Barriers and Facilitators to the Implementation of a Personalized Breast Cancer Screening Program: Views of Spanish Health Professionals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Study Population
2.2. Survey Instrument
- Sociodemographic data: age, gender, professional field (nurse, doctor, other), medical specialty or professional profile, years of practice, type of work center (public, private, both, university, other), type of relationship or employment contract, and work relation with early detection of breast cancer (yes/no);
- Advantages of risk-based screening for the health of women with an individual risk of breast cancer higher (6 items)/lower (6 items) than the population average;
- Disadvantages of risk-based screening for women’s health (6 items);
- Advantages of risk-based screening, in relation to current screening, for the Spanish National Health System (4 items);
- Barriers (15 items) and facilitators (6 items) for the implementation of risk-based screening;
- Implementation of shared decision-making in breast cancer screening (12 items);
- Aspects of the organizational structure to consider for the implementation of a risk-based screening program (9 items);
- Communication of the benefits and harms of breast cancer screening (7 items);
- Coordination of the risk-based screening program (3 items);
- Considering the advantages and disadvantages, how important is it for you to move from the current Screening Program to a personalized Breast Cancer Screening Program? Answer: 1 to 5 Likert scale, where 1-very little or nothing and 5-a lot;
- Given the current Breast Cancer Screening Program, do you think Primary Care should be the gateway to a future personalized breast cancer screening program? Answer: Yes/No.
2.3. Data Analysis
3. Results
3.1. Participants Characteristics
3.2. Advantages and Disadvantages of Risk-Based Breast Cancer Screening
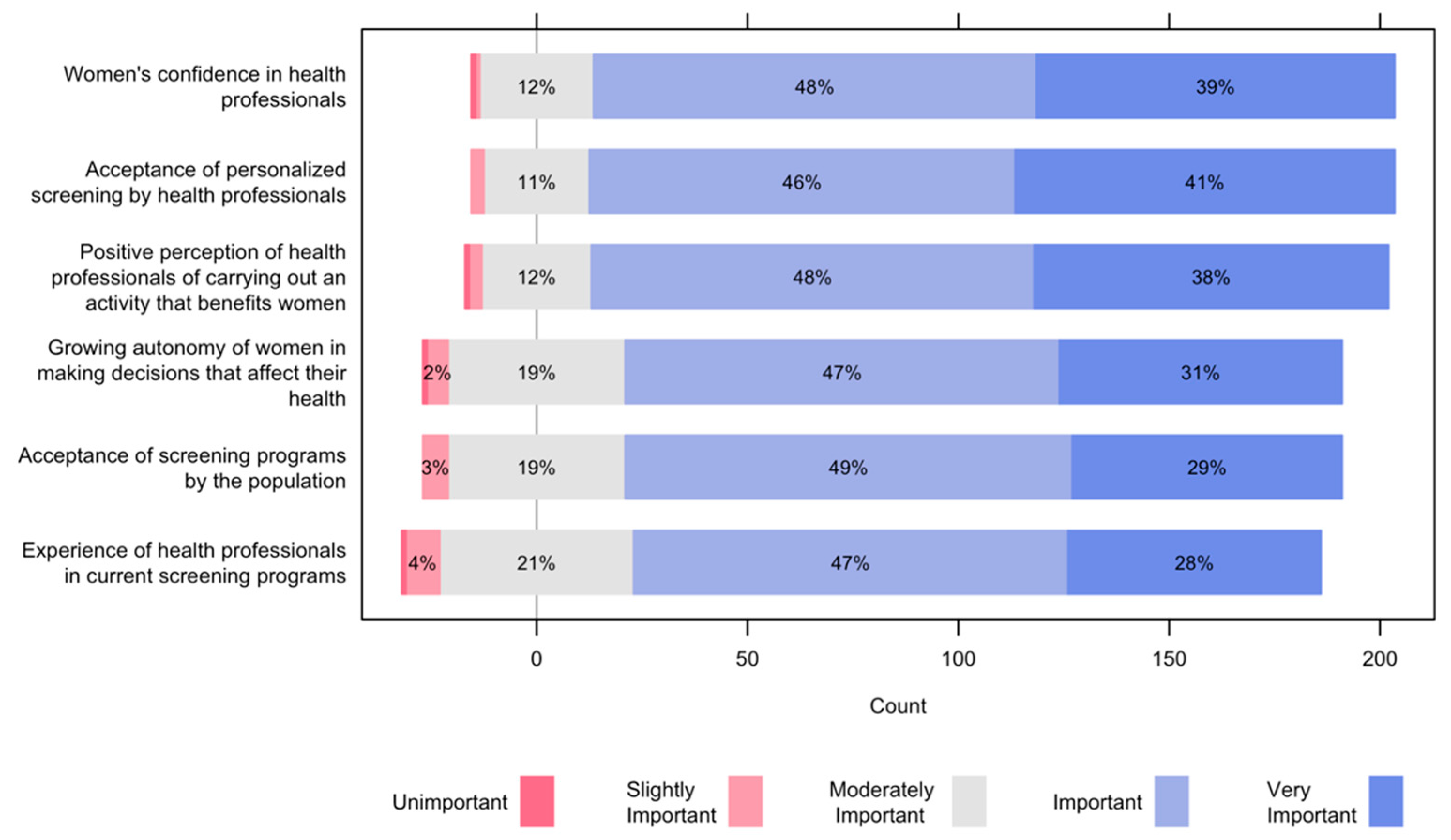
3.3. Facilitators and Barriers for the Implementation of a Risk-Based Screening Program
3.4. Implementation of Shared Decision-Making in Breast Cancer Screening
3.5. Aspects of the Organizational Structure to Consider for the Implementation of a Risk-Based Screening Program
3.6. Communication of the Benefits and Harms of Breast Cancer Screening and Coordination of the Risk-Based Screening Program
4. Discussion
4.1. Summary of Main Findings
4.2. Comparison with Previous Studies
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marmot, M.G.; Altman, D.G.; Cameron, D.; Dewar, J.; Thompson, S.G.; Wilcox, M. The benefits and harms of breast cancer screening: An independent review. Br. J. Cancer 2013, 108, 2205–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotzsche, P.C.; Jorgensen, K.J. Screening for breast cancer with mammography. Cochrane Database Syst. Rev. 2013, 6, CD001877. [Google Scholar] [CrossRef] [PubMed]
- Myers, E.R.; Moorman, P.G.; Gierisch, J.M.; Havrilesky, L.J.; Grimm, L.; Ghate, S.V.; Davidson, B.; Mongtomery, R.C.; Crowley, M.J.; McCrory, D.C.; et al. Benefits and Harms of Breast Cancer Screening. JAMA 2015, 314, 1615–1634. [Google Scholar] [CrossRef] [PubMed]
- Vilaprinyo, E.; Forne, C.; Carles, M.; Sala, M.; Pla, R.; Castells, X.; Domingo, L.; Rue, M.; The Interval Cancer (INCA) Study Group. Cost-Effectiveness and Harm-Benefit Analyses of Risk-Based Screening Strategies for Breast Cancer. PLoS ONE 2014, 9, e86858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Román, M.; Sala, M.; Domingo, L.; Posso, M.; Louro, J.; Castells, X. Personalized breast cancer screening strategies: A systematic review and quality assessment. PLoS ONE 2019, 14, e0226352. [Google Scholar] [CrossRef] [Green Version]
- Rainey, L.; van der Waal, D.; Jervaeus, A.; Wengström, Y.; Evans, D.G.; Donnelly, L.S.; Broeders, M.J. Are we ready for the challenge of implementing risk-based breast cancer screening and primary prevention? Breast 2018, 39, 24–32. [Google Scholar] [CrossRef]
- Alam Khan, S.; Hernandez-Villafuerte, K.V.; Muchadeyi, M.T.; Schlander, M. Cost-effectiveness of risk-based breast cancer screening: A systematic review. Int. J. Cancer 2021, 149, 790–810. [Google Scholar] [CrossRef]
- Puzhko, S.; Gagnon, J.; Simard, J.; Knoppers, B.M.; Siedlikowski, S.; Bartlett, G. Health professionals’ perspectives on breast cancer risk stratification: Understanding evaluation of risk versus screening for disease. Public Health Rev. 2019, 40, 2. [Google Scholar] [CrossRef] [Green Version]
- Rainey, L.; Van Der Waal, D.; Donnelly, L.S.; Evans, D.G.; Wengström, Y.; Broeders, M. Women’s decision-making regarding risk-stratified breast cancer screening and prevention from the perspective of international healthcare professionals. PLoS ONE 2018, 13, e0197772. [Google Scholar] [CrossRef] [Green Version]
- Esquivel-Sada, D.; Lévesque, E.; Hagan, J.; Knoppers, B.M.; Simard, B.M.K.A.J. Envisioning Implementation of a Personalized Approach in Breast Cancer Screening Programs: Stakeholder Perspectives. Healthc. Policy 2019, 15, 39–54. [Google Scholar] [CrossRef]
- Collins, I.M.; Steel, E.; Mann, G.B.; Emery, J.D.; Bickerstaffe, A.; Trainer, A.; Butow, P.; Pirotta, M.; Antoniou, A.C.; Cuzick, J.; et al. Assessing and managing breast cancer risk: Clinicians’ current practice and future needs. Breast 2014, 23, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Pons-Rodriguez, A.; Izquierdo, C.F.; Vilaplana-Mayoral, J.; Cruz-Esteve, I.; Sánchez-López, I.; Reñé-Reñé, M.; Cazorla, C.; Hernández-Andreu, M.; Galindo-Ortego, G.; Gabandé, M.L.; et al. Feasibility and acceptability of personalised breast cancer screening (DECIDO study): Protocol of a single-arm proof-of-concept trial. BMJ Open 2020, 10, e044597. [Google Scholar] [CrossRef] [PubMed]
- Heiberger, R.M. Statistical Analysis and Data Display: Heiberger and Holland. 2020. Available online: https://cran.r-project.org/web/packages/HH/HH.pdf (accessed on 26 January 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 26 January 2022).
- Rainey, L.; Van Der Waal, D.; Jervaeus, A.; Donnelly, L.S.; Evans, D.G.; Hammarström, M.; Hall, P.; Wengström, Y.; Broeders, M.J.M. European women’s perceptions of the implementation and organisation of risk-based breast cancer screening and prevention: A qualitative study. BMC Cancer 2020, 20, 247. [Google Scholar] [CrossRef] [PubMed]
- Rainey, L.; Jervaeus, A.; Donnelly, L.S.; Evans, D.G.; Hammarström, M.; Hall, P.; Wengström, Y.; Broeders, M.J.; van der Waal, D. Women’s perceptions of personalized risk-based breast cancer screening and prevention: An international focus group study. Psycho-Oncology 2019, 28, 1056–1062. [Google Scholar] [CrossRef] [Green Version]
- Evans, D.G.R.; Donnelly, L.; Harkness, E.F.; Astley, S.M.; Stavrinos, P.; Dawe, S.; Watterson, D.; Fox, L.; Sergeant, J.C.; Ingham, S.; et al. Breast cancer risk feedback to women in the UK NHS breast screening population. Br. J. Cancer 2016, 114, 1045–1052. [Google Scholar] [CrossRef]
- French, D.P.; Southworth, J.; Howell, A.; Harvie, M.; Stavrinos, P.; Watterson, D.; Sampson, S.; Evans, G.; Donnelly, L. Psychological impact of providing women with personalised 10-year breast cancer risk estimates. Br. J. Cancer 2018, 118, 1648–1657. [Google Scholar] [CrossRef] [Green Version]
- Mathioudakis, A.G.; Salakari, M.; Pylkkanen, L.; Saz-Parkinson, Z.; Bramesfeld, A.; Deandrea, S.; Lerda, D.; Neamtiu, L.; Pardo-Hernandez, H.; Solà, I.; et al. Systematic review on women’s values and preferences concerning breast cancer screening and diagnostic services. Psycho-Oncology 2019, 28, 939–947. [Google Scholar] [CrossRef] [Green Version]
- Petrova, D.; Mas, G.; Navarrete, G.; Rodriguez, T.T.; Ortiz, P.J.; Garcia-Retamero, R. Cancer screening risk literacy of physicians in training: An experimental study. PLoS ONE 2019, 14, e0218821. [Google Scholar] [CrossRef] [Green Version]
- Willems, B.; Bracke, P. Participants, Physicians or Programmes: Participants’ educational level and initiative in cancer screening. Health Policy 2018, 122, 422–430. [Google Scholar] [CrossRef]
- Lévesque, E.; Hagan, J.; Knoppers, B.M.; Simard, J. Organizational challenges to equity in the delivery of services within a new personalized risk-based approach to breast cancer screening. New Genet. Soc. 2018, 38, 38–59. [Google Scholar] [CrossRef] [Green Version]
- McWilliams, L.; Woof, V.G.; Donnelly, L.S.; Howell, A.; Evans, D.G.; French, D.P. Risk stratified breast cancer screening: UK healthcare policy decision-making stakeholders’ views on a low-risk breast screening pathway. BMC Cancer 2020, 20, 680. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Leal, M.J.; Pérez-Lacasta, M.J.; Feijoo-Cid, M.; Ramos-García, V.; Carles-Lavila, M. Healthcare professionals’ behaviour regarding the implementation of shared decision-making in screening programmes: A systematic review. Patient Educ. Couns. 2021, 104, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Allweis, T.M.; Hermann, N.; Berenstein-Molho, R.; Guindy, M. Personalized Screening for Breast Cancer: Rationale, Present Practices, and Future Directions. Ann. Surg. Oncol. 2021, 28, 4306–4317. [Google Scholar] [CrossRef] [PubMed]
- Silver, E.; Wenger, N.; Xie, Z.; Elashoff, D.; Lee, K.; Madlensky, L.; Trent, J.; Petruse, A.; Johansen, L.; Naeim, A. Implementing a Population-Based Breast Cancer Risk Assessment Program. Clin. Breast Cancer 2019, 19, 246–253.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagan, J.; Lévesque, E.; Knoppers, B.M. Influence of organizational factors on implementation of a personalized approach to breast cancer screening. St. Publique 2016, 28, 353–361. [Google Scholar] [CrossRef]
- Yi, H.; Xiao, T.; Thomas, P.S.; Aguirre, A.N.; Smalletz, C.; Dimond, J.; Finkelstein, J.; Infante, K.; Trivedi, M.; David, R.; et al. Barriers and Facilitators to Patient-Provider Communication When Discussing Breast Cancer Risk to Aid in the Development of Decision Support Tools. AMIA Annu. Symp. Proc. 2015, 2015, 1352–1360. [Google Scholar]
- Lévesque, E.; Kirby, E.; Bolt, I.; Knoppers, B.M.; De Beaufort, I.; Pashayan, N.; Widschwendter, M. Ethical, Legal, and Regulatory Issues for the Implementation of Omics-Based Risk Prediction of Women’s Cancer: Points to Consider. Public Health Genom. 2018, 21, 37–44. [Google Scholar] [CrossRef]
- Chowdhury, S.; Dent, T.; Pashayan, N.; Hall, A.; Lyratzopoulos, G.; Hallowell, N.; Hall, P.; Pharoah, P.; Burton, H. Incorporating genomics into breast and prostate cancer screening: Assessing the implications. Genet. Med. 2013, 15, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, R.M.; Vanbeselaere, C. Enciclopedia of Social Measurement: Web-Based Survey; Elsevier Inc.: Amsterdam, The Netherlands, 2005; pp. 955–962. [Google Scholar]







| Variable | N (%) | Number of Responses |
|---|---|---|
| Gender, Female | 151 (76.3) | 198 |
| Age, Median [Q1, Q3] | 53 [44.8, 60.0] | 220 |
| Years of work experience, Median [Q1, Q3] | 25.0 [16.0;33.0] | 220 |
| Work area | 195 | |
| Medicine | 102 (52.3) | |
| Nursing | 72 (36.9) | |
| Other | 21 (10.8) | |
| Health-related specialty | 195 | |
| Oncology | 38 (19.5) | |
| Epidemiology/Preventive Medicine and Public Health | 37 (19.0) | |
| Family and Community Medicine | 29 (14.9) | |
| No specialty | 21 (10.8) | |
| Gynecology and Obstetrics | 25 (12.8) | |
| Radiology | 7 (3.6) | |
| Health economics | 6 (3.1) | |
| Surgery | 3 (1.5) | |
| Other | 29 (14.9) | |
| Workplace | 199 | |
| Public health center | 170 (85.4) | |
| Private health center | 7 (3.52) | |
| Both public and private health center | 10 (5.03) | |
| University | 8 (4.02) | |
| Other | 4 (2.01) | |
| Work-related to breast cancer early detection | 91 (41.6) | 219 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laza-Vásquez, C.; Hernández-Leal, M.J.; Carles-Lavila, M.; Pérez-Lacasta, M.J.; Cruz-Esteve, I.; Rué, M.; on behalf of the DECIDO Group. Barriers and Facilitators to the Implementation of a Personalized Breast Cancer Screening Program: Views of Spanish Health Professionals. Int. J. Environ. Res. Public Health 2022, 19, 1406. https://doi.org/10.3390/ijerph19031406
Laza-Vásquez C, Hernández-Leal MJ, Carles-Lavila M, Pérez-Lacasta MJ, Cruz-Esteve I, Rué M, on behalf of the DECIDO Group. Barriers and Facilitators to the Implementation of a Personalized Breast Cancer Screening Program: Views of Spanish Health Professionals. International Journal of Environmental Research and Public Health. 2022; 19(3):1406. https://doi.org/10.3390/ijerph19031406
Chicago/Turabian StyleLaza-Vásquez, Celmira, María José Hernández-Leal, Misericòrdia Carles-Lavila, Maria José Pérez-Lacasta, Inés Cruz-Esteve, Montserrat Rué, and on behalf of the DECIDO Group. 2022. "Barriers and Facilitators to the Implementation of a Personalized Breast Cancer Screening Program: Views of Spanish Health Professionals" International Journal of Environmental Research and Public Health 19, no. 3: 1406. https://doi.org/10.3390/ijerph19031406
APA StyleLaza-Vásquez, C., Hernández-Leal, M. J., Carles-Lavila, M., Pérez-Lacasta, M. J., Cruz-Esteve, I., Rué, M., & on behalf of the DECIDO Group. (2022). Barriers and Facilitators to the Implementation of a Personalized Breast Cancer Screening Program: Views of Spanish Health Professionals. International Journal of Environmental Research and Public Health, 19(3), 1406. https://doi.org/10.3390/ijerph19031406






