Climate Change Impacts on Microbiota in Beach Sand and Water: Looking Ahead
Abstract
:1. Introduction and Broad Climate Change Projections
1.1. Characterizing Climate Change Impacts on Microbiota
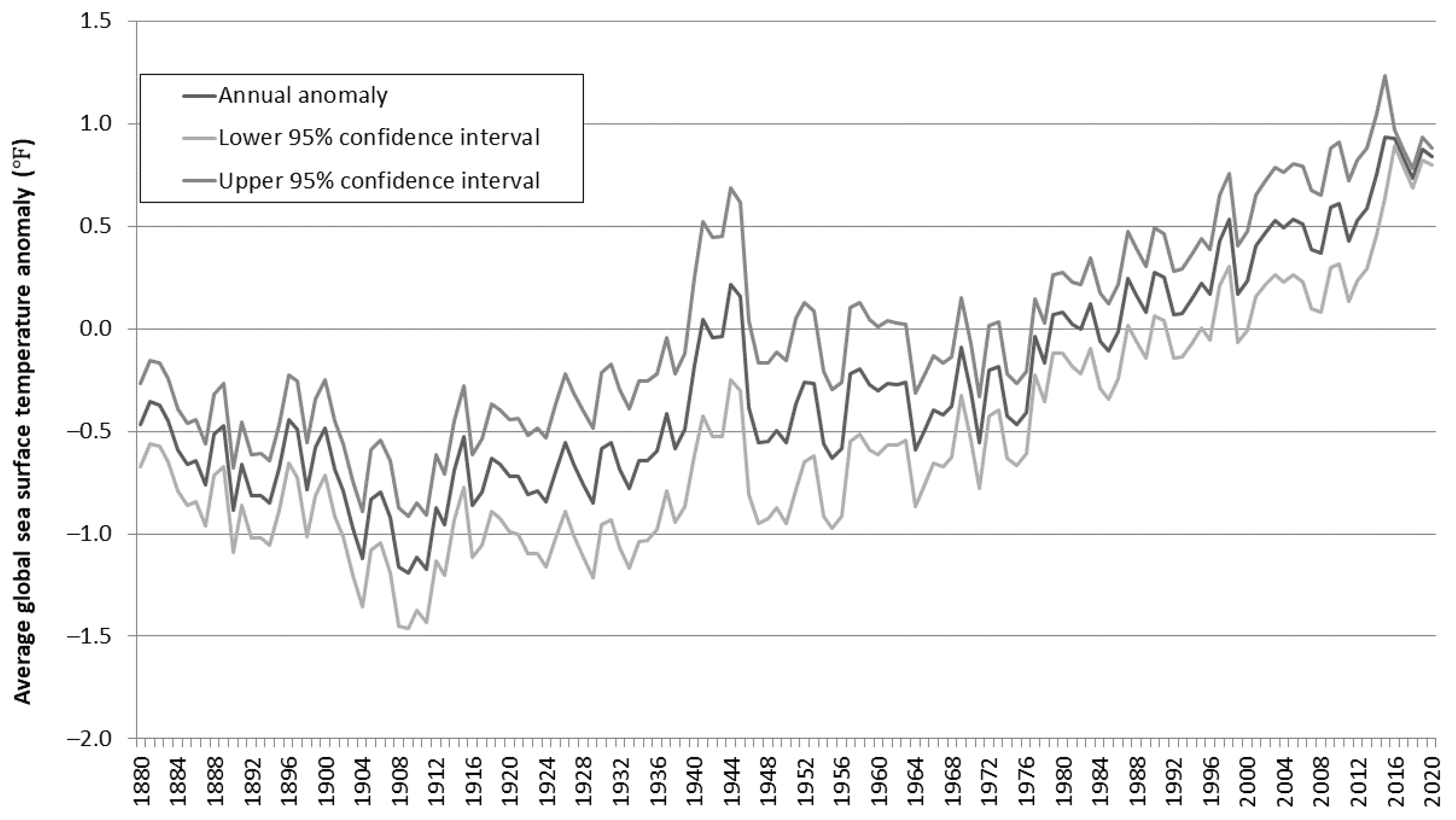
1.2. Temperature Increases
1.3. Precipitation Increases
1.4. Wave Activity and Sea Level Rise
1.5. Economic Impact
2. Recommendations for the Future
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change Synthesis Report 2014; Pachauri, R.K., Meyer, L., Eds.; Intergovernmental Panel on Climate Change: New York, NY, USA, 2014. [Google Scholar]
- IPCC. Climate Change the Physical Science Basis 2013; Stocker, T.F., Qin, D., Eds.; Intergovernmental Panel on Climate Change: New York, NY, USA, 2013. [Google Scholar]
- NOAA. Extended Reconstructed Sea Surface Temperature (ERSST.v5). 2021. Available online: https://www.epa.gov/climate-indicators/climate-change-indicators-sea-surface-temperature (accessed on 10 December 2021).
- Johansson, M.M.; Pellikka, H.; Kahma, K.K.; Ruosteenoja, K. Global sea level rise scenarios adapted to the Finnish coast. J. Mar. Syst. 2014, 129, 35–46. [Google Scholar] [CrossRef]
- O’Reilly, C.M.; Sharma, S.; Gray, D.K.; Hampton, S.E.; Read, J.S.; Rowley, R.J.; Schneider, P.; Lenters, J.D.; McIntyre, P.B.; Kraemer, B.M.; et al. Rapid and highly variable warming of lake surface waters around the globe. Geophys. Res. Lett. 2015, 42, 10773–10781. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Gray, D.K.; Read, J.S.; O’Reilly, C.M.; Schneider, P.; Qudrat, A.; Gries, C.; Stefanoff, S.; Hampton, S.E.; Hook, S.; et al. A global database of lake surface temperatures collected by in situ and satellite methods from 1985–2009. Sci. Data 2015, 2, 19. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.X.; Cai, W.J.; Zhang, L.P.; Nakamura, H.; Timmermann, A.; Joyce, T.; McPhaden, M.J.; Alexander, M.; Qiu, B.; Visbecks, M.; et al. Enhanced warming over the global subtropical western boundary currents. Nat. Clim. Chang. 2012, 2, 161–166. [Google Scholar] [CrossRef]
- Young, I.R.; Zieger, S.; Babanin, A.V. Global trends in wind speed and wave height. Science 2011, 332, 451–455. [Google Scholar] [CrossRef]
- Tokinaga, H.; Xie, S.P. Wave and anemometer-based sea surface wind (WASWind) for climate change analysis. J. Clim. 2011, 24, 267–285. [Google Scholar] [CrossRef] [Green Version]
- Byrnes, J.E.; Reed, D.C.; Cardinale, B.J.; Cavanaugh, K.C.; Holbrook, S.J.; Schmitt, R.J. Climate-driven increases in storm frequency simplify kelp forest food webs. Glob. Chang. Biol. 2011, 17, 2513–2524. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.R.; Gast, R.J.; Fujioka, R.S.; Solo-Gabriele, H.M.; Meschke, J.S.; Amaral-Zettler, L.A.; del Castillo, E.; Polz, M.F.; Collier, T.K.; Strom, M.S.; et al. The coastal environment and human health: Microbial indicators, pathogens, sentinels and reservoirs. Environ. Health 2008, 7, S3. [Google Scholar] [CrossRef] [Green Version]
- Weiskerger, C.J.; Brandao, J.; Ahmed, W.; Aslan, A.; Avolio, L.; Badgley, B.D.; Boehm, A.B.; Edge, T.A.; Fleisher, J.M.; Heaney, C.D.; et al. Impacts of a changing earth on microbial dynamics and human health risks in the continuum between beach water and sand. Water Res. 2019, 162, 456–470. [Google Scholar] [CrossRef]
- Casadevall, A.; Kontoyiannis, D.P.; Robert, V. On the emergence of Candida auris: Climate change, azoles, swamps, and birds. mBio 2019, 10, e01397-19. [Google Scholar] [CrossRef] [Green Version]
- Arora, P.; Singh, P.; Wang, Y.; Yadav, A.; Pawar, K.; Singh, A.; Padmavati, G.; Xu, J.; Chowdhary, A. Environmental isolation of Candida auris from the coastal wetlands of Andaman Islands, India. mBio 2021, 12, e03181-20. [Google Scholar] [CrossRef]
- Sabino, R.; Veríssimo, C.; Cunha, M.; Wergikoski, B.; Ferreira, F.; Rodrigues, R.; Parada, H.; Falcão, L.; Rosado, L.; Pinheiro, C.; et al. Pathogenic fungi: An unacknowledged risk at coastal resorts? New insights on microbiological sand quality in Portugal. Mar. Pollut. Bull. 2011, 62, 1506–1511. [Google Scholar] [CrossRef] [Green Version]
- Brandão, J.; Gangneux, J.P.; Arikan-Akdagli, S.; Barac, A.; Bostanaru, A.C.; Brito, S.; Bull, M.; Çerikçioğlu, N.; Chapman, B.; Efstratiou, M.A.; et al. Mycosands: Fungal diversity and abundance in beach sand and recreational waters—Relevance to human health. Sci. Total Environ. 2021, 781, 146598. [Google Scholar] [CrossRef]
- Byrne, R.H.; Mecking, S.; Feely, R.A.; Liu, X.W. Direct observations of basin-wide acidification of the North Pacific Ocean. Geophys. Res. Lett. 2010, 37, 5. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.J.; Hu, X.P.; Huang, W.J.; Murrell, M.C.; Lehrter, J.C.; Lohrenz, S.E.; Chou, W.C.; Zhai, W.D.; Hollibaugh, J.T.; Wang, Y.C.; et al. Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 2011, 4, 766–770. [Google Scholar] [CrossRef]
- Henson, S.A.; Cael, B.B.; Allen, S.R.; Dutkiewicz, S. Future phytoplankton diversity in a changing climate. Nat. Commun. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Williamson, C.E.; Madronich, S.; Lal, A.; Zepp, R.G.; Lucas, R.M.; Overholt, E.P.; Rose, K.C.; Schladow, S.G.; Lee-Taylor, J. Climate change-induced increases in precipitation are reducing the potential for solar ultraviolet radiation to inactivate pathogens in surface waters. Sci. Rep. 2017, 7, 12. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Impacts of Climate Change on the Occurrence of Harmful Algal Blooms. (2013, May). Available online: https://www.epa.gov/sites/default/files/documents/climatehabs.pdf (accessed on 17 November 2021).
- Rastogi, R.P.; Madamwar, D.; Incharoensakdi, A. Bloom dynamics of cyanobacteria and their toxins: Environmental health impacts and mitigation strategies. Front. Microbiol. 2015, 6, 1254. [Google Scholar] [CrossRef] [Green Version]
- Richer, R.; Banack, S.A.; Metcalf, J.S.; Cox, P.A. The persistence of cyanobacterial toxins in desert soils. J. Arid Environ. 2015, 112, 134–139. [Google Scholar] [CrossRef]
- Phillips, M.C.; Feng, Z.; Vogel, L.J.; Reniers, A.J.H.M.; Haus, B.K.; Enns, A.A.; Zhang, Y.; Hernandez, D.B.; Solo-Gabriele, H.M. Microbial release from seeded beach sediments during wave conditions. Mar. Pollut. Bull. 2014, 79, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Vogel, L.J.; O’Carroll, D.M.; Edge, T.A.; Robinson, C.E. Release of Escherichia coli from foreshore sand and pore water during intensified wave conditions at a recreational beach. Environ. Sci. Technol. 2016, 50, 5676–5684. [Google Scholar] [CrossRef]
- Kauppinen, A.; Al-Hello, H.; Zacheus, O.; Kilponen, J.; Maunula, L.; Huusko, S.; Lappalainen, M.; Miettinen, I.; Blomqvist, S.; Rimhanen-Finne, R. Increase in outbreaks of gastroenteritis linked to bathing water in Finland in summer 2014. Eurosurveillance 2017, 22, 13–20. [Google Scholar] [CrossRef]
- Moreno, A.; Amelung, B.; Santamarta, L. Linking beach recreation to weather conditions: A case study in Zandvoort, Netherlands. Tour. Mar. Environ. 2009, 5, 111–119. [Google Scholar] [CrossRef]
- Smith, K. The influence of weather and climate on recreation and tourism. Weather 1993, 48, 398–404. [Google Scholar] [CrossRef]
- Shuval, H. Estimating the global burden of thalassogenic diseases: Human infectious diseases caused by wastewater pollution of the marine environment. J. Water Health 2003, 1, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Perkins, T.L.; Clements, K.; Baas, J.H.; Jago, C.F.; Jones, D.L.; Malham, S.K.; McDonald, J.E. Sediment composition influences spatial variation in the abundance of human pathogen indicator bacteria within an estuarine environment. PLoS ONE 2014, 9, 9. [Google Scholar] [CrossRef]
- Brookes, J.D.; Carey, C.C. Resilience to blooms. Science 2011, 334, 46–47. [Google Scholar] [CrossRef]
- Rigosi, A.; Hanson, P.; Hamilton, D.P.; Hipsey, M.; Rusak, J.A.; Bois, J.; Sparber, K.; Chorus, I.; Watkinson, A.J.; Qin, B.; et al. Determining the probability of cyanobacterial blooms: The application of Bayesian networks in multiple lake systems. Ecol. Appl. 2015, 25, 186–199. [Google Scholar] [CrossRef]
- Bussi, G.; Whitehead, P.G.; Thomas, A.R.C.; Masante, D.; Jones, L.; Cosby, B.J.; Emmett, B.A.; Malham, S.K.; Prudhomme, C.; Prosser, H. Climate and land-use change impact on faecal indicator bacteria in a temperate maritime catchment (the River Conwy, Wales). J. Hydrol. 2017, 553, 248–261. [Google Scholar] [CrossRef] [Green Version]
- Noble, R.T.; Lee, I.M.; Schiff, K.C. Inactivation of indicator micro-organisms from various sources of faecal contamination in seawater and freshwater. J. Appl. Microbiol. 2004, 96, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Sokolova, E.; Astrom, J.; Pettersson, T.J.R.; Bergstedt, O.; Hermansson, M. Decay of Bacteroidales genetic markers in relation to traditional fecal indicators for water quality modeling of drinking water sources. Environ. Sci. Technol. 2012, 46, 892–900. [Google Scholar] [CrossRef] [Green Version]
- Viau, E.J.; Goodwin, K.D.; Yamahara, K.M.; Layton, B.A.; Sassoubre, L.M.; Burns, S.L.; Tong, H.I.; Wong, S.H.C.; Lu, Y.A.; Boehm, A.B. Bacterial pathogens in Hawaiian coastal streams—Associations with fecal indicators, land cover, and water quality. Water Res. 2011, 45, 3279–3290. [Google Scholar] [CrossRef]
- Hokajärvi, A.M.; Pitkanen, T.; Siljanen, H.M.P.; Nakari, U.M.; Torvinen, E.; Siitonen, A.; Miettinen, I.T. Occurrence of thermotolerant Campylobacter spp. and adenoviruses in Finnish bathing waters and purified sewage effluents. J. Water Health 2013, 11, 120–134. [Google Scholar] [CrossRef] [Green Version]
- Baker-Austin, C.; Trinanes, J.A.; Taylor, N.G.H.; Hartnell, R.; Siitonen, A.; Martinez-Urtaza, J. Emerging Vibrio risk at high latitudes in response to ocean warming. Nat. Clim. Chang. 2013, 3, 73–77. [Google Scholar] [CrossRef]
- Huehn, S.; Eichhorn, C.; Urmersbach, S.; Breidenbach, J.; Bechlars, S.; Bier, N.; Alter, T.; Bartelt, E.; Frank, C.; Oberheitmann, B.; et al. Pathogenic vibrios in environmental, seafood and clinical sources in Germany. Int. J. Med. Microbiol. 2014, 304, 843–850. [Google Scholar] [CrossRef]
- Sterk, A.; Schets, F.M.; Husman, A.M.D.; de Nijs, T.; Schijven, J.F. Effect of climate change on the concentration and associated risks of Vibrio spp. in Dutch recreational waters. Risk Anal. 2015, 35, 1717–1729. [Google Scholar] [CrossRef]
- Desvars, A.; Jego, S.; Chiroleu, F.; Bourhy, P.; Cardinale, E.; Michault, A. Seasonality of human Leptospirosis in Reunion Island (Indian Ocean) and its association with meteorological data. PLoS ONE 2011, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Sumi, A.; Telan, E.F.O.; Chagan-Yasutan, H.; Piolo, M.B.; Hattori, T.; Kobayashi, N. Effect of temperature, relative humidity and rainfall on dengue fever and leptospirosis infections in Manila, the Philippines. Epidemiol. Infect. 2017, 145, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Liao, J.S.; Huang, X.; Wang, Y.P.; Ren, J.H.; Wang, X.Y.; Ding, F. Mapping risk of leptospirosis in China using environmental and socioeconomic data. BMC Infect. Dis. 2016, 16, 10. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, K.D.; McNay, M.; Cao, Y.P.; Ebentier, D.; Madison, M.; Griffith, J.F. A multi-beach study of Staphylococcus aureus, MRSA, and enterococci in seawater and beach sand. Water Res. 2012, 46, 4195–4207. [Google Scholar] [CrossRef] [Green Version]
- Maghsoudi, E.; Prevost, M.; Duy, S.V.; Sauve, S.; Dorner, S. Adsorption characteristics of multiple microcystins and cylindrospermopsin on sediment: Implications for toxin monitoring and drinking water treatment. Toxicon 2015, 103, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Akar, P.J.; Jirka, G.H. Lateral spreading with ambient current advection: Processus d’étalement en surface dans le transport et le mélange de polluants lere partie: Etalement lateral dans un écoulement ambiant. J. Hydraul. Res. 1994, 32, 815–831. [Google Scholar] [CrossRef]
- Hellberg, R.S.; Chu, E. Effects of climate change on the persistence and dispersal of foodborne bacterial pathogens in the outdoor environment: A review. Crit. Rev. Microbiol. 2016, 42, 548–572. [Google Scholar] [CrossRef] [PubMed]
- Hofstra, N. Quantifying the impact of climate change on enteric waterborne pathogen concentrations in surface water. Curr. Opin. Environ. Sustain. 2011, 3, 471–479. [Google Scholar] [CrossRef]
- Curriero, F.C.; Patz, J.A.; Rose, J.B.; Lele, S. The association between extreme precipitation and waterborne disease outbreaks in the United States, 1948–1994. Am. J. Public Health 2001, 91, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Patz, J.A.; Vavrus, S.J.; Uejio, C.K.; McLellan, S.L. Climate change and waterborne disease risk in the Great Lakes region of the US. Am. J. Prev. Med. 2008, 35, 451–458. [Google Scholar] [CrossRef]
- Mimura, N. Sea-level rise caused by climate change and its implications for society. Proc. Japan Acad. Ser. B-Phys. Biol. Sci. 2013, 89, 281–301. [Google Scholar] [CrossRef] [Green Version]
- Steets, B.M.; Holden, P.A. A mechanistic model of runoff-associated fecal coliform fate and transport through a coastal lagoon. Water Res. 2003, 37, 589–608. [Google Scholar] [CrossRef]
- Evanson, M.; Ambrose, R.F. Sources and growth dynamics of fecal indicator bacteria in a coastal wetland system and potential impacts to adjacent waters. Water Res. 2006, 40, 475–486. [Google Scholar] [CrossRef]
- Halliday, E.; Gast, R.J. Bacteria in beach sands: An emerging challenge in protecting coastal water quality and bather health. Environ. Sci. Technol. 2011, 45, 370–379. [Google Scholar] [CrossRef] [Green Version]
- Rippy, M.A.; Stein, R.; Sanders, B.F.; Davis, K.; McLaughlin, K.; Skinner, J.F.; Kappeler, J.; Grant, S.B. Small drains, big problems: The impact of dry weather runoff on shoreline water quality at enclosed beaches. Environ. Sci. Technol. 2014, 48, 14168–14177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edge, T.A.; Crowe, A.; Hill, S.; Seto, P.; Marsalek, J. Surveillance for Potential Sources of E. coli at Toronto’s Bluffer’s Park Beach 2005–2006; Environment Canada: Burlington, ON, Canada, 2007. [Google Scholar]
- Ackerman, D.; Weisberg, S. Relationship between rainfall and beach bacterial concentrations on Santa Monica Bay beaches. J. Water Health 2003, 1, 85–89. [Google Scholar] [CrossRef] [Green Version]
- McLellan, S.L.; Salmore, A.K. Evidence for localized bacterial loading as the cause of chronic beach closings in a freshwater marina. Water Res. 2003, 37, 2700–2708. [Google Scholar] [CrossRef]
- O’Dwyer, J.; Downes, M.M.; Adley, C.C. The impact of meteorology on the occurrence of waterborne outbreaks of vero cytotoxin-producing Escherichia coli (VTEC): A logistic regression approach. J. Water Health 2016, 14, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeij, W.; van der Wiele, J.; van Moorselaar, I.; van der Grinten, E. Impact of Climate Change on Water Quality in The Netherlands; National Institute for Public Health and Environment: Bilthoven, The Netherlands, 2010. [Google Scholar]
- Shah, A.H.; Abdelzaher, A.M.; Phillips, M.; Hernandez, R.; Solo-Gabriele, H.M.; Kish, J.; Scorzetti, G.; Fell, J.W.; Diaz, M.R.; Scott, T.M.; et al. Indicator microbes correlate with pathogenic bacteria, yeasts and helminthes in sand at a subtropical recreational beach site. J. Appl. Microbiol. 2011, 110, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Lemonte, J.J.; Stuckey, J.W.; Sanchez, J.Z.; Tappero, R.; Rinklebe, J.; Sparks, D.L. Sea Level Rise Induced Arsenic Release from Historically Contaminated Coastal Soils. Environ. Sci. Technol. 2017, 51, 5913–5922. [Google Scholar] [CrossRef]
- Powers, N.C.; Pinchback, J.; Flores, L.; Huang, Y.; Wetz, M.S.; Turner, J.W. Long-term water quality analysis reveals correlation between bacterial pollution and sea level rise in the northwestern Gulf of Mexico. Mar. Pollut. Bull. 2021, 166, 112231. [Google Scholar] [CrossRef]
- Phillips, M.C.; Solo-Gabriele, H.M.; Piggot, A.M.; Klaus, J.S.; Zhang, Y. Relationships between sand and water quality at recreational beaches. Water Res. 2011, 45, 6763–6769. [Google Scholar] [CrossRef] [Green Version]
- Piggot, A.M.; Klaus, J.S.; Johnson, S.; Phillips, M.C.; Solo-Gabriele, H.M. Relationship between enterococcal levels and sediment biofilms at recreational beaches in south Florida. Appl. Environ. Microbiol. 2012, 78, 5973–5982. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, T.D.; Nowosielski, K.; Cuvelier, M.; Hartz, A.; Green, M.; Esiobu, N.; McCorquodale, D.S.; Fleisher, J.M.; Rogerson, A. Prevalence and distribution of fecal indicator organisms in South Florida beach sand and preliminary assessment of health effects associated with beach sand exposure. Mar. Pollut. Bull. 2007, 54, 1472–1482. [Google Scholar] [CrossRef]
- Heaney, C.D.; Exum, N.G.; Dufour, A.P.; Brenner, K.P.; Haugland, R.A.; Chern, E.; Schwab, K.J.; Love, D.C.; Serre, M.L.; Noble, R.; et al. Water quality, weather and environmental factors associated with fecal indicator organism density in beach sand at two recreational marine beaches. Sci. Total Environ. 2014, 497, 440–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamahara, K.M.; Sassoubre, L.M.; Goodwin, K.D.; Boehm, A.B. Occurrence and persistence of bacterial pathogens and indicator organisms in beach sand along the California coast. Appl. Environ. Microbiol. 2012, 78, 1733–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, A.; Oliver, D.M.; Bivins, A.; Sherchan, S.P.; Pitkänen, T. Bathing water quality monitoring practices in europe and the United States. Int. J. Environ. Res. Public Health 2021, 18, 5513. [Google Scholar] [CrossRef] [PubMed]
- Lutz, C.; Erken, M.; Noorian, P.; Sun, S.; McDougald, D. Environmental reservoirs and mechanisms of persistence of Vibrio cholerae. Front. Microbiol. 2013, 4, 375. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, A.; Kauppinen, A.; Pitkänen, T. Decay of Enterococcus faecalis, vibrio cholerae and MS2 coliphage in a laboratory mesocosm under brackish beach conditions. Front. Public Health 2019, 7, 269. [Google Scholar] [CrossRef] [Green Version]
- Topić, N.; Cenov, A.; Jozić, S.; Glad, M.; Mance, D.; Lušić, D.; Kapetanović, D.; Mance, D.; Lušić, D.V. Staphylococcus aureus—An additional parameter of bathing water quality for crowded urban beaches. Int. J. Environ. Res. Public Health 2021, 18, 5234. [Google Scholar] [CrossRef]
- Liu, L.; Phanikumar, M.S.; Molloy, S.L.; Whitman, R.L.; Shively, D.A.; Nevers, M.B.; Schwab, D.J.; Rose, J.B. Modeling the transport and inactivation of E. coli and enterococci in the near-shore region of Lake Michigan. Environ. Sci. Technol. 2006, 40, 5022–5028. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines on Recreational Water Quality: Volume 1: Coastal and Fresh Waters; WHO: Geneva, Switzerland, 2021; Volume 1, ISBN 9789240031302. [Google Scholar]
- Moore, S.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health 2008, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Tryjanowski, P.; Sparks, T.H.; Kuźniak, S.; Czechowski, P.; Jerzak, L. Bird Migration Advances More Strongly in Urban Environments. PLoS ONE 2013, 8, e63482. [Google Scholar] [CrossRef]
- Conover, M.R. Population growth and movements of Canada geese in New Haven County, Connecticut, during a 25-year period. Waterbirds 2011, 34, 412–421. [Google Scholar] [CrossRef]
- Converse, R.R.; Kinzelman, J.L.; Sams, E.A.; Hudgens, E.; Dufour, A.P.; Ryu, H.; Santo-Domingo, J.W.; Kelty, C.A.; Shanks, O.C.; Siefring, S.D. Dramatic improvements in beach water quality following gull removal. Environ. Sci. Technol. 2012, 46, 10206–10213. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.; Grémillet, D.; Afán, I.; Miranda, F.; Bouten, W.; Forero, M.G.; Figuerola, J. Pathogen transmission risk by opportunistic gulls moving across human landscapes. Sci. Rep. 2019, 9, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hubálek, Z. Pathogenic microorganisms associated with gulls and terns (Laridae). J. Vertebr. Biol. 2021, 70. [Google Scholar] [CrossRef]
- Zeballos-Gross, D.; Rojas-Sereno, Z.; Salgado-Caxito, M.; Poeta, P.; Torres, C.; Benavides, J.A. The Role of Gulls as Reservoirs of Antibiotic Resistance in Aquatic Environments: A Scoping Review. Front. Microbiol. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kurittu, P.; Khakipoor, B.; Brouwer, M.S.M.; Heikinheimo, A. Plasmids conferring resistance to extended-spectrum beta-lactamases including a rare IncN+IncR multireplicon carrying blaCTX-M-1 in Escherichia coli recovered from migrating barnacle geese (Branta leucopsis). Open Res. Eur. 2021, 1, 46. [Google Scholar] [CrossRef]
- Fowler, A.M.; Hennessy, K.J. Potential impacts of global warming on the frequency and magnitude of heavy precipitation. Nat. Hazards 1995, 11, 283–303. [Google Scholar] [CrossRef]
- Mearns, L.O.; Giorgi, F.; McDaniel, L.; Shields, C. Analysis of daily variability of precipitation in a nested regional climate model-comparison with observations and doubled CO2 results. Glob. Planet. Change 1995, 10, 55–78. [Google Scholar] [CrossRef]
- Trenberth, K.E. Conceptual framework for changes of extremes of the hydrological cycle with climate change. Clim. Change 1999, 42, 327–339. [Google Scholar] [CrossRef]
- McBride, G.B.; Stott, R.; Miller, W.; Bambic, D.; Wuertz, S. Discharge-based QMRA for estimation of public health risks from exposure to stormwater-borne pathogens in recreational waters in the United States. Water Res. 2013, 47, 5282–5297. [Google Scholar] [CrossRef]
- Paule-Mercado, M.A.; Ventura, J.S.; Memon, S.A.; Jahng, D.; Kang, J.H.; Lee, C.H. Monitoring and predicting the fecal indicator bacteria concentrations from agricultural, mixed land use and urban stormwater runoff. Sci. Total Environ. 2016, 550, 1171–1181. [Google Scholar] [CrossRef]
- Selvakumar, A.; Borst, M. Variation of microorganism concentrations in urban stormwater runoff with land use and seasons. J. Water Health 2006, 4, 109–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, J.C.; Hamlet, A.F.; Lettenmaier, D.P. Implications of global climate change for snowmelt hydrology in the twenty-first century. Hydrol. Process. 2009, 23, 962–972. [Google Scholar] [CrossRef]
- Lo Iacono, G.; Armstrong, B.; Fleming, L.E.; Elson, R.; Kovats, S.; Vardoulakis, S.; Nichols, G.L. Challenges in developing methods for quantifying the effects of weather and climate on water-associated diseases: A systematic review. PLoS Negl. Trop. Dis. 2017, 11, 35. [Google Scholar] [CrossRef]
- Solo-Gabriele, H.M.; Harwood, V.J.; Kay, D.; Fujioka, R.S.; Sadowsky, M.J.; Whitman, R.L.; Wither, A.; Canica, M.; Da Fonseca, R.C.; Duarte, A.; et al. Beach sand and the potential for infectious disease transmission: Observations and recommendations. J. Mar. Biol. Assoc. UK 2016, 96, 101–120. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.R.; Ryu, H.D.; Hill, S.; Schoen, M.; Ashbolt, N.; Edge, T.A.; Domingo, J.S. Distribution and potential significance of a gull fecal marker in urban coastal and riverine areas of southern Ontario, Canada. Water Res. 2011, 45, 3960–3968. [Google Scholar] [CrossRef] [PubMed]
- Alm, E.W.; Daniels-Witt, Q.R.; Learman, D.R.; Ryu, H.; Jordan, D.W.; Gehring, T.M.; Santo Domingo, J. Potential for gulls to transport bacteria from human waste sites to beaches. Sci. Total Environ. 2018, 615, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Rytkönen, A.; Tiwari, A.; Hokajärvi, A.M.; Uusheimo, S.; Vepsäläinen, A.; Tulonen, T.; Pitkänen, T. The Use of Ribosomal RNA as a Microbial Source Tracking Target Highlights the Assay Host-Specificity Requirement in Water Quality Assessments. Front. Microbiol. 2021, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Neumann, B.; Vafeidis, A.T.; Zimmermann, J.; Nicholls, R.J. Future coastal population growth and exposure to sea-level rise and coastal flooding—A global assessment. PLoS ONE 2015, 10, e0131375. [Google Scholar] [CrossRef] [Green Version]
- DeFlorio-Barker, S.; Wing, C.; Jones, R.M.; Dorevitch, S. Estimate of incidence and cost of recreational waterborne illness on United States surface waters. Environ. Health 2018, 17, 3. [Google Scholar] [CrossRef] [Green Version]
- Burnham, J.P. Climate change and antibiotic resistance: A deadly combination. Ther. Adv. Infect. Dis. 2021, 8, 1–7. [Google Scholar] [CrossRef]
- Simpkins, G. The costs of infrastructure adaptation. Nat. Rev. Earth Environ. 2021, 2, 661. [Google Scholar] [CrossRef]
- Neumann, J.E.; Chinowsky, P.; Helman, J.; Black, M.; Fant, C.; Strzepek, K.; Martinich, J. Climate effects on US infrastructure: The economics of adaptation for rail, roads, and coastal development. Clim. Change 2021, 167, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Collier, S.A.; Deng, L.; Adam, E.A.; Benedict, K.M.; Beshearse, E.M.; Blackstock, A.J.; Bruce, B.B.; Derado, G.; Edens, C.; Fullerton, K.E.; et al. Estimate of Burden and Direct Healthcare Cost of Infectious Waterborne Disease in the United States. Emerg. Infect. Dis. 2021, 27, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.; Roser, M. Water Use and Stress 2017 Published Online at OurWorldInData.org. Available online: https://ourworldindata.org/water-use-stress (accessed on 10 November 2021).
- Mannocci, A.; La Torre, G.; Spagnoli, A.; Solimini, A.G.; Palazzo, C.; De Giusti, M. Is swimming in recreational water associated with the occurrence of respiratory illness? A systematic review and meta-analysis. J. Water Health 2016, 14, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Gerba, C.P.; Rose, J.B.; Haas, C.N. Sensitive populations: Who is at the greatest risk? Int. J. Food Microbiol. 1996, 30, 113–123. [Google Scholar] [CrossRef]
- Yamahara, K.M.; Layton, B.A.; Santoro, A.E.; Boehm, A.B. Beach sands along the California coast are diffuse sources of fecal bacteria to coastal waters. Environ. Sci. Technol. 2007, 41, 4515–4521. [Google Scholar] [CrossRef]
- Zampieri, B.D.B.; Oliviera, R.S.D.; Pinto, A.B.; Andrade, V.D.C.; Barbieri, E.; Chinellato, R.M.; Oliviera, A.J.F.C.D. Comparison of bacterial densities and resistance in different beach compartments: Should water by our main concern? O Mundo Saúde São Paulo 2017, 40A, 461–482. [Google Scholar] [CrossRef]
- Whitman, R.L.; Nevers, M.B. Foreshore sand as a source of Escherichia coli in nearshore water of a Lake Michigan beach. Appl. Environ. Microbiol. 2003, 69, 5555–5562. [Google Scholar] [CrossRef] [Green Version]
- Heaney, C.D.; Sams, E.; Wing, S.; Marshall, S.; Brenner, K.; Dufour, A.P.; Wade, T.J. Contact with beach sand among beachgoers and risk of illness. Am. J. Epidemiol. 2009, 170, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Heaney, C.D.; Sams, E.; Dufour, A.P.; Brenner, K.P.; Haugland, R.A.; Chern, E.; Wing, S.; Marshall, S.; Love, D.C.; Serre, M.; et al. Fecal Indicators in Sand, Sand Contact, and Risk of Enteric Illness Among Beachgoers. Epidemiology 2012, 23, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Sabino, R.; Rodrigues, R.; Costa, I.; Carneiro, C.; Cunha, M.; Duarte, A.; Faria, N.; Ferreira, F.C.; Gargate, M.J.; Julio, C.; et al. Routine screening of harmful microorganisms in beach sands: Implications to public health. Sci. Total Environ. 2014, 472, 1062–1069. [Google Scholar] [CrossRef]
- Graham, K.E.; Prussin, A.J.; Marr, L.C.; Sassoubre, L.M.; Boehm, A.B. Microbial community structure of sea spray aerosols at three California beaches. FEMS Microbiol. Ecol. 2018, 94, 10. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Bansal, C.; Kaintura, M. Sinonasal Mucormycosis: A to Z. Indian J. Otolaryngol. Head Neck Surg. Off. Publ. Assoc. Otolaryngol. India 2019, 71, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Kamali Sarvestani, H.; Seifi, A.; Falahatinejad, M.; Mahmoudi, S. Black aspergilli as causes of otomycosis in the era of molecular diagnostics, a mini-review. J. Mycologie Medicale. 2021, 32, 101240. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, A.E.; Field, K.G. Identification of nonpoint sources of fecal pollution in coastal waters by using host-specific 16S ribosomal DNA genetic markers from fecal anaerobes. Appl. Environ. Microbiol. 2000, 66, 1587–1594. [Google Scholar] [CrossRef] [Green Version]
- Harwood, V.J.; Staley, C.; Badgley, B.D.; Borges, K.; Korajkic, A. Microbial source tracking markers for detection of fecal contamination in environmental waters: Relationships between pathogens and human health outcomes. FEMS Microbiol. Rev. 2014, 38, 1–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valério, E.; Santos, M.L.; Teixeira, P.; Matias, R.; Mendonça, J.; Ahmed, W.; Brandão, J. Beach sand contaminated by dog walking—A molecular case study. Sci. Total Environ. (submitted).
- Haas, C.N.; Rose, J.B.; Gerba, C.P. Quantitative Microbial Risk Assessment; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Mylius, S.D.; Nauta, M.J.; Havelaar, A.H. Cross-contamination during food preparation: A mechanistic model applied to chicken-borne Campylobacter. Risk Anal. 2007, 27, 803–813. [Google Scholar] [CrossRef]
- Ashbolt, N.J. Risk analysis of drinking water microbial contamination versus disinfection by-products (DBPs). Toxicology 2004, 198, 255–262. [Google Scholar] [CrossRef]
- Schijven, J.F.; Teunis, P.P.M.; Rutjes, S.A.; Bouwknegt, M.; Husman, A.M.D. QMRAspot: A tool for quantitative microbial risk assessment from surface water to potable water. Water Res. 2011, 45, 5564–5576. [Google Scholar] [CrossRef]
- Juntunen, J.; Merilainen, P.; Simola, A. Public health and economic risk assessment of waterborne contaminants and pathogens in Finland. Sci. Total Environ. 2017, 599, 873–882. [Google Scholar] [CrossRef]
- Ashbolt, N.J.; Schoen, M.E.; Soller, J.A.; Rose, D.J. Predicting pathogen risks to aid beach management: The real value of quantitative microbial risk assessment (QMRA). Water Res. 2010, 44, 4692–4703. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Solo-Gabriele, H.M. Quantitative microbial risk assessment of human illness from exposure to marine beach sand. Environ. Sci. Technol. 2012, 46, 2799–2805. [Google Scholar] [CrossRef] [PubMed]
- Schoen, M.E.; Ashbolt, N.J. Assessing pathogen risk to swimmers at non-sewage impacted recreational beaches. Environ. Sci. Technol. 2010, 44, 2286–2291. [Google Scholar] [CrossRef]
- Jang, C.S.; Liang, C.P. Characterizing health risks associated with recreational swimming at Taiwanese beaches by using quantitative microbial risk assessment. Water Sci. Technol. 2018, 77, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Brandão, J.; Solo-Gabriele, H.M.; Gordon, B.; Ferguson, A.C. Recreational environment-pathogenic fungi in public places, information gaps in assessing public health risk. In Environmental Mycology in Public Health; Viegas, C., Pinheiro, C., Verissimo, C., Sabino, R., Brandão, J., Viegas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Sterk, A.; Schijven, J.; de Nijs, T.; Husman, A.M.D. Direct and indirect effects of climate change on the risk of infection by water-transmitted pathogens. Environ. Sci. Technol. 2013, 47, 12648–12660. [Google Scholar] [CrossRef] [PubMed]
- Soller, J.A.; Schoen, M.E.; Bartrand, T.; Ravenscroft, J.E.; Ashbolt, N.J. Estimated human health risks from exposure to recreational waters impacted by human and non-human sources of faecal contamination. Water Res. 2010, 44, 4674–4691. [Google Scholar] [CrossRef]
- Soller, J.A.; Eftim, S.; Wade, T.J.; Ichida, A.M.; Clancy, J.L.; Johnson, T.B.; Schwab, K.; Ramirez-Toro, G.; Nappier, S.; Ravenscroft, J.E. Use of quantitative microbial risk assessment to improve interpretation of a recreational water epidemiological study. Microb. Risk Anal. 2016, 1, 2–11. [Google Scholar] [CrossRef]
- Zhang, Q.; Gallard, J.; Wu, B.; Harwood, V.J.; Sadowsky, M.J.; Hamilton, K.A.; Ahmed, W. Synergy between quantitative microbial source tracking (qMST) and quantitative microbial risk assessment (QMRA): A review and prospectus. Environ Int. 2019, 130, 104703. [Google Scholar] [CrossRef]
- Weiskerger, C.J.; Brandão, J. Fungal contaminants in water and sand: A new frontier for quantitative microbial risk assessment. Curr. Opin. Environ. Sci. Health 2020, 16, 73–81. [Google Scholar] [CrossRef]
- Auld, H.; MacIver, D.; Klaassen, J. Heavy rainfall and waterborne disease outbreaks: The Walkerton example. J. Toxicol. Environ. Health A-Curr. Issues 2004, 67, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Cann, K.F.; Thomas, D.R.; Salmon, R.L.; Wyn-Jones, A.P.; Kay, D. Extreme water-related weather events and waterborne disease. Epidemiol. Infect. 2013, 141, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Herrador, B.R.G.; de Blasio, B.F.; MacDonald, E.; Nichols, G.; Sudre, B.; Vold, L.; Semenza, J.C.; Nygard, K. Analytical studies assessing the association between extreme precipitation or temperature and drinking water-related waterborne infections: A review. Environ. Health 2015, 14, 12. [Google Scholar] [CrossRef] [Green Version]
- Levy, K.; Woster, A.P.; Goldstein, R.S.; Carlton, E.J. Untangling the impacts of climate change on waterborne diseases: A systematic review of relationships between diarrheal diseases and temperature, rainfall, flooding, and drought. Environ. Sci. Technol. 2016, 50, 4905–4922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, M.K.; Charron, D.F.; Waltner-Toews, D.; Schuster, C.; Maarouf, A.R.; Holt, J.D. A role of high impact weather events in waterborne disease outbreaks in Canada, 1975-2001. Int. J. Environ. Health Res. 2006, 16, 167–180. [Google Scholar] [CrossRef]
- Floury, M.; Usseglio-Polatera, P.; Ferreol, M.; Delattre, C.; Souchon, Y. Global climate change in large European rivers: Long-term effects on macroinvertebrate communities and potential local confounding factors. Glob. Chang. Biol. 2013, 19, 1085–1099. [Google Scholar] [CrossRef]
- Groffman, P.M.; Rustad, L.E.; Templer, P.H.; Campbell, J.L.; Christenson, L.M.; Lany, N.K.; Socci, A.M.; Vadeboncoeur, M.A.; Schaberg, P.G.; Wilson, G.F.; et al. Long-term integrated studies show complex and surprising effects of climate change in the northern hardwood forest. Bioscience 2012, 62, 1056–1066. [Google Scholar] [CrossRef] [Green Version]
- Foley, B.; Jones, I.D.; Maberly, S.C.; Rippey, B. Long-term changes in oxygen depletion in a small temperate lake: Effects of climate change and eutrophication. Freshw. Biol. 2012, 57, 278–289. [Google Scholar] [CrossRef]
- USEPA. Environmental Monitoring in the Everglades, Everglades REMAP Web Page. 1993–2014 (2021). Available online: https://www.epa.gov/everglades/environmental-monitoring-everglades (accessed on 12 November 2021).
- Bezirtzoglou, C.; Dekas, K.; Charvalos, E. Climate changes, environment and infection: Facts, scenarios and growing awareness from the public health community within Europe. Anaerobe 2011, 17, 337–340. [Google Scholar] [CrossRef]
- Wu, B.; Wang, C.; Zhang, C.; Sadowsky, M.J.; Dzakpasu, M.; Wang, X.C. Source-Associated Gastroenteritis Risk from Swimming Exposure to Aging Fecal Pathogens. Environ. Sci. Technol. 2020, 54, 921–929. [Google Scholar] [CrossRef]
| RCP Emissions Scenario | Range of Projected Sea Level Rise (m) |
|---|---|
| RCP2.6 | 0.26–0.55 |
| RCP4.5 | 0.32–0.63 |
| RCP6.0 | 0.33–0.63 |
| RCP8.5 | 0.45–0.82 |
| Hydrometeorological Variable | Projected Change in Variable | Impacts on Beach Microbes and Public Health | References |
|---|---|---|---|
| Air Temperature | Increased Air Temperature | Direct Effects: | [26,27,28] |
| |||
| Indirect Effects: | [29,30] | ||
| |||
| Water Temperature | Increased Water Temperature | Direct Effects: | [5,6,7,31,32,33,34,35,36,37] |
| |||
| [36,38,39,40,41,42,43,44] | ||
| [19,22,31,32,45] | ||
| Indirect Effects: | [46] | ||
| |||
| Precipitation | Increased Frequency and Intensity of Storm Events | Direct Effects: | [47,48,49,50,51] |
| |||
| [52,53,54] | ||
| [47,55,56] | ||
| Indirect Effects: | [20,49,57] | ||
| |||
| [50,58] | ||
| Increased Drought Conditions | Direct Effects: | [59,60] | |
| |||
| [15,61] | ||
| Sea Level | Sea Level Rise | Direct Effects: | [62,63] |
| |||
| Indirect Effects: | [64] | ||
| |||
| [12,65] | ||
| Waves | Increased Wave Activity | Direct Effects: | [54,66,67] |
| |||
| [54,68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandão, J.; Weiskerger, C.; Valério, E.; Pitkänen, T.; Meriläinen, P.; Avolio, L.; Heaney, C.D.; Sadowsky, M.J. Climate Change Impacts on Microbiota in Beach Sand and Water: Looking Ahead. Int. J. Environ. Res. Public Health 2022, 19, 1444. https://doi.org/10.3390/ijerph19031444
Brandão J, Weiskerger C, Valério E, Pitkänen T, Meriläinen P, Avolio L, Heaney CD, Sadowsky MJ. Climate Change Impacts on Microbiota in Beach Sand and Water: Looking Ahead. International Journal of Environmental Research and Public Health. 2022; 19(3):1444. https://doi.org/10.3390/ijerph19031444
Chicago/Turabian StyleBrandão, João, Chelsea Weiskerger, Elisabete Valério, Tarja Pitkänen, Päivi Meriläinen, Lindsay Avolio, Christopher D. Heaney, and Michael J. Sadowsky. 2022. "Climate Change Impacts on Microbiota in Beach Sand and Water: Looking Ahead" International Journal of Environmental Research and Public Health 19, no. 3: 1444. https://doi.org/10.3390/ijerph19031444
APA StyleBrandão, J., Weiskerger, C., Valério, E., Pitkänen, T., Meriläinen, P., Avolio, L., Heaney, C. D., & Sadowsky, M. J. (2022). Climate Change Impacts on Microbiota in Beach Sand and Water: Looking Ahead. International Journal of Environmental Research and Public Health, 19(3), 1444. https://doi.org/10.3390/ijerph19031444







