Cochlear Implant Results in Older Adults with Post-Lingual Deafness: The Role of “Top-Down” Neurocognitive Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample and Setting
2.2. Measurements and Instruments
2.2.1. Neuropsychological Assessment
2.2.2. Stimuli-Specific Procedures
2.2.3. Audiological Examination
2.2.4. Statistical Analysis
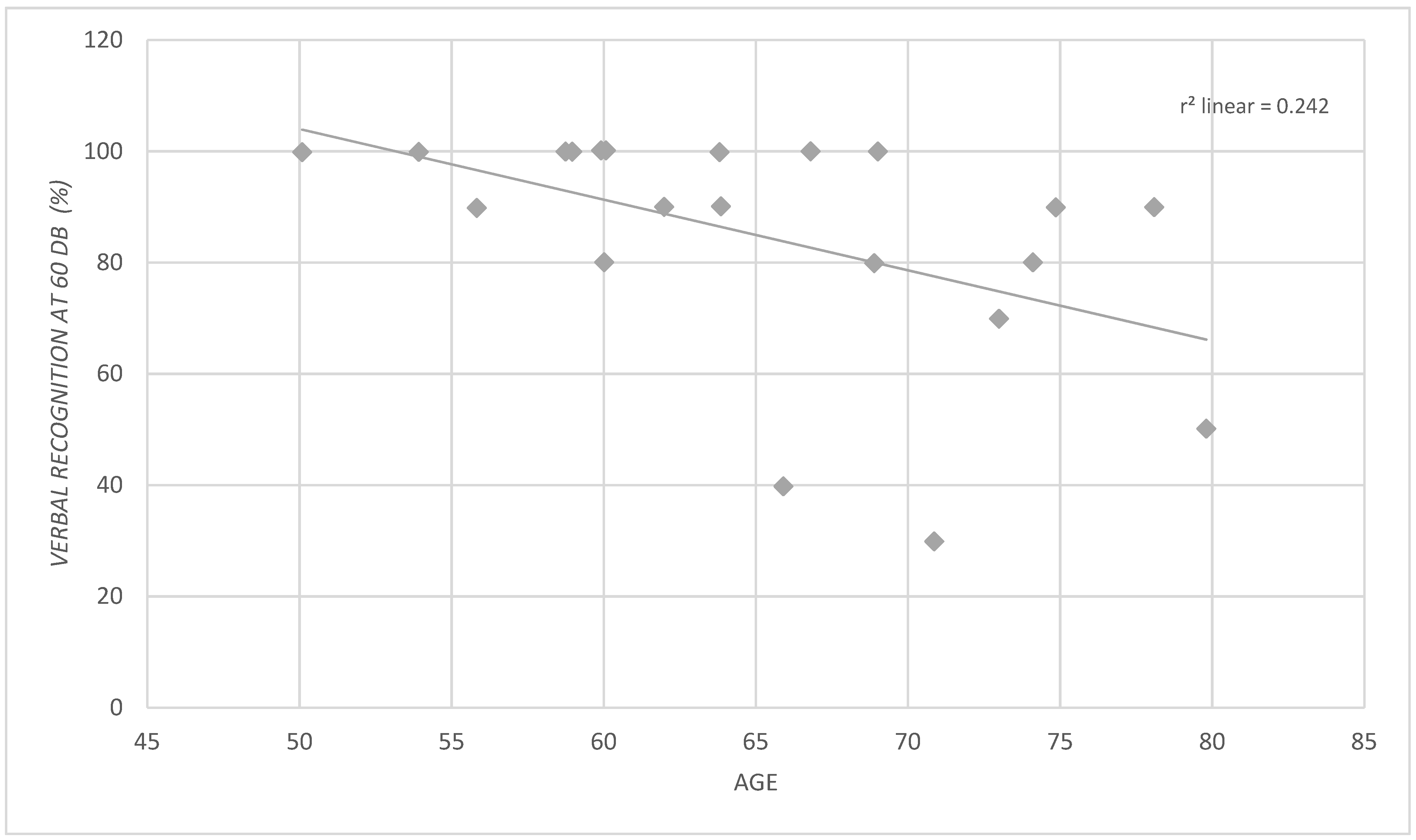
3. Results
3.1. Relation between CI Audiological Outcome at T12 and Clinical Features of Patients after Stratification of Sample by Various Characteristics of Interest
3.2. Identification of Clinical Predictors of CI Audiological Outcome at T12 through Linear Regression Analyses
3.3. Relation between CI Audiological Outcome at T12 and Cognitive Abilities at T0 after Stratification of the Patients’ Cohort Based on Percentage of CI Verbal Recognition at One Year of Implantation
3.4. Identification of Cognitive Predictors of CI Audiological Outcome at T12 through Linear Regression Analyses
4. Discussion
4.1. Clinical Characteristics and Predictors
4.2. Cognitive Abilities and Predictors
4.3. Processing Speed and Quality of Audiological Outcome in Older Adult CI Users: A New Hypothesis Based on a Multi-Modal Model for Language Understanding
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tun, P.A.; McCoy, S.; Wingfield, A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol. Aging 2009, 24, 761–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panza, F.; Solfrizzi, V.; Logroscino, G. Age-related hearing impairment—A risk factor and frailty marker for dementia and AD. Nat. Rev. Neurol. 2015, 11, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.R.; Yaffe, K.; Xia, J.; Xue, Q.-L.; Harris, T.B.; Purchase-Helzner, E.; Satterfield, S.; Ayonayon, H.N.; Ferrucci, L.; Simonsick, E.M.; et al. Hearing loss and cognitive decline in older adults. JAMA Intern. Med. 2013, 173, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.R.; Albert, M. Hearing loss and dementia-who is listening? Aging Ment. Health 2014, 18, 671–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, F.R.; Metter, E.J.; O’Brien, R.J.; Resnick, S.M.; Zonderman, A.B.; Ferrucci, L. Hearing loss and incident dementia. Arch. Neurol. 2011, 68, 214–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallacher, J.; Ilubaera, V.; Ben-Shlomo, Y.; Bayer, A.; Fish, M.; Babisch, W.; Elwood, P. Auditory threshold, phonologic demand, and incident dementia. Neurology 2012, 79, 1583–1590. [Google Scholar] [CrossRef]
- Johnson, J.C.S.; Marshall, C.R.; Weil, R.S.; Bamiou, D.E.; Hardy, C.J.D.; Warren, J.D. Hearing and dementia: From ears to brain. Brain 2021, 144, 391–401. [Google Scholar] [CrossRef]
- Rabbitt, P.M. Channel-capacity, intelligibility and immediate memory. Q. J. Exp. Psychol. 1968, 20, 241–248. [Google Scholar] [CrossRef]
- Gao, X.; Stine-Morrow, E.A.L.; Noh, S.R.; Eskew, R.T. Visual noise disrupts conceptual integration in reading. Psychon. Bull. Rev. 2011, 18, 83–88. [Google Scholar] [CrossRef]
- Berrettini, S.; Baggiani, A.; Bruschini, L.; Cassandro, E.; Cuda, D.; Filipo, R.; Palla, I.; Quaranta, N.; Forli, F. Systematic review of the literature on the clinical effectiveness of the cochlear implant procedure in adult patients. Acta Otorhinolaryngol. Ital. 2011, 31, 299–310. [Google Scholar]
- Blamey, P.; Artieres, F.; Başkent, D.; Bergeron, F.; Beynon, A.; Burke, E.; Dillier, N.; Dowell, R.; Fraysse, B.; Gallégo, S.; et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: An update with 2251 patients. Audiol. Neurootol. 2013, 18, 36–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holden, L.K.; Finley, C.C.; Firszt, J.B.; Holden, T.A.; Brenner, C.; Potts, L.G.; Gotter, B.D.; Vanderhoof, S.S.; Mispagel, K.; Heydebrand, G.; et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 2013, 34, 342–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smulders, Y.E.; Hendriks, T.; Eikelboom, R.H.; Stegeman, I.; Santa Maria, P.L.; Atlas, M.D.; Friedland, P.L. Predicting Sequential Cochlear Implantation Performance: A Systematic Review. Audiol. Neurootol. 2017, 22, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Debruyne, J.A.; Janssen, A.M.; Brokx, J.P.L. Systematic Review on Late Cochlear Implantation in arly-Deafened Adults and Adolescents: Predictors of Performance. Ear Hear. 2020, 41, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Grayden, D.; McDonnell, M. Unifying information theory and machine learning in a model of electrode discrimination in cochlear implants. PLoS ONE 2021, 16, e0257568. [Google Scholar] [CrossRef] [PubMed]
- Rönnberg, J.; Holmer, E.; Rudner, M. Cognitive hearing science and ease of language understanding. Int. J. Audiol. 2019, 58, 247–261. [Google Scholar] [CrossRef]
- Moore, D.R. Auditory processing disorder (APD): Definition, diagnosis, neural basis, and intervention. Audiol. Med. 2006, 4, 4–11. [Google Scholar] [CrossRef]
- Knutson, J.F.; Hinrichs, J.V.; Tyler, R.S.; Gantz, B.J.; Schartz, H.A.; Woodworth, G. Psychological predictors of audiological outcomes of multichannel cochlear implants: Preliminary findings. Ann. Otol. Rhinol. Laryngol. 1991, 100, 817–822. [Google Scholar] [CrossRef]
- Lyxell, B.; Andersson, J.; Arlinger, S.; Bredberg, G.; Harder, H.; Ronnberg, J. Verbal information-processing capabilities and cochlear implants: Implications for preoperative predictors of speech understanding. J. Deaf Stud. Deaf Educ. 1996, 1, 190–201. [Google Scholar] [CrossRef] [Green Version]
- Heydebrand, G.; Hale, S.; Potts, L.; Gotter, B.; Skinner, M. Cognitive predictors of improvements in adults’ spoken word recognition six months after cochlear implant activation. Audiol. Neurootol. 2007, 12, 254–264. [Google Scholar] [CrossRef]
- Moberly, A.C.; Houston, D.M.; Castellanos, I. Non-auditory neurocognitive skills contribute to speech recognition in adults with cochlear implants. Laryngoscope Investig. Otolaryngol. 2016, 1, 154–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosetti, M.K.; Pinkston, J.B.; Flores, J.M.; Friedmann, D.R.; Jones, C.B.; Roland, J.T.; Waltzman, S.B. Neurocognitive testing and cochlear implantation: Insights into performance in older adults. Clin. Interv. Aging 2016, 11, 603–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moberly, A.C.; Harris, M.S.; Boyce, L.; Nittrouer, S. Speech Recognition in Adults With Cochlear Implants: The Effects of Working Memory, Phonological Sensitivity, and Aging. J. Speech Lang. Hear. Res. 2017, 60, 1046–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moberly, A.C.; Houston, D.M.; Harris, M.S.; Adunka, O.F.; Castellanos, I. Verbal working memory and inhibition-concentration in adults with cochlear implants. Laryngoscope Investig. Otolaryngol. 2017, 2, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Zhan, K.Y.; Lewis, J.H.; Vasil, K.J.; Tamati, T.N.; Harris, M.S.; Pisoni, D.B.; Kronenberger, W.G.; Ray, C.; Moberly, A.C. Cognitive Functions in Adults Receiving Cochlear Implants: Predictors of Speech Recognition and Changes After Implantation. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2020, 41, e322–e329. [Google Scholar] [CrossRef]
- Commissione Nazionale per la Definizione e L’aggiornamento dei Livelli Essenziali di Assistenza, Impianto Cocleare. Ministero Della Salute. 2003. Available online: www.salute.gov.it/ (accessed on 20 September 2021).
- Quaranta, A.; Arslan, E.; Burdo, S.; Cuda, D.; Filipo, R.; Quaranta, N. Documento del Gruppo SIO Impianti Cocleari: Linee Guida per l’applicazione dell’Impianto Cocleare e la gestione del centro Impianti Cocleari. Acta Otorhinolaryngol. Ital. 2009, 3, 1–5. [Google Scholar]
- Canale, A.; Dalmasso, G.; Dagna, F.; Lacilla, M.; Montuschi, C.; Rosa, R.D.; Albera, R. Monaural or binaural sound deprivation in postlingual hearing loss: Cochlear implant in the worse ear. Laryngoscope 2016, 126, 1905–1910. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Freedman, M.; Leach, L.; Kaplan, E.; Winocur, G.; Shulman, K.I.; Delis, D.C. Clock Drawing: A Neuropsychological Analysis; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Shulman, K.I. Clock-drawing: Is it the ideal cognitive screening test? Int. J. Geriatr. Psychiatry 2000, 15, 548–561. [Google Scholar] [CrossRef]
- Carlesimo, G.A.; Caltagirone, C.; Gainotti, G. The mental deterioration battery: Normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur. Neurol. 1996, 36, 378–384. [Google Scholar] [CrossRef]
- Wechsler, D. The Measurement of Adult Intelligence; Williams & Witkins: Baltimore, MD, USA, 1939; 229p. [Google Scholar]
- Milner, B. Interhemispheric differences in the localization of psychological processes in man. Br. Med. Bull. 1971, 27, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Mondini, S.; Mapelli, D.; Vestri, A.; Bisiacchi, P.S. Esame Neuropsicologico Breve (ENB) Una Batteria di Test per lo Screening Neuropsicologico; Raffello Cortina Editore: Milano, Italy, 2003. [Google Scholar]
- Orsini, A.; Grossi, D.; Capitani, E.; Laiacona, M.; Papagno, C.; Vallar, G. Verbal and spatial immediate memory span: Normative data from 1355 adults and 1112 children. Ital. J. Neurol. Sci. 1987, 8, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Spinnler, H.; Tognoni, G. Standardizzazione italiana e taratura di test neuropsicologici. Ital. J. Neurol. Sci. 1987, 6, 120. [Google Scholar]
- Benton, A.L.; Hamsher, K.D.S.; Sivan, A.B. Multilingual Aphasia Examination, 3rd ed.; AJA Associates: Iowa City, IA, USA, 1994. [Google Scholar]
- Reitan, R.M.; Wolfson, D.A. Selective and critical review of neuropsychological deficits and the frontal lobes. Neuropsychol. Rev. 1994, 4, 161–198. [Google Scholar] [CrossRef]
- Bocca, E.; Pellegrini, A. Studio statistico sulla composizione fonetica della lingua italiana e sua applicazione pratica all’audiometria con la parola [Statistical study of the phonetic composition of the Italian language and its practical application to audiometry of words]. Arch. Ital. Otol. Rinol. Laringol. 1950, 61, 116–141. [Google Scholar]
- Assiri, M.; Khurayzi, T.; Alshalan, A.; Alsanosi, A. Cochlear implantation among patients with otosclerosis: A systematic review of clinical characteristics and outcomes. Eur. Arch. Otorhinolaryngol. 2021, 1–13. [Google Scholar] [CrossRef]
- Lazard, D.S.; Vincent, C.; Venail, F.; Van de Heyning, P.; Truy, E.; Sterkers, O.; Skarzynski, P.H.; Skarzynski, H.; Schauwers, K.; O’Leary, S.; et al. Pre-, per- and postoperative factors affecting performance of postlinguistically deaf adults using cochlear implants: A new conceptual model over time. PLoS ONE 2012, 7, e48739. [Google Scholar] [CrossRef] [Green Version]
- Blamey, P.; Arndt, P.; Bergeron, F.; Bredberg, G.; Brimacombe, J.; Facer, G.; Larky, J.; Lindström, B.; Nedzelski, J.; Peterson, A.; et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiol. Neurotol. 1996, 1, 293–306. [Google Scholar] [CrossRef]
- Mosnier, I.; Bebear, J.P.; Marx, M.; Fraysse, B.; Truy, E.; Lina-Granade, G.; Mondain, M.; Sterkers-Artières, F.; Bordure, P.; Robier, A.; et al. Predictive Factors of Cochlear Implant Outcomes in the Elderly. Audiol. Neurotol. 2014, 19, 15–20. [Google Scholar] [CrossRef]
- Boisvert, I.; Lyxell, B.; Mäki-Torkko, E.; McMahon, C.M.; Dowell, R.C. Choice of ear for cochlear implantation in adults with monaural sound-deprivation and unilateral hearing aid. Otol. Neurotol. 2012, 33, 572–579. [Google Scholar] [CrossRef]
- Ketterer, M.C.; Häussler, S.M.; Hildenbrand, T.; Speck, I.; Peus, D.; Rosner, B.; Knopke, S.; Graebel, S.; Olze, H. Binaural Hearing Rehabilitation Improves Speech Perception, Quality of Life, Tinnitus Distress, and Psychological Comorbidities. Otol. Neurotol. 2020, 41, e563–e574. [Google Scholar] [CrossRef] [PubMed]
- Hilly, O.; Hwang, E.; Smith, L.; Shipp, D.; Nedzelski, J.M.; Chen, J.M.; Lin, V.W. Cochlear implantation in elderly patients: Stability of outcome over time. J. Laryngol. Otol. 2016, 130, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.S.; Lin, H.W.; Herrmann, B.S.; Lee, D.J. Differential cochlear implant outcomes in older adults. Laryngoscope 2013, 123, 1952–1956. [Google Scholar] [CrossRef] [PubMed]
- Manrique-Huarte, R.; Calavia, D.; Huarte Irujo, A.; Girón, L.; Manrique-Rodríguez, M. Treatment for Hearing Loss among the Elderly: Auditory Outcomes and Impact on Quality of Life. Audiol. Neurootol. 2016, 21, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Canale, A.; Macocco, F.; Ndrev, D.; Gabella, G.; Scozzari, G.; Albera, R.; Pecorari, G.; Albera, A. Cochlear implant outcomes in prelingually deafened adults with and without sound deprivation: Are there differences in quality of life? Med. Sci. Monit. 2021, 27, e930232-1–e930232-7. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Johansson, B.; Magnusson, L.; Lyxell, B.; Ellis, R.J. Speech Recognition and Cognitive Skills in Bimodal Cochlear Implant Users. J. Speech Lang. Hear. Res. 2017, 60, 2752–2763. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Molander, P.; Rönnberg, J.; Lyxell, B.; Andersson, G.; Lunner, T. Predicting Speech-in-Noise Recognition from Performance on the Trail Making Test: Results From a Large-Scale Internet Study. Ear Hear. 2016, 37, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, R.A.; Marsiske, M.M.; Smith, G.E. Neuropsychology of aging. Handb. Clin. Neurol. 2019, 167, 149–180. [Google Scholar] [CrossRef]
- Pickora-Fuller, M.K. Processing speed and timing in aging adults: Psychoacoustics, speech perception, and comprehension. Int. J. Audiol. 2003, 42, S59–S67. [Google Scholar] [CrossRef]
- Ellis, R.J.; Munro, K.J. Does cognitive function predict frequency compressed speech recognition in listeners with normal hearing and normal cognition? Int. J. Audiol. 2013, 52, 14–22. [Google Scholar] [CrossRef]
- Ellis, R.J.; Munro, K.J. Predictors of aided speech recognition, with and without frequency compression, in older adults. Int. J. Audiol. 2015, 54, 467–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Füllgrabe, C.; Moore, B.C.J.; Stone, M.A. Age-group differences in speech identification despite matched audiometrically normal hearing: Contributions from auditory temporal processing and cognition. Front. Aging Neurosci. 2014, 6, 347. [Google Scholar] [CrossRef]
- Baddeley, A. Exploring the central executive. Q. J. Exp. Psychol. 1996, 49, 5–28. [Google Scholar] [CrossRef]
- McCabe, D.P.; Roediger, H.L.; McDaniel, M.A.; Balota, D.A.; Hambrick, D.Z. The relationship between working memory capacity and executive functioning: Evidence for a common executive attention construct. Neuropsychology 2010, 24, 222–243. [Google Scholar] [CrossRef] [Green Version]
- Barrouillet, P.; Bernardin, S.; Camos, V. Time constraints and resource sharing in adults’ working memory spans. J. Exp. Psychol. Gen. 2004, 133, 83–100. [Google Scholar] [CrossRef] [Green Version]
- Barrouillet, P.; De Paepe, A.; Langerock, N. Time causes forgetting from working memory. Psychon. Bull. Rev. 2012, 19, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Wingfield, A.; Amichetti, N.M.; Lash, A. Cognitive aging and hearing acuity: Modeling spoken language comprehension. Front. Psychol. 2015, 6, 684. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, M.V.; Mirz, F.; Gjedde, A. Restored speech comprehension linked to activity in left inferior prefrontal and right temporal cortices in postlingual deafness. Neuroimage 2006, 31, 842–852. [Google Scholar] [CrossRef]
- Wagner, A.D.; Paré-Blagoev, E.J.; Clark, J.; Poldrack, R.A. Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron 2001, 31, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Foley, J.A.; Foltynie, T.; Zrinzo, L.; Hyam, J.A.; Limousin, P.; Cipolotti, L. Apathy and Reduced Speed of Processing Underlie Decline in Verbal Fluency following DBS. Behav. Neurol. 2017, 2017, 7348101. [Google Scholar] [CrossRef]
| Patient | Age (Years) | Education (Years) | Aetiology | IS | HL Duration (Years) | AD in IS (Years) | Controlateral HA |
|---|---|---|---|---|---|---|---|
| 1 | 54 | 11 | Sudden Hearing Loss | Right | 10 | 3 | Yes |
| 2 | 69 | 18 | Idiopatic | Right | 8 | 0 | No |
| 3 | 73 | 5 | Autoimmune | Right | 2 | 0 | Yes |
| 4 | 56 | 9 | Chronic Otitis Media | Right | 30 | 30 | Yes |
| 5 | 71 | 12 | Sudden Hearing Loss | Right | 15 | 1 | Yes |
| 6 | 64 | 5 | Idiopatic | Left | 20 | 15 | Yes |
| 7 | 74 | 5 | Otosclerosis | Left | 40 | 4 | Yes |
| 8 | 50 | 18 | Infantile Meningitis | Right | 45 | 20 | No |
| 9 | 64 | 12 | Idiopatic | Left | 55 | 35 | No |
| 10 | 69 | 18 | Idiopatic | Right | 30 | 15 | No |
| 11 | 60 | 8 | Otosclerosis | Left | 25 | 4 | Yes |
| 12 | 66 | 8 | Otosclerosis | Left | 35 | 22 | No |
| 13 | 59 | 8 | Otosclerosis | Right | 40 | 15 | No |
| 14 | 78 | 18 | Sudden Hearing Loss | Right | 15 | 3 | Yes |
| 15 | 62 | 16 | Idiopatic | Right | 20 | 5 | Yes |
| 16 | 67 | 13 | Idiopatic | Left | 25 | 0 | Yes |
| 17 | 59 | 8 | Autoimmune | Right | 4 | 0 | Yes |
| 18 | 80 | 3 | Menière Disease | Right | 20 | 0 | Yes |
| 19 | 60 | 13 | Sudden Hearing Loss | Left | 15 | 1 | Yes |
| 20 | 75 | 8 | Idiopatic | Right | 20 | 3 | Yes |
| 21 | 60 | 13 | Menière Disease | Left | 25 | 4 | Yes |
| n | PTA Aided Side (dB) | SRS with HA (%) |
|---|---|---|
| 1 | 101.25 | 10 |
| 3 | 93.75 | 20 |
| 4 | 92.5 | 20 |
| 5 | 100 | 10 |
| 6 | 91.25 | 10 |
| 7 | 70 | 50 |
| 11 | 78.75 | 30 |
| 14 | 101.25 | 10 |
| 15 | 73.75 | 40 |
| 16 | 90 | 10 |
| 17 | 71.25 | 50 |
| 18 | 101.25 | 10 |
| 19 | 71.25 | 50 |
| 20 | 106.25 | 10 |
| 21 | 76.25 | 50 |
| Mean | 87.92 | 25.33 |
| SD | 13.05 | 17.67 |
| Cognitive Tests (T0) | Verbal Recognition at 60 dB > 80% (T12) | Verbal Recognition at 60 dB ≤ 80% (T12) | p-Value |
|---|---|---|---|
| MMSE | 27.4 ± 2.3 | 25.6 ± 4.4 | 0.545 |
| CDT | 13.2 ± 1.7 | 10.6 ± 4.4 | 0.117 |
| RAVLT Immediate | 35.0 ± 8.0 | 30.5 ± 11.3 | 0.343 |
| RAVLT Differite | 6.7 ± 3.0 | 5.8 ± 4.3 | 0.455 |
| Digit-Span Test Forward | 5.2 ± 1.1 | 4.4 ± 1.3 | 0.199 |
| Digit-Span Test Backward | 4.1 ± 1.4 | 3.4 ± 1.1 | 0.382 |
| Corsi block-tapping test Forward | 5.1 ± 1.0 | 4.4 ± 0.8 | 0.220 |
| Corsi block-tapping test Backward | 4.3 ± 1.5 | 3.8 ± 1.3 | 0.588 |
| Verbal phonemic Fluency Test | 35.5 ± 12.0 | 30.1 ± 11.0 | 0.218 |
| Verbal semantic Fluency Test | 24.3 ± 4.0 | 19.1 ± 5.5 | 0.052 |
| TMT-A | 37.2 ± 18.4 | 51.8 ± 15.6 | 0.115 |
| TMT-B | 114.3 ± 71.9 | 241.8 ± 171.1 | 0.087 |
| TMT B-A | 77.3 ± 60.0 | 190.0 ± 157.6 | 0.138 |
| Cognitve Tests (T0) | r2 | β | p-Value |
|---|---|---|---|
| MMSE | 0.061 | 0.247 | 0.280 |
| CDT | 0.177 | 0.421 | 0.058 |
| RAVLT Immediate | 0.049 | 0.222 | 0.346 |
| RAVLT Differite | 0.110 | 0.331 | 0.154 |
| Digit-Span Test Forward | 0.003 | 0.051 | 0.826 |
| Digit-Span Test Backward | 0.036 | 0.190 | 0.410 |
| Corsi block-tapping test Forward | 0.081 | 0.284 | 0.212 |
| Corsi block-tapping test Backward | 0.103 | 0.321 | 0.156 |
| Verbal phonemic Fluency Test | 0.002 | 0.049 | 0.834 |
| Verbal semantic Fluency Test | 0.165 | 0.407 | 0.067 |
| TMT-A | 0.236 | −0.486 | 0.035 |
| TMT-B | 0.086 | −0.370 | 0.119 |
| TMT B-A | 0.108 | −0.328 | 0.170 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zucca, M.; Albera, A.; Albera, R.; Montuschi, C.; Della Gatta, B.; Canale, A.; Rainero, I. Cochlear Implant Results in Older Adults with Post-Lingual Deafness: The Role of “Top-Down” Neurocognitive Mechanisms. Int. J. Environ. Res. Public Health 2022, 19, 1343. https://doi.org/10.3390/ijerph19031343
Zucca M, Albera A, Albera R, Montuschi C, Della Gatta B, Canale A, Rainero I. Cochlear Implant Results in Older Adults with Post-Lingual Deafness: The Role of “Top-Down” Neurocognitive Mechanisms. International Journal of Environmental Research and Public Health. 2022; 19(3):1343. https://doi.org/10.3390/ijerph19031343
Chicago/Turabian StyleZucca, Milena, Andrea Albera, Roberto Albera, Carla Montuschi, Beatrice Della Gatta, Andrea Canale, and Innocenzo Rainero. 2022. "Cochlear Implant Results in Older Adults with Post-Lingual Deafness: The Role of “Top-Down” Neurocognitive Mechanisms" International Journal of Environmental Research and Public Health 19, no. 3: 1343. https://doi.org/10.3390/ijerph19031343
APA StyleZucca, M., Albera, A., Albera, R., Montuschi, C., Della Gatta, B., Canale, A., & Rainero, I. (2022). Cochlear Implant Results in Older Adults with Post-Lingual Deafness: The Role of “Top-Down” Neurocognitive Mechanisms. International Journal of Environmental Research and Public Health, 19(3), 1343. https://doi.org/10.3390/ijerph19031343






