The Diagnosis and Management of Autism Spectrum Disorder (ASD) in Adult Females in the Presence or Absence of an Intellectual Disability
Abstract
:1. Introduction
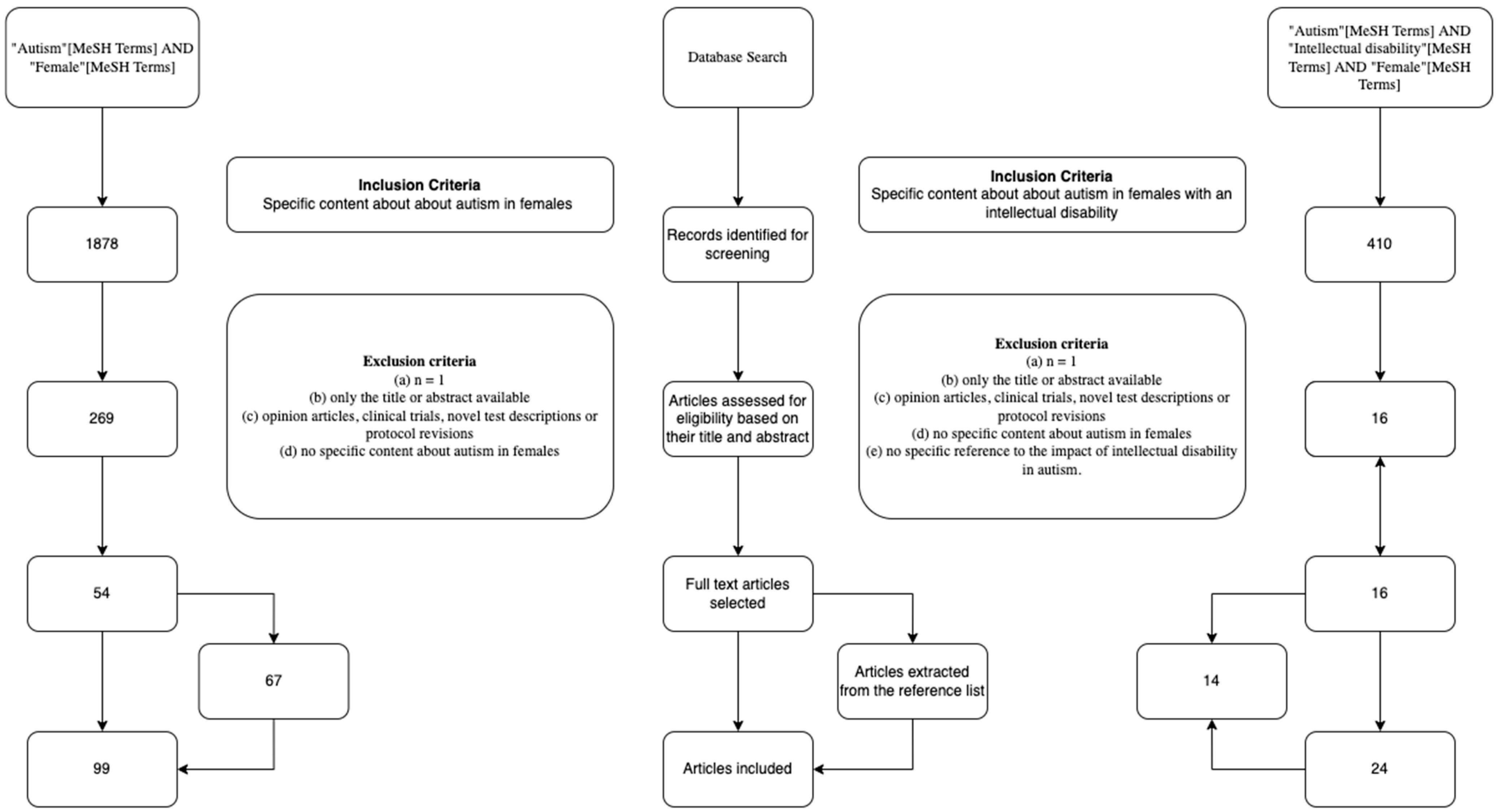
2. Methods
3. Explaining the Difference between the Sexes
3.1. Female Protective Effect (FPE) Theory
3.2. Female Autism Profile (FAP) Theory
4. Implications of ASD Being Perceived as a ‘Male Disorder’
5. Associated Conditions
6. Diagnosis in Women
Impact of Diagnosis in Women
7. Management
7.1. Current Management
7.2. Future Management
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Psychiatric Management of Autism in Adults. Available online: https://www.rcpsych.ac.uk/docs/default-source/improving-care/better-mh-policy/college-reports/college-report-cr228.pdf?sfvrsn=c64e10e3_2 (accessed on 30 May 2021).
- Richards, C.; Jones, C.; Groves, L.; Moss, J.; Oliver, C. Prevalence of autism spectrum disorder phenomenology in genetic disorders: A systematic review and meta-analysis. Lancet Psychiatry 2015, 2, 909–916. [Google Scholar] [CrossRef] [Green Version]
- Autsin, R.; Pisano, G. Neurodiversity as a Competitive Advantage. Harv. Bus. Rev. 2017, 95, 96–103. [Google Scholar]
- Kenny, L.; Hattersley, C.; Molins, B.; Buckley, C.; Povey, C.; Pellicano, E. Which terms should be used to describe autism? Perspectives from the UK autism community. Autism 2016, 20, 442–462. [Google Scholar] [CrossRef] [PubMed]
- Happé, F.G.; Mansour, H.; Barrett, P.; Brown, T.; Abbott, P.; Charlton, R.A. Demographic and Cognitive Profile of Individuals Seeking a Diagnosis of Autism Spectrum Disorder in Adulthood. J. Autism Dev. Disord. 2016, 46, 3469–3480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, D.L.; Braun, K.; Baio, J.; Bilder, D.; Charles, J. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1279. [Google Scholar] [CrossRef]
- Russell, G.; Rodgers, L.R.; Ukoumunne, O.C.; Ford, T. Prevalence of parent-reported ASD and ADHD in the UK: Findings from the millennium cohort study. J. Autism Dev. Disord. 2014, 44, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Elsabbagh, M.; Divan, G.; Koh, Y.J.; Kim, Y.S.; Kauchali, S.; Marcín, C.; Montiel-Nava, C.; Patel, V.; Paula, C.S.; Wang, C.; et al. Global Prevalence of Autism and Other Pervasive Developmental Disorders. Autism Res. 2012, 5, 160–179. [Google Scholar] [CrossRef] [Green Version]
- Mpaka, D.M.; Okitundu, D.L.E.A.; Ndjukendi, A.O.; N’situ, A.M.; Kinsala, S.Y.; Mukau, J.E.; Ngoma, V.M.; Kashala-Abotnes, E.; Ma-Miezi-Mampunza, S.; Vogels, A.; et al. Prevalence and comorbidities of autism among children referred to the outpatient clinics for neurodevelopmental disorders. Pan Afr. Med. J. 2016, 25, 82. [Google Scholar] [CrossRef]
- Hossain, M.D.; Ahmed, H.U.; Uddin, M.M.J.; Chowdhury, W.A.; Iqbal, M.S.; Kabir, R.I.; Chowdhury, I.A.; Aftab, A.; Datta, P.G.; Rabbani, G.; et al. Autism Spectrum disorders (ASD) in South Asia: A systematic review. BMC Psychiatry 2017, 17, 281. [Google Scholar] [CrossRef] [Green Version]
- Werling, D.M.; Geschwind, D.H. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 2013, 26, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halladay, A.K.; Bishop, S.; Constantino, J.N.; Daniels, A.M.; Koenig, K.; Palmer, K.; Messinger, D.; Pelphrey, K.; Sanders, S.J.; Singer, A.T.; et al. Sex and gender differences in autism spectrum disorder: Summarizing evidence gaps and identifying emerging areas of priority. Mol. Autism 2015, 6, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeargin-Allsopp, M.; Rice, C.; Karapurkar, T.; Doernberg, N.; Boyle, C.; Murphy, C. Prevalence of autism in a US metropolitan area. J. Am. Med. Assoc. 2003, 289, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Giarelli, E.; Wiggins, L.D.; Rice, C.E.; Levy, S.E.; Kirby, R.S.; Pinto-Martin, J.; Mandell, D. Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disabil. Health J. 2010, 3, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Kirkovski, M.; Enticott, P.G.; Fitzgerald, P.B. A review of the role of female gender in autism spectrum disorders. J. Autism Dev. Disord. 2013, 43, 2584–2603. [Google Scholar] [CrossRef]
- Rutherford, M.; McKenzie, K.; Johnson, T.; Catchpole, C.; O’Hare, A.; McClure, I.; Forsyth, K.; McCartney, D.; Murray, A. Gender ratio in a clinical population sample, age of diagnosis and duration of assessment in children and adults with autism spectrum disorder. Autism 2016, 20, 628–634. [Google Scholar] [CrossRef] [Green Version]
- Missed Diagnoses and Misdiagnoses of Adults with Autism Spectrum Disorder. Available online: https://link.springer.com/article/10.1007%2Fs00406-020-01189-w#citeas (accessed on 30 May 2021).
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Altman, D.G.; Booth, A.; et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [Green Version]
- Driver, B.; Chester, V. The presentation, recognition and diagnosis of autism in women and girls. Adv. Autism 2020, 7, 194–207. [Google Scholar] [CrossRef]
- Robinson, E.B.; Lichtenstein, P.; Anckarsäter, H.; Happé, F.; Ronald, A. Examining and interpreting the female protective effect against autistic behavior. Proc. Natl. Acad. Sci. USA 2013, 110, 5258–5262. [Google Scholar] [CrossRef] [Green Version]
- Gilman, S.R.; Iossifov, I.; Levy, D.; Ronemus, M.; Wigler, M.; Vitkup, D. Rare De Novo Variants Associated with Autism Implicate a Large Functional Network of Genes Involved in Formation and Function of Synapses. Neuron 2011, 70, 898–907. [Google Scholar] [CrossRef] [Green Version]
- Levy, D.; Ronemus, M.; Yamrom, B.; Lee, Y.H.; Leotta, A.; Kendall, J.; Marks, S.; Lakshmi, B.; Pai, D.; Ye, K.; et al. Rare De Novo and Transmitted Copy-Number Variation in Autistic Spectrum Disorders. Neuron 2011, 70, 886–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jack, A.; Sullivan, C.A.W.; Aylward, E.; Bookheimer, S.Y.; Dapretto, M.; Gaab, N.; Van Horn, J.D.; Eilbott, J.; Jacokes, Z.; Torgerson, C.M.; et al. A neurogenetic analysis of female autism. Brain 2021, 144, 1911–1926. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S. The extreme male brain theory of autism. Trends Cogn. Sci. 2002, 6, 248–254. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Lombardo, M.V.; Auyeung, B.; Ashwin, E.; Chakrabarti, B.; Knickmeyer, R. Why are Autism Spectrum conditions more prevalent in Males? PLoS Biol. 2011, 9, e1001081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron-Cohen, S.; Auyeung, B.; Nørgaard-Pedersen, B.; Hougaard, D.M.; Abdallah, M.W.; Melgaard, L.; Cohen, A.S.; Chakrabarti, B.; Ruta, L.; Lombardo, M.V. Elevated fetal steroidogenic activity in autism. Mol. Psychiatry 2015, 20, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Knickmeyer, R.; Baron-Cohen, S.; Fane, B.A.; Wheelwright, S.; Mathews, G.A.; Conway, G.S.; Brook, C.G.D.; Hines, M. Androgens and autistic traits: A study of individuals with congenital adrenal hyperplasia. Horm. Behav. 2006, 50, 148–153. [Google Scholar] [CrossRef]
- Schwarz, E.; Guest, P.C.; Rahmoune, H.; Wang, L.; Levin, Y.; Ingudomnukul, E.; Ruta, L.; Kent, L.; Spain, M.; Baron-Cohen, S.; et al. Sex-specific serum biomarker patterns in adults with Asperger’s syndrome. Mol. Psychiatry 2011, 16, 1213–1220. [Google Scholar] [CrossRef]
- Guyatt, A.L.; Heron, J.; Knight, B.L.C.; Golding, J.; Rai, D. Digit ratio and autism spectrum disorders in the Avon Longitudinal Study of Parents and Children: A birth cohort study. BMJ Open 2015, 5, e007433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skuse, D. Imprinting, the X-Chromosome, and the Male Brain: Explaining Sex Differences in the Liability to Autism. Pediatr. Res. 2000, 47, 9. [Google Scholar] [CrossRef] [Green Version]
- Cataldo, I.; Azhari, A.; Esposito, G. A review of oxytocin and arginine-vasopressin receptors and their modulation of autism spectrum disorder. Front. Mol. Neurosci. 2018, 11, 27. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.C.; Glymour, M.M.; Rewak, M.; Cornelis, M.C.; Walter, S.; Koenen, K.C.; Kawachi, I.; Liang, L.; Tchetgen, E.J.T.; Kubzansky, L.D. Are genetic variations in OXTR, AVPR1A, and CD38 genes important to social integration? Results from two large U.S. cohorts. Psychoneuroendocrinology 2014, 39, 257–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veenema, A.H.; Beiderbeck, D.I.; Lukas, M.; Neumann, I.D. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm. Behav. 2010, 58, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Desachy, G.; Croen, L.A.; Torres, A.R.; Kharrazi, M.; Delorenze, G.N.; Windham, G.C.; Yoshida, C.K.; Weiss, L.A. Increased female autosomal burden of rare copy number variants in human populations and in autism families. Mol. Psychiatry 2015, 20, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Frazier, T.W.; Youngstrom, E.A.; Hardan, A.Y.; Georgiades, S.; Constantino, J.N.; Eng, C. Quantitative autism symptom patterns recapitulate differential mechanisms of genetic transmission in single and multiple incidence families. Mol. Autism 2015, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Ozonoff, S.; Young, G.S.; Carter, A.; Messinger, D.; Yirmiya, N.; Zwaigenbaum, L.; Bryson, S.; Carver, L.J.; Constantino, J.N.; Dobkins, K.; et al. Recurrence risk for autism spectrum disorders: A baby siblings research consortium study. Pediatrics 2011, 128, e488–e495. [Google Scholar] [CrossRef] [Green Version]
- Russell, G.; Steer, C.; Golding, J. Social and demographic factors that influence the diagnosis of autistic spectrum disorders. Soc. Psychiatry Psychiatr. Epidemiol. 2011, 46, 1283–1293. [Google Scholar] [CrossRef]
- Dworzynski, K.; Ronald, A.; Bolton, P.; Happé, F. How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 788–797. [Google Scholar] [CrossRef]
- Duvekot, J.; van der Ende, J.; Verhulst, F.C.; Slappendel, G.; van Daalen, E.; Maras, A.; Greaves-Lord, K. Factors influencing the probability of a diagnosis of autism spectrum disorder in girls versus boys. Autism 2017, 21, 646–658. [Google Scholar] [CrossRef]
- Shattuck, P.T.; Durkin, M.; Maenner, M.; Newschaffer, C.; Mandell, D.S.; Wiggins, L.; Lee, L.C.; Rice, C.; Giarelli, E.; Kirby, R.; et al. Timing of identification among children with an autism spectrum disorder: Findings from a population-based surveillance study. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 474–483. [Google Scholar] [CrossRef] [Green Version]
- Geelhand, P.; Bernard, P.; Klein, O.; Van Tiel, B.; Kissine, M. The role of gender in the perception of autism symptom severity and future behavioral development. Mol. Autism 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Mattila, M.L.; Kielinen, M.; Linna, S.L.; Jussila, K.; Ebeling, H.; Bloigu, R.; Joseph, R.M.; Moilanen, I. Autism spectrum disorders according to DSM-IV-TR and comparison with DSM-5 draft criteria: An epidemiological study. J. Am. Acad. Child Adolesc. Psychiatry 2011, 50, 583–592.e11. [Google Scholar] [CrossRef] [PubMed]
- Kopp, S.; Gillberg, C. The Autism Spectrum Screening Questionnaire (ASSQ)-Revised Extended Version (ASSQ-REV): An instrument for better capturing the autism phenotype in girls? A preliminary study involving 191 clinical cases and community controls. Res. Dev. Disabil. 2011, 32, 2875–2888. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.M.; Attwood, T.; Garnett, M.; Stokes, M.A. Am I Autistic? Utility of the Girls Questionnaire for Autism Spectrum Condition as an Autism Assessment in Adult Women. Autism Adulthood 2020, 2, 216–226. [Google Scholar] [CrossRef]
- Hull, L.; Mandy, W.; Petrides, K.V. Behavioural and cognitive sex/gender differences in autism spectrum condition and typically developing males and females. Autism 2017, 21, 706–727. [Google Scholar] [CrossRef]
- Lai, M.C.; Lombardo, M.V.; Pasco, G.; Ruigrok, A.N.V.; Wheelwright, S.J.; Sadek, S.A.; Chakrabarti, B.; Baron-Cohen, S. A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PLoS ONE 2011, 6, e20835. [Google Scholar] [CrossRef] [Green Version]
- Head, A.M.; McGillivray, J.A.; Stokes, M.A. Gender differences in emotionality and sociability in children with autism spectrum disorders. Mol. Autism 2014, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hiller, R.M.; Young, R.L.; Weber, N. Sex Differences in Autism Spectrum Disorder based on DSM-5 Criteria: Evidence from Clinician and Teacher Reporting. J. Abnorm. Child Psychol. 2014, 42, 1381–1393. [Google Scholar] [CrossRef] [Green Version]
- Estrin, G.L.; Milner, V.; Spain, D.; Happé, F.; Colvert, E. Barriers to Autism Spectrum Disorder Diagnosis for Young Women and Girls: A Systematic Review. Rev. J. Autism Dev. Disord. 2021, 8, 454–470. [Google Scholar] [CrossRef]
- Sedgewick, F.; Hill, V.; Pellicano, E. ‘It’s different for girls’: Gender differences in the friendships and conflict of autistic and neurotypical adolescents. Autism 2019, 23, 1119–1132. [Google Scholar] [CrossRef] [Green Version]
- Tillmann, J.; Ashwood, K.; Absoud, M.; Bölte, S.; Bonnet-Brilhault, F.; Buitelaar, J.K.; Calderoni, S.; Calvo, R.; Canal-Bedia, R.; Canitano, R.; et al. Evaluating Sex and Age Differences in ADI-R and ADOS Scores in a Large European Multi-site Sample of Individuals with Autism Spectrum Disorder. J. Autism Dev. Disord. 2018, 48, 2490–2505. [Google Scholar] [CrossRef] [Green Version]
- Hattier, M.A.; Matson, J.L.; Tureck, K.; Horovitz, M. The effects of gender and age on repetitive and/or restricted behaviors and interests in adults with autism spectrum disorders and intellectual disability. Res. Dev. Disabil. 2011, 32, 2346–2351. [Google Scholar] [CrossRef]
- Antezana, L.; Factor, R.S.; Condy, E.E.; Strege, M.V.; Scarpa, A.; Richey, J.A. Gender differences in restricted and repetitive behaviors and interests in youth with autism. Autism Res. 2019, 12, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Mandy, W.; Chilvers, R.; Chowdhury, U.; Salter, G.; Seigal, A.; Skuse, D. Sex differences in autism spectrum disorder: Evidence from a large sample of children and adolescents. J. Autism Dev. Disord. 2012, 42, 1304–1313. [Google Scholar] [CrossRef]
- Grove, R.; Hoekstra, R.A.; Wierda, M.; Begeer, S. Special interests and subjective wellbeing in autistic adults. Autism Res. 2018, 11, 766–775. [Google Scholar] [CrossRef] [Green Version]
- Nowell, S.W.; Jones, D.R.; Harrop, C. Circumscribed interests in autism: Are there sex differences? Adv. Autism 2019, 5, 187–198. [Google Scholar] [CrossRef]
- McFayden, T.C.; Albright, J.; Muskett, A.E.; Scarpa, A. Brief Report: Sex Differences in ASD Diagnosis—A Brief Report on Restricted Interests and Repetitive Behaviors. J. Autism Dev. Disord. 2019, 49, 1693–1699. [Google Scholar] [CrossRef]
- Sutherland, R.; Hodge, A.; Bruck, S.; Costley, D.; Klieve, H. Parent-reported differences between school-aged girls and boys on the autism spectrum. Autism 2017, 21, 785–794. [Google Scholar] [CrossRef]
- Hull, L.; Petrides, K.V.; Mandy, W. The Female Autism Phenotype and Camouflaging: A Narrative Review. Rev. J. Autism Dev. Disord. 2020, 7, 306–317. [Google Scholar] [CrossRef] [Green Version]
- Hull, L.; Petrides, K.V.; Allison, C.; Smith, P.; Baron-Cohen, S.; Lai, M.C.; Mandy, W. “Putting on My Best Normal”: Social Camouflaging in Adults with Autism Spectrum Conditions. J. Autism Dev. Disord. 2017, 47, 2519–2534. [Google Scholar] [CrossRef]
- Kreiser, N.L.; White, S.W. ASD in Females: Are We Overstating the Gender Difference in Diagnosis? Clin. Child Fam. Psychol. Rev. 2014, 17, 67–84. [Google Scholar] [CrossRef]
- Mandy, W.; Tchanturia, K. Do women with eating disorders who have social and flexibility difficulties really have autism? A case series. Mol. Autism 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, M.C.; Baron-Cohen, S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2015, 2, 1013–1027. [Google Scholar] [CrossRef]
- Livingston, L.A.; Happé, F. Conceptualising compensation in neurodevelopmental disorders: Reflections from autism spectrum disorder. Neurosci. Biobehav. Rev. 2017, 80, 729–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hull, L.; Petrides, K.V.; Mandy, W. Cognitive Predictors of Self-Reported Camouflaging in Autistic Adolescents. Autism Res. 2021, 14, 523–532. [Google Scholar] [CrossRef]
- Milner, V.; McIntosh, H.; Colvert, E.; Happé, F. A Qualitative Exploration of the Female Experience of Autism Spectrum Disorder (ASD). J. Autism Dev. Disord. 2019, 49, 2389–2402. [Google Scholar] [CrossRef] [Green Version]
- Dean, M.; Harwood, R.; Kasari, C. The art of camouflage: Gender differences in the social behaviors of girls and boys with autism spectrum disorder. Autism 2017, 21, 678–689. [Google Scholar] [CrossRef]
- Cassidy, S.; Bradley, L.; Shaw, R.; Baron-Cohen, S. Risk markers for suicidality in autistic adults. Mol. Autism 2018, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bargiela, S.; Steward, R.; Mandy, W. The Experiences of Late-diagnosed Women with Autism Spectrum Conditions: An Investigation of the Female Autism Phenotype. J. Autism Dev. Disord. 2016, 46, 3281–3294. [Google Scholar] [CrossRef] [Green Version]
- Navot, N.; Jorgenson, A.G.; Webb, S.J. Maternal experience raising girls with autism spectrum disorder: A qualitative study. Child Care Health Dev. 2017, 43, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Riley-Hall, E. Parenting Girls on the Autism Spectrum: Overcoming the Challenges and Celebrating the Gifts; Jessica Kingsley Publishers: London, UK, 2012. [Google Scholar]
- Mandell, D. Dying before their time: Addressing premature mortality among autistic people. Autism 2018, 22, 234–235. [Google Scholar] [CrossRef] [Green Version]
- Brugha, T.S.; Spiers, N.; Bankart, J.; Cooper, S.A.; McManus, S.; Scott, F.J.; Smith, J.; Tyrer, F. Epidemiology of autism in adults across age groups and ability levels. Br. J. Psychiatry 2016, 209, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croen, L.A.; Zerbo, O.; Qian, Y.; Massolo, M.L.; Rich, S.; Sidney, S.; Kripke, C. The health status of adults on the autism spectrum. Autism 2015, 19, 814–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gesi, C.; Migliarese, G.; Torriero, S.; Capellazzi, M.; Omboni, A.C.; Cerveri, G.; Mencacci, C. Gender differences in misdiagnosis and delayed diagnosis among adults with autism spectrum disorder with no language or intellectual disability. Brain Sci. 2021, 11, 912. [Google Scholar] [CrossRef]
- Kovacs, M.; Devlin, B. Internalizing disorders in childhood. J. Child Psychol. Psychiatry Allied Discip. 1998, 39, 47–63. [Google Scholar] [CrossRef]
- Chandler, S.; Howlin, P.; Simonoff, E.; O’Sullivan, T.; Tseng, E.; Kennedy, J.; Charman, T.; Baird, G. Emotional and behavioural problems in young children with autism spectrum disorder. Dev. Med. Child Neurol. 2016, 58, 202–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotham, K.; Brunwasser, S.M.; Lord, C. Depressive and anxiety symptom trajectories from school age through young adulthood in samples with autism spectrum disorder and developmental delay. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 369–376.e3. [Google Scholar] [CrossRef] [Green Version]
- Oswald, T.M.; Winter-Messiers, M.A.; Gibson, B.; Schmidt, A.M.; Herr, C.M.; Solomon, M. Sex Differences in Internalizing Problems During Adolescence in Autism Spectrum Disorder. J. Autism Dev. Disord. 2016, 46, 624–636. [Google Scholar] [CrossRef]
- May, T.; Cornish, K.; Rinehart, N.J. Gender Profiles of Behavioral Attention in Children With Autism Spectrum Disorder. J. Atten. Disord. 2016, 20, 627–635. [Google Scholar] [CrossRef]
- Brown, C.M.; Stokes, M.A. Intersection of Eating Disorders and the Female Profile of Autism. Child Adolesc. Psychiatr. Clin. N. Am. 2020, 29, 409–417. [Google Scholar] [CrossRef]
- Vagni, D.; Moscone, D.; Travaglione, S.; Cotugno, A. Using the Ritvo Autism Asperger Diagnostic Scale-Revised (RAADS-R) disentangle the heterogeneity of autistic traits in an Italian eating disorder population. Res. Autism Spectr. Disord. 2016, 32, 143–155. [Google Scholar] [CrossRef]
- Zucker, N.L.; Losh, M.; Bulik, C.M.; LaBar, K.S.; Piven, J.; Pelphrey, K.A. Anorexia Nervosa and Autism Spectrum Disorders: Guided Investigation of Social Cognitive Endophenotypes. Psychol. Bull. 2007, 133, 976–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerr-Gaffney, J.; Hayward, H.; Jones, E.J.H.; Halls, D.; Murphy, D.; Tchanturia, K. Autism symptoms in anorexia nervosa: A comparative study with females with autism spectrum disorder. Mol. Autism 2021, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Jaffa, T.; Davies, S.; Auyeung, B.; Allison, C.; Wheelwright, S. Do girls with anorexia nervosa have elevated autistic traits? Mol. Autism 2013, 4, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentz, M.; Westwood, H.; Jepsen, J.R.M.; Plessen, K.J.; Tchanturia, K. The autism diagnostic observation schedule: Patterns in individuals with anorexia nervosa. Eur. Eat. Disord. Rev. 2020, 28, 571–579. [Google Scholar] [CrossRef]
- Cederlöf, M.; Larsson, H.; Lichtenstein, P.; Almqvist, C.; Serlachius, E.; Ludvigsson, J.F. Nationwide population-based cohort study of psychiatric disorders in individuals with Ehlers-Danlos syndrome or hypermobility syndrome and their siblings. BMC Psychiatry 2016, 16, 207. [Google Scholar] [CrossRef] [Green Version]
- Casanova, E.L.; Baeza-Velasco, C.; Buchanan, C.B.; Casanova, M.F. The relationship between autism and ehlers-danlos syndromes/hypermobility spectrum disorders. J. Pers. Med. 2020, 10, 260. [Google Scholar] [CrossRef]
- Casanova, E.L.; Sharp, J.L.; Edelson, S.M.; Kelly, D.P.; Sokhadze, E.M.; Casanova, M.F. Immune, Autonomic, and Endocrine Dysregulation in Autism and Ehlers-Danlos Syndrome/Hypermobility Spectrum Disorders Versus Unaffected Controls. J. Reatt. Ther. Dev. Divers. 2019, 82–95. [Google Scholar] [CrossRef]
- Baeza-Velasco, C.; Cohen, D.; Hamonet, C.; Vlamynck, E.; Diaz, L.; Cravero, C.; Cappe, E.; Guinchat, V. Autism, Joint Hypermobility-Related Disorders and Pain. Front. Psychiatry 2018, 9, 656. [Google Scholar] [CrossRef]
- Strang, J.F.; Kenworthy, L.; Dominska, A.; Sokoloff, J.; Kenealy, L.E.; Berl, M.; Walsh, K.; Menvielle, E.; Slesaransky-Poe, G.; Kim, K.E.; et al. Increased Gender Variance in Autism Spectrum Disorders and Attention Deficit Hyperactivity Disorder. Arch. Sex. Behav. 2014, 43, 1525–1533. [Google Scholar] [CrossRef]
- Holt, V.; Skagerberg, E.; Dunsford, M. Young people with features of gender dysphoria: Demographics and associated difficulties. Clin. Child Psychol. Psychiatry 2016, 21, 108–118. [Google Scholar] [CrossRef]
- Kaltiala-Heino, R.; Sumia, M.; Työläjärvi, M.; Lindberg, N. Two years of gender identity service for minors: Overrepresentation of natal girls with severe problems in adolescent development. Child Adolesc. Psychiatry Ment. Health 2015, 9, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vries, A.L.C.; Noens, I.L.J.; Cohen-Kettenis, P.T.; Van Berckelaer-Onnes, I.A.; Doreleijers, T.A. Autism spectrum disorders in gender dysphoric children and adolescents. J. Autism Dev. Disord. 2010, 40, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.; Smith, L.G.E.; Russell, A.J. Gender Identity in Autism: Sex Differences in Social Affiliation with Gender Groups. J. Autism Dev. Disord. 2018, 48, 3995–4006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandy, W.; Lai, M.C. Towards sex- and gender-informed autism research. Autism 2017, 21, 643–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tromans, S.; Chester, V.; Kapugama, C.; Elliott, A.; Robertson, S.; Barrett, M. The PAAFID project: Exploring the perspectives of autism in adult females among intellectual disability healthcare professionals. Adv. Autism 2019, 5, 157–170. [Google Scholar] [CrossRef]
- Eckerd, M. Detection and Diagnosis of ASD in Females. J. Health Serv. Psychol. 2020, 46, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Ormond, S.; Brownlow, C.; Garnett, M.S.; Rynkiewicz, A.; Attwood, T. Profiling Autism Symptomatology: An Exploration of the Q-ASC Parental Report Scale in Capturing Sex Differences in Autism. J. Autism Dev. Disord. 2018, 48, 389–403. [Google Scholar] [CrossRef]
- Lai, M.C.; Lombardo, M.V.; Auyeung, B.; Chakrabarti, B.; Baron-Cohen, S. Sex/Gender Differences and Autism: Setting the Scene for Future Research. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Hull, L.; Mandy, W.; Lai, M.C.; Baron-Cohen, S.; Allison, C.; Smith, P.; Petrides, K.V. Development and Validation of the Camouflaging Autistic Traits Questionnaire (CAT-Q). J. Autism Dev. Disord. 2019, 49, 819–833. [Google Scholar] [CrossRef] [Green Version]
- Cage, E.; Troxell-Whitman, Z. Understanding the Reasons, Contexts and Costs of Camouflaging for Autistic Adults. J. Autism Dev. Disord. 2019, 49, 1899–1911. [Google Scholar] [CrossRef] [Green Version]
- Hull, L.; Lai, M.C.; Baron-Cohen, S.; Allison, C.; Smith, P.; Petrides, K.V.; Mandy, W. Gender differences in self-reported camouflaging in autistic and non-autistic adults. Autism 2020, 24, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Green, R.M.; Travers, A.M.; Howe, Y.; McDougle, C.J. Women and Autism Spectrum Disorder: Diagnosis and Implications for Treatment of Adolescents and Adults. Curr. Psychiatry Rep. 2019, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.R. Invisible at the End of the Spectrum: Shadows, Residues, ‘Bap’, and the Female Aspergers Experience. Proc. Conf. Autism Unlocking Potential 2003, 6, 1–14. [Google Scholar]
- Balfe, M.; Tantam, D. A descriptive social and health profile of a community sample of adults and adolescents with Asperger syndrome. BMC Res. Notes 2010, 3, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howes, O.D.; Rogdaki, M.; Findon, J.L.; Wichers, R.H.; Charman, T.; King, B.H.; Loth, E.; McAlonan, G.M.; McCracken, J.T.; Parr, J.R.; et al. Autism spectrum disorder: Consensus guidelines on assessment, treatment and research from the British Association for Psychopharmacology. J. Psychopharmacol. 2018, 32, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Pilling, S.; Baron-Cohen, S.; Megnin-Viggars, O.; Lee, R.; Taylor, C. Recognition, referral, diagnosis, and management of adults with autism: Summary of NICE guidance. BMJ 2012, 344, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Sandler, A. Placebo effects in developmental disabilities: Implications for research and practice. Ment. Retard. Dev. Disabil. Res. Rev. 2005, 11, 164–170. [Google Scholar] [CrossRef]
- Tsakanikos, E.; Underwood, L.; Kravariti, E.; Bouras, N.; McCarthy, J. Gender differences in co-morbid psychopathology and clinical management in adults with autism spectrum disorders. Res. Autism Spectr. Disord. 2011, 5, 803–808. [Google Scholar] [CrossRef]
- Owen, R.; Sikich, L.; Marcus, R.N.; Corey-Lisle, P.; Manos, G.; McQuade, R.D.; Carson, W.H.; Findling, R.L. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics 2009, 124, 1533–1540. [Google Scholar] [CrossRef] [Green Version]
- Salzman, C.; Kochansky, G.E.; Van Der Kolk, B.A.; Shader, R.I. The effect of marijuana on small group process. Am. J. Drug Alcohol Abuse 1977, 4, 251–255. [Google Scholar] [CrossRef]
- Aran, A.; Harel, M.; Cassuto, H.; Polyansky, L.; Schnapp, A.; Wattad, N.; Shmueli, D.; Golan, D.; Castellanos, F.X. Cannabinoid treatment for autism: A proof-of-concept randomized trial. Mol. Autism 2021, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.; Wozniak, J.; Faraone, S.V.; Fried, R.; Chan, J.; Furtak, S.; Grimsley, E.; Conroy, K.; Kilcullen, J.R.; Woodworth, K.Y.; et al. A prospective open-label trial of memantine hydrochloride for the treatment of social deficits in intellectually capable adults with autism spectrum disorder. J. Clin. Psychopharmacol. 2016, 36, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Hosenbocus, S.; Chahal, R. Memantine: A review of possible uses in child and adolescent psychiatry. J. Can. Acad. Child Adolesc. Psychiatry 2013, 22, 166–171. [Google Scholar] [PubMed]
- Ghaleiha, A.; Asadabadi, M.; Mohammadi, M.R.; Shahei, M.; Tabrizi, M.; Hajiaghaee, R.; Hassanzadeh, E.; Akhondzadeh, S. Memantine as adjunctive treatment to risperidone in children with autistic disorder: A randomized, double-blind, placebo-controlled trial. Int. J. Neuropsychopharmacol. 2013, 16, 783–789. [Google Scholar] [CrossRef] [Green Version]
- Van Hoorn, A.; Carpenter, T.; Oak, K.; Laugharne, R.; Ring, H.; Shankar, R. Neuromodulation of autism spectrum disorders using vagal nerve stimulation. J. Clin. Neurosci. 2019, 63, 8–12. [Google Scholar] [CrossRef]
- Al-Beltagi, M. Autism medical comorbidities. World J. Clin. Pediatr. 2021, 10, 15–28. [Google Scholar] [CrossRef]
- Chernikova, M.A.; Flores, G.D.; Kilroy, E.; Labus, J.S.; Mayer, E.A.; Aziz-zadeh, L. The Brain-Gut-Microbiome System: Pathways and Implications for Autism Spectrum Disorder. Nutrients 2021, 13, 4497. [Google Scholar] [CrossRef]
- Fattorusso, A.; Di Genova, L.; Dell’isola, G.B.; Mencaroni, E.; Esposito, S. Autism spectrum disorders and the gut microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef] [Green Version]
- Loth, E.; Murphy, D.G.; Spooren, W. Defining precision medicine approaches to autism spectrum disorders: Concepts and challenges. Front. Psychiatry 2016, 7, 188. [Google Scholar] [CrossRef] [Green Version]
- Voineagu, I.; Wang, X.; Johnston, P.; Lowe, J.K.; Tian, Y.; Horvath, S.; Mill, J.; Cantor, R.M.; Blencowe, B.J.; Geschwind, D.H. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 2011, 474, 380–386. [Google Scholar] [CrossRef]
| Aspect or Feature | Male | Female |
|---|---|---|
| Prevalence | 4-2 | 1 |
| Impaired Social Communication Abilities | Present | Present, but generally females have higher social abilities |
| Restricted Repetitive Behaviours | Present | Present, but either lower or displayed in more sociably acceptable areas (i.e., more relational than mechanical) |
| Camouflaging | Present | More prevalent than males |
| Associated Conditions | Externalising co-occurring disorders (e.g., behavioural problems and inattention) more likely | Internalising co-occurring disorders (e.g., anxiety, depression and eating disorders) more likely |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rujeedawa, T.; Zaman, S.H. The Diagnosis and Management of Autism Spectrum Disorder (ASD) in Adult Females in the Presence or Absence of an Intellectual Disability. Int. J. Environ. Res. Public Health 2022, 19, 1315. https://doi.org/10.3390/ijerph19031315
Rujeedawa T, Zaman SH. The Diagnosis and Management of Autism Spectrum Disorder (ASD) in Adult Females in the Presence or Absence of an Intellectual Disability. International Journal of Environmental Research and Public Health. 2022; 19(3):1315. https://doi.org/10.3390/ijerph19031315
Chicago/Turabian StyleRujeedawa, Tanzil, and Shahid H. Zaman. 2022. "The Diagnosis and Management of Autism Spectrum Disorder (ASD) in Adult Females in the Presence or Absence of an Intellectual Disability" International Journal of Environmental Research and Public Health 19, no. 3: 1315. https://doi.org/10.3390/ijerph19031315
APA StyleRujeedawa, T., & Zaman, S. H. (2022). The Diagnosis and Management of Autism Spectrum Disorder (ASD) in Adult Females in the Presence or Absence of an Intellectual Disability. International Journal of Environmental Research and Public Health, 19(3), 1315. https://doi.org/10.3390/ijerph19031315






