel theory for the evolutionary emergence of human bipedalism. Front. Neurosci. 2014, 8, 84. [Google Scholar] [CrossRef][Green Version]
- Tavano, A.; Fabbro, F.; Borgatti, R. 9. Speaking without the cerebellum: Language skills in a young adult with near total cerebellar agenesis. In Studies in Language Companion Series; Schalley, A.C., Khlentzos, D., Eds.; John Benjamins Publishing Company: Amsterdam, The Netherlands, 2007; Volume 92, pp. 171–189. ISBN 978-90-272-3102-4. [Google Scholar]
- Ventura, P.; Presicci, A.; Perniola, T.; Campa, M.G.; Margari, L. Mental Retardation and Epilepsy in Patients with Isolated Cerebellar Hypoplasia. J. Child Neurol. 2006, 21, 776–781. [Google Scholar] [CrossRef]
- Tavano, A.; Grasso, R.; Gagliardi, C.; Triulzi, F.; Bresolin, N.; Fabbro, F.; Borgatti, R. Disorders of cognitive and affective development in cerebellar malformations. Brain 2007, 130, 2646–2660. [Google Scholar] [CrossRef]
- Tavano, A.; Borgatti, R. Evidence for a link among cognition, language and emotion in cerebellar malformations. Cortex 2010, 46, 907–918. [Google Scholar] [CrossRef]
- von Bernhardi, R.; Bernhardi, L.E.; Eugenín, J. What Is Neural Plasticity? In The Plastic Brain; von Bernhardi, R., Eugenín, J., Muller, K.J., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Germany, 2017; Volume 1015, pp. 1–15. ISBN 978-3-319-62815-8. [Google Scholar]
- Gomez-Beldarrain, M.; Garcia-Monco, J.C.; Rubio, B.; Pascual-Leone, A. Effect of focal cerebellar lesions on procedural learning in the serial reaction time task. Exp. Brain Res. 1998, 120, 25–30. [Google Scholar] [CrossRef]
- Menghini, D.; Hagberg, G.E.; Caltagirone, C.; Petrosini, L.; Vicari, S. Implicit learning deficits in dyslexic adults: An fMRI study. NeuroImage 2006, 33, 1218–1226. [Google Scholar] [CrossRef]
- Menghini, D.; Di Paola, M.; Murri, R.; Costanzo, F.; Caltagirone, C.; Vicari, S.; Petrosini, L. Cerebellar vermis abnormalities and cognitive functions in individuals with Williams syndrome. Res. Dev. Disabil. 2013, 34, 2118–2126. [Google Scholar] [CrossRef]
- Bo, J.; Peltier, S.; Noll, D.; Seidler, R. Symbolic representations in motor sequence learning. NeuroImage 2011, 54, 417–426. [Google Scholar] [CrossRef][Green Version]
- Wilkinson, L.; Khan, Z.; Jahanshahi, M. The role of the basal ganglia and its cortical connections in sequence learning: Evidence from implicit and explicit sequence learning in Parkinson’s disease. Neuropsychologia 2009, 47, 2564–2573. [Google Scholar] [CrossRef] [PubMed]
- Gobel, E.W.; Blomeke, K.; Zadikoff, C.; Simuni, T.; Weintraub, S.; Reber, P.J. Implicit perceptual-motor skill learning in mild cognitive impairment and Parkinson’s disease. Neuropsychology 2013, 27, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Steinlin, M.; Imfeld, S.; Zulauf, P.; Boltshauser, E.; Lovblad, K.-O.; Lüthy, A.R.; Perrig, W.; Kaufmann, F. Neuropsychological long-term sequelae after posterior fossa tumour resection during childhood. Brain 2003, 126, 1998–2008. [Google Scholar] [CrossRef] [PubMed]
- Steinlin, M. Non-progressive congenital ataxia with or without cerebellar hypoplasia: A review of 34 subjects. Dev. Med. Child Neurol. 2008, 40, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Ward, J. The Student’s Guide to Cognitive Neuroscience; Taylor & Francis: London, UK, 2020. [Google Scholar] [CrossRef]
- Carta, I.; Chen, C.H.; Schott, A.L.; Dorizan, S.; Khodakhah, K. Cerebellar modulation of the reward circuitry and social behavior. Science 2019, 363, eaav0581. [Google Scholar] [CrossRef]
- Nicolson, R.I.; Fawcett, A.J. Dyslexia, dysgraphia, procedural learning and the cerebellum. Cortex 2011, 47, 117–127. [Google Scholar] [CrossRef]
- Boscariol, M.; Guimarães, C.A.; Hage, S.; Cendes, F.; Guerreiro, M.M. Processamento temporal auditivo: Relação com dislexia do desenvolvimento e malformação cortical. Pró-Fono Rev. Atualização Cient. 2010, 22, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Boscariol, M.; Guimarães, C.A.; Hage, S.R.D.V.; Garcia, V.L.; Schmutzler, K.M.R.; Cendes, F.; Guerreiro, M.M. Auditory processing disorder in patients with language-learning impairment and correlation with malformation of cortical development. Brain Dev. 2011, 33, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.; Leonard, G.; Bastos, A.C.; Esposito-Festen, J.E.; Tampieri, D.; Watkins, K.; Andermann, F.; Andermann, E. Cognitive functioning in bilateral perisylvian polymicrogyria (BPP): Clinical and radiological correlations. Epilepsy Behav. 2005, 6, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.C. Cognitive deficits and developmental language disorders in patients with malformations of cortical development. Epilepsia 2010, 51, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Saletti, V.; Bulgheroni, S.; D’Incerti, L.; Franceschetti, S.; Molteni, B.; Airaghi, G.; Pantaleoni, C.; D’Arrigo, S.; Riva, D. Verbal and Gestural Communication in Children With Bilateral Perisylvian Polymicrogyria. J. Child Neurol. 2007, 22, 1090–1098. [Google Scholar] [CrossRef]
- Hage, S.R.D.V.; Cendes, F.; Montenegro, M.A.; Abramides, D.V.; Guimarães, C.A.; Guerreiro, M.M. Specific language impairment: Linguistic and neurobiological aspects. Arq. Neuro-Psiquiatr. 2006, 64, 173–180. [Google Scholar] [CrossRef]
- Parrini, E.; Conti, V.; Dobyns, W.B.; Guerrini, R. Genetic Basis of Brain Malformations. Mol. Syndromol. 2016, 7, 220–233. [Google Scholar] [CrossRef]
- Chai, W.J.; Hamid, A.I.A.; Abdullah, J.M. Working Memory From the Psychological and Neurosciences Perspectives: A Review. Front. Psychol. 2018, 9, 401. [Google Scholar] [CrossRef]
- Eliez, S.; Rumsey, J.M.; Giedd, J.N.; Schmitt, J.E.; Patwardhan, A.J.; Reiss, A.L. Morphological Alteration of Temporal Lobe Gray Matter in Dyslexia: An MRI Study. J. Child Psychol. Psychiatry 2000, 41, 637–644. [Google Scholar] [CrossRef]
- Raschle, N.M.; Zuk, J.; Gaab, N. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proc. Natl. Acad. Sci. USA 2012, 109, 2156–2161. [Google Scholar] [CrossRef]
- Ye, Z.; Rüsseler, J.; Gerth, I.; Münte, T.F. Audiovisual speech integration in the superior temporal region is dysfunctional in dyslexia. Neuroscience 2017, 356, 1–10. [Google Scholar] [CrossRef]
- TotalBoox. TBX the Cerebellum and Cognition; Elsevier Science: Amsterdam, The Netherlands, 1997; ISBN 978-0-08-085775-6. [Google Scholar]
- Mazza, M.G.; Rossetti, A.; Crespi, G.; Clerici, M. Prevalence of co-occurring psychiatric disorders in adults and adolescents with intellectual disability: A systematic review and meta-analysis. J. Appl. Res. Intellect. Disabil. 2020, 33, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Thomas, M.; Bright, C. Mental disorder in adults with intellectual disability. 2: The rate of behaviour disorders among a community-based population aged between 16 and 64 years. J. Intellect. Disabil. Res. 2001, 45, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Smiley, E. Epidemiology of mental health problems in adults with learning disability: An update. Adv. Psychiatr. Treat. 2005, 11, 214–222. [Google Scholar] [CrossRef]
- Schmahmann, J.D. The cerebellar cognitive affective syndrome. Brain 1998, 121, 561–579. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D. Disorders of the Cerebellum: Ataxia, Dysmetria of Thought, and the Cerebellar Cognitive Affective Syndrome. J. Neuropsychiatry Clin. Neurosci. 2004, 16, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D.; Weilburg, J.B.; Sherman, J.C. The neuropsychiatry of the cerebellum—Insights from the clinic. Cerebellum 2007, 6, 254–267. [Google Scholar] [CrossRef]
- Cukier, H.N.; Dueker, N.D.; Slifer, S.H.; Lee, J.M.; Whitehead, P.L.; Lalanne, E.; Leyva, N.; Konidari, I.; Gentry, R.C.; Hulme, W.F.; et al. Exome sequencing of extended families with autism reveals genes shared across neurodevelopmental and neuropsychiatric disorders. Mol. Autism 2014, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Cohen-Woods, S.; Chen, Q.; Noor, A.; Knight, J.; Hosang, G.; Parikh, S.V.; De Luca, V.; Tozzi, F.; Muglia, P.; et al. Genome-wide association study of bipolar disorder in Canadian and UK populations corroborates disease loci including SYNE1 and CSMD1. BMC Med. Genet. 2014, 15, 2. [Google Scholar] [CrossRef]
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016, 352, 712–716. [Google Scholar] [CrossRef]
- Ruiz-Martínez, J.; Azcona, L.J.; Bergareche, A.; Martí-Massó, J.F.; Paisán-Ruiz, C. Whole-exome sequencing associates novel CSMD1 gene mutations with familial Parkinson disease. Neurol. Genet. 2017, 3, e177. [Google Scholar] [CrossRef]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, X.; Tang, Z.; Li, C.; Xu, Y.; Zhang, F.; Zhou, D.; Zhu, C. Altered expression of the CSMD1 gene in the peripheral blood of schizophrenia patients. BMC Psychiatry 2019, 19, 113. [Google Scholar] [CrossRef]
- Kraus, D.M.; Elliott, G.S.; Chute, H.; Horan, T.; Pfenninger, K.H.; Sanford, S.D.; Foster, S.; Scully, S.; Welcher, A.A.; Holers, V.M. CSMD1 Is a Novel Multiple Domain Complement-Regulatory Protein Highly Expressed in the Central Nervous System and Epithelial Tissues. J. Immunol. 2006, 176, 4419–4430. [Google Scholar] [CrossRef]
- Steen, V.M.; Nepal, C.; Ersland, K.M.; Holdhus, R.; Nævdal, M.; Ratvik, S.M.; Skrede, S.; Håvik, B. Neuropsychological Deficits in Mice Depleted of the Schizophrenia Susceptibility Gene CSMD1. PLoS ONE 2013, 8, e79501. [Google Scholar] [CrossRef]
- Luykx, J.; Bakker, S.C.; Lentjes, E.; Neeleman, M.; Strengman, E.; Mentink, L.; Deyoung, J.; De Jong, S.; Sul, J.H.; Eskin, E.; et al. Genome-wide association study of monoamine metabolite levels in human cerebrospinal fluid. Mol. Psychiatry 2013, 19, 228–234. [Google Scholar] [CrossRef]
- Loh, K.H.; Stawski, P.S.; Draycott, A.S.; Udeshi, N.D.; Lehrman, E.K.; Wilton, D.K.; Svinkina, T.; Deerinck, T.J.; Ellisman, M.H.; Stevens, B.; et al. Proteomic Analysis of Unbounded Cellular Compartments: Synaptic Clefts. Cell 2016, 166, 1295–1307.e21. [Google Scholar] [CrossRef]
- Gutierrez, M.A.; Dwyer, B.E.; Franco, S.J. Csmd2 Is a Synaptic Transmembrane Protein that Interacts with PSD-95 and Is Required for Neuronal Maturation. Eneuro 2019, 6, ENEURO.0434-18.2019. [Google Scholar] [CrossRef]
- Mizukami, T.; Kohno, T.; Hattori, M. CUB and Sushi multiple domains 3 regulates dendrite development. Neurosci. Res. 2016, 110, 11–17. [Google Scholar] [CrossRef]
- Koiliari, E.; Roussos, P.; Pasparakis, E.; Lencz, T.; Malhotra, A.; Siever, L.J.; Giakoumaki, S.; Bitsios, P. The CSMD1 genome-wide associated schizophrenia risk variant rs10503253 affects general cognitive ability and executive function in healthy males. Schizophr. Res. 2014, 154, 42–47. [Google Scholar] [CrossRef]
- Athanasiu, L.; Giddaluru, S.; Fernandes, C.; Christoforou, A.; Reinvang, I.; Lundervold, A.J.; Nilsson, L.-G.; Kauppi, K.; Adolfsson, R.; Eriksson, E.; et al. A genetic association study of CSMD1 and CSMD2 with cognitive function. Brain Behav. Immun. 2017, 61, 209–216. [Google Scholar] [CrossRef]
- Crippa, M.; Malatesta, P.; Bonati, M.T.; Trapasso, F.; Fortunato, F.; Annesi, G.; Larizza, L.; Labate, A.; Finelli, P.; Perrotti, N.; et al. A familial t(4;8) translocation segregates with epilepsy and migraine with aura. Ann. Clin. Transl. Neurol. 2020, 7, 855–859. [Google Scholar] [CrossRef]
- Bartley, J.; Friedrich, E. Partial duplication of CSMD1 (CUB and sushi multiple domains 1) (arr cgh 8p23.2((2,756,521->3,188,217))x3) associated with myoclonic seizures in a one year old female. In Proceedings of the 59th Annual Meeting of The American Society of Human Genetics, Honolulu, Hawaii, 20–24 October 2009. [Google Scholar]
- Giddaluru, S.; Espeseth, T.; Salami, A.; Westlye, L.T.; Lundquist, A.; Christoforou, A.; Cichon, S.; Adolfsson, R.; Steen, V.M.; Reinvang, I.; et al. Genetics of structural connectivity and information processing in the brain. Brain Struct. Funct. 2016, 221, 4643–4661. [Google Scholar] [CrossRef]
- Meda, S.A.; Ruaño, G.; Windemuth, A.; O’Neil, K.; Berwise, C.; Dunn, S.M.; Boccaccio, L.E.; Narayanan, B.; Kocherla, M.; Sprooten, E.; et al. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc. Natl. Acad. Sci. USA 2014, 111, E2066–E2075. [Google Scholar] [CrossRef]
- Argyropoulos, G.P.D.; Van Dun, K.; Adamaszek, M.; Leggio, M.; Manto, M.; Masciullo, M.; Molinari, M.; Stoodley, C.J.; Van Overwalle, F.; Ivry, R.B.; et al. The Cerebellar Cognitive Affective/Schmahmann Syndrome: A Task Force Paper. Cerebellum 2020, 19, 102–125. [Google Scholar] [CrossRef]
| Neuropsychological Assessment | First Evaluation (5.7 yrs) | Lower Limit Of 95% Tolerance Interval For Chronological Age Norms (5.8)/*CA-1 Matched Controls (N = 12), Mean (SD) | Lower Limit of 95% Tolerance Interval for Mental Age Norms (3.7)/*MA-1 Matched Controls (n = 10), Mean (SD) | Second Evaluation (11 yrs) | Lower Limit of 95% Tolerance Interval for Chronological Age Norms (11.7)/*CA-2 Matched Controls (n = 12), Mean (SD) | Lower Limit of 95% Tolerance Interval for Mental Age Norms (6.7)/*MA-2 Matched Controls (n = 12), Mean (SD) |
|---|---|---|---|---|---|---|
| Language | ||||||
| Expression | ||||||
| Lexical | 9 aa,bb | 15.06 | 10.88 | 27 aa | 31.8 | 12 |
| Morphosyntactic | 7.5 aa | 10.42 | 3.46 | 10 aa,bb | N.A. | 11.9 |
| Comprehension | ||||||
| Lexical | 27 aa,b | 52 | 11 | 84 aa,b | 117 | 84 |
| Morphosyntactic | 67.2 | 33 | 43.9 aa,b | N.A. | 37.9 | |
| Phonological Awareness | ||||||
| Syllabic Blending | 12 | 11 | ||||
| Syllabic Segmentation | 14 | 8 | ||||
| Phonological Blending | 0 aa,bb | N.A. | 5 | |||
| Phonological Segmentation | 0 aa,bb | N.A. | 0 | |||
| Memory | ||||||
| Short-term and Working Memory | ||||||
| Word Span | 3 | 2.6 | 3 aa,b | 3.6 | 2.8 | |
| Nonword Repetition | 7 aa,bb | 13 | *26.6 (9.5) | 28 a | 28 | 20 |
| Visual Span | 0.4 aa | 2.6 | *1.82 (1.23) | 3 a | 3 | 2.6 |
| Spatial Span | 2.8 | 2.6 | 4.2 | 3 | ||
| Explicit Long-term Memory | ||||||
| Word Recall Immediate | 11 | 7 | 14 aa | 19 | 11 | |
| Word Recall Delayed | 0 aa,bb | 1 | *3.8 (1.5) | 4 aa | 6 | 2 |
| Semantic | 7 aa,bb | 14 | *19.9 (4.6) | 18 aa,b | 33 | 18 |
| Visual Immediate | 15 | 5 | 24 a | 24 | 13 | |
| Visual Delayed | 7 | 2 | 9 a | 9 | 4 | |
| Spatial Immediate | 13 a | 8 | *26.3 (10.3) | 44 | 22 | |
| Spatial Delayed | 0 aa,b | 1 | *7.5 (3.4) | 15 | 9 | |
| Implicit Long-term Memory | ||||||
| SRTT I (random) | 840 | *829 (171) | 641 | *471 (61) | ||
| SRTT II (ordered) | 820 | *659 (167) | 688 | *441 (42) | ||
| SRTT III (ordered) | 766 | *579 (118) | 605 | *417 (71) | ||
| SRTT IV (ordered) | 734 | *528 (156) | 716 | *405 (77) | ||
| SRTT V (random) | 781 | *727 (161) | 855 | *442 (55) | ||
| Executive Functions | ||||||
| Attention | ||||||
| Selective | 19 aa | 21.8 | 11.6 | 26 aa,b | 43 | 24.9 |
| Sustained | 46 aa | 65.8 | 41.1 | 96 aa | 119.9 | 78.5 |
| Planning | ||||||
| TOL | 3 aa,bb | 16 | 13 | 15 aa,bb | 23 | 18 |
| Inhibition | ||||||
| Go RTs | 615 | *455 (103) | *612 (236) | 570 aa | *223 (98) | *453 (101) |
| Go omissions | 1 | *0.5 (1.24) | *1.2 (1.2) | 0 | *0.3 (0.2) | *0.8 (1.2) |
| NoGo RTs | 801 | *701 (185) | *843 (151) | 625 aa | *328 (122) | *612 (82) |
| NoGo errors | 2 | *3.66 (6.82) | *3.8 (4.2) | 6 aa,bb | *1.2 (0.6) | *2.3 (1.3) |
| NoGo omissions | 33 aa,bb | *1.25 (2.1) | *3.8 (3.1) | 1 | *0.4 (1.1) | *5.3 (4.1) |
| Visual-Spatial Abilities | ||||||
| Visual-motor integration | ||||||
| Integration | 6 aa | 8 | 2 | 12 aa | 14 | 9 |
| Visual perception | 16 | 9 | 13 aa | 16 | 10 | |
| Motor coordination | 9 a | 9 | 4 | 13 aa | 16 | 9 |
| Perceptual abilities | ||||||
| Spatial Positions | 5 | 4 | 15 aa | 16 | 7 | |
| Confounding Background | 6 | 5 | 16 | 9 |
| Neuropsychological and Behavioural Assessment | 5 ys | 8 ys | 11 ys | 15 ys | |
|---|---|---|---|---|---|
| Neuropsychological Measures | |||||
| Language | Lexical expression |  |  | ||
| Morphosyntactic expression |  |  | |||
| Lexical comprehension |  |  | |||
| Morphosyntactic comprehension |  |  | |||
| Phonogical awareness | Syllabic blending |  |  | ||
| Syllabic segmentation |  |  | |||
| Memory | Short-term verbal |  |  | ||
| Short-term visual |  |  | |||
| Short-term spatial |  |  | |||
| Phonological working memory |  |  | |||
| Episodic verbal memory (immediate) |  |  | |||
| Episodic verbal memory (delayed) |  |  | |||
| Semantic verbal memory |  |  | |||
| Episodic visual memory (immediate) |  |  | |||
| Episodic visual memory (delayed) |  |  | |||
| Episodic spatial memory (immediate) |  |  | |||
| Episodic spatial memory (delayed) |  |  | |||
| Procedural learning |  |  | |||
| Executive | Selective visual attention |  |  | ||
| Sustained visual attention |  |  | |||
| Planning abilities |  |  | |||
| Inhibition |  |  | |||
| Perceptual/visual-spatial | Visual-motor integration |  |  | ||
| Perceptual abilities |  |  | |||
| Academic abilities | |||||
| Reading |  |  | |||
| Writing |  |  | |||
| Math |  |  | |||
| Adaptive level | |||||
| Communication |  |  |  |  | |
| Daily living skills |  |  |  |  | |
| Socialization domain |  |  |  |  | |
| Motor abilities |  |  |  |  | |
| Psychopathological evaluation | |||||
| Mood |  |  |  |  | |
| Anxious/fobia |  |  |  |  | |
| Attention |  |  |  |  | |
| Aggressive behavior |  |  |  |  | |
| PTSD |  |  |  |  | |
| Obsessive |  |  |  |  | |
| Social problems |  |  |  |  | |
| Conduct/dyscontrol |  |  |  |  | |
| Hyperactivity |  |  |  |  | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costanzo, F.; Zanni, G.; Fucà, E.; Di Paola, M.; Barresi, S.; Travaglini, L.; Colafati, G.S.; Gambardella, A.; Bellacchio, E.; Bertini, E.; et al. Cerebellar Agenesis and Bilateral Polimicrogyria Associated with Rare Variants of CUB and Sushi Multiple Domains 1 Gene (CSMD1): A Longitudinal Neuropsychological and Neuroradiological Case Study. Int. J. Environ. Res. Public Health 2022, 19, 1224. https://doi.org/10.3390/ijerph19031224
Costanzo F, Zanni G, Fucà E, Di Paola M, Barresi S, Travaglini L, Colafati GS, Gambardella A, Bellacchio E, Bertini E, et al. Cerebellar Agenesis and Bilateral Polimicrogyria Associated with Rare Variants of CUB and Sushi Multiple Domains 1 Gene (CSMD1): A Longitudinal Neuropsychological and Neuroradiological Case Study. International Journal of Environmental Research and Public Health. 2022; 19(3):1224. https://doi.org/10.3390/ijerph19031224
Chicago/Turabian StyleCostanzo, Floriana, Ginevra Zanni, Elisa Fucà, Margherita Di Paola, Sabina Barresi, Lorena Travaglini, Giovanna Stefania Colafati, Antonio Gambardella, Emanuele Bellacchio, Enrico Bertini, and et al. 2022. "Cerebellar Agenesis and Bilateral Polimicrogyria Associated with Rare Variants of CUB and Sushi Multiple Domains 1 Gene (CSMD1): A Longitudinal Neuropsychological and Neuroradiological Case Study" International Journal of Environmental Research and Public Health 19, no. 3: 1224. https://doi.org/10.3390/ijerph19031224
APA StyleCostanzo, F., Zanni, G., Fucà, E., Di Paola, M., Barresi, S., Travaglini, L., Colafati, G. S., Gambardella, A., Bellacchio, E., Bertini, E., Menghini, D., & Vicari, S. (2022). Cerebellar Agenesis and Bilateral Polimicrogyria Associated with Rare Variants of CUB and Sushi Multiple Domains 1 Gene (CSMD1): A Longitudinal Neuropsychological and Neuroradiological Case Study. International Journal of Environmental Research and Public Health, 19(3), 1224. https://doi.org/10.3390/ijerph19031224







