1. Introduction
The total population of older adults in the European Union (EU), defined as those aged 65 years or older, is projected to increase significantly and will reach 129.8 million by 2050. During this transition, the number of people in the EU aged 75–84 years is projected to expand by 56.1%, while the number aged 65–74 years is expected to increase by 16.6% [
1]. This complex process is caused by low fertility rates, increased life expectancy, and, in some cases, migratory patterns. This transition to an aged demographic pyramid in the coming decades will represent a challenge that will have to be managed in every country to provide health and social coverage to many older adults with multimorbidity, due to an increase of chronic conditions associated with age [
2,
3].
Lower urinary tract symptoms (LUTS) are a wide variety of symptoms that indicate poor bladder health. These symptoms can be divided into three groups: (a) storage symptoms, experienced during the storage phase of the bladder cycle and include daytime frequency, urinary incontinence (UI) and nocturia; (b) voiding symptoms experienced during the voiding phase including slow stream, hesitancy, and straining to start micturition; and (c) post-micturition symptoms experienced immediately after voiding, including incomplete emptying and post-micturition dribbling [
4]. LUTS are highly prevalent world-wide but induce low levels of medical consultation [
5,
6,
7,
8]. UI is twice as prevalent in women than men, due largely to the impact of pregnancy, childbirth, and menopause, and their possible effects on pelvic organs and pelvic floor muscles [
5,
6,
7,
8,
9]. LUTS and UI can happen at any age, but they are more common in older age. The prevalence is expected to increase with expected increases in mean life expectancy over the next decades, together with economic and psychosocial impacts on health care systems [
2,
8,
9].
UI is the objectively demonstrable involuntary loss of urine that increases the subject’s frailty, their physical inactivity, their risk of falls, and their immobility; decreases their functional independence; and has negative physiological effects due to hygienic problems [
4,
9,
10,
11,
12,
13,
14,
15,
16,
17]. Also, UI greatly impacts on psychological health, severely affects normal social interaction and leisure activities, increases the risk of self-imposed isolation and cognitive impairment, and decreases satisfaction and quality of life (QoL) [
10,
18,
19,
20,
21,
22]. There are five types of UI: (a) stress UI is the involuntary leakage of urine that occurs with increases in intra-abdominal pressure (e.g., with exertion, effort, sneezing, or coughing); (b) urgency UI is the involuntary leakage of urine that may be preceded or accompanied by a sense of urinary urgency; (c) mixed UI is the involuntary leakage of urine caused by a combination of stress and urgency UI; (d) overflow UI is the involuntary leakage of urine from an overdistended bladder; and functional UI is the involuntary leakage of urine due to environmental, cognitive, or physical barriers to toileting [
23,
24]. In the nursing home (NH) population, the functional UI type stands out, caused by the inability to move to the bathroom independently, whether due to a physical or cognitive problems such as dementia [
17].
In the NH population, UI is strongly associated with cognitive decline, inactivity, immobility, and impairment in activities of daily living performance that could lead to a decrease in physical activity, and an increase of sedentary behavior (SB) [
20,
21,
25]. SB is considered a risk factor for cardiovascular disease, metabolic disease, obesity, frailty, disability, psychological disorders, and mortality decline [
26,
27,
28,
29,
30,
31,
32,
33]. Concerning the relationship between SB and pelvic health, a previous study found an association between urgency UI and the average duration of SB bouts, and another study found that low levels of physical activity were associated with greater nocturia and nocturnal enuresis; both studies were carried out on community-dwelling older women [
34,
35]. In addition, many authors suggest that low levels of physical activity and prolonged patterns of SB could be direct risk factors for UI in older adults [
33,
35,
36,
37,
38].
To our knowledge there is no evidence on the association between SB and UI in NH residents. Consequently, new research is needed to analyse this relationship, with the aim of developing strategies to approach UI to improve resident’s health and QoL and reduce the UI burden on social and health services. The main objective of the study is to determine the prevalence of UI and its associated factors in a cohort of NH residents. Also, we aimed to verify the prevalence of the different types of LUTS and UI, as well as their impact on residents’ QoL.
3. Results
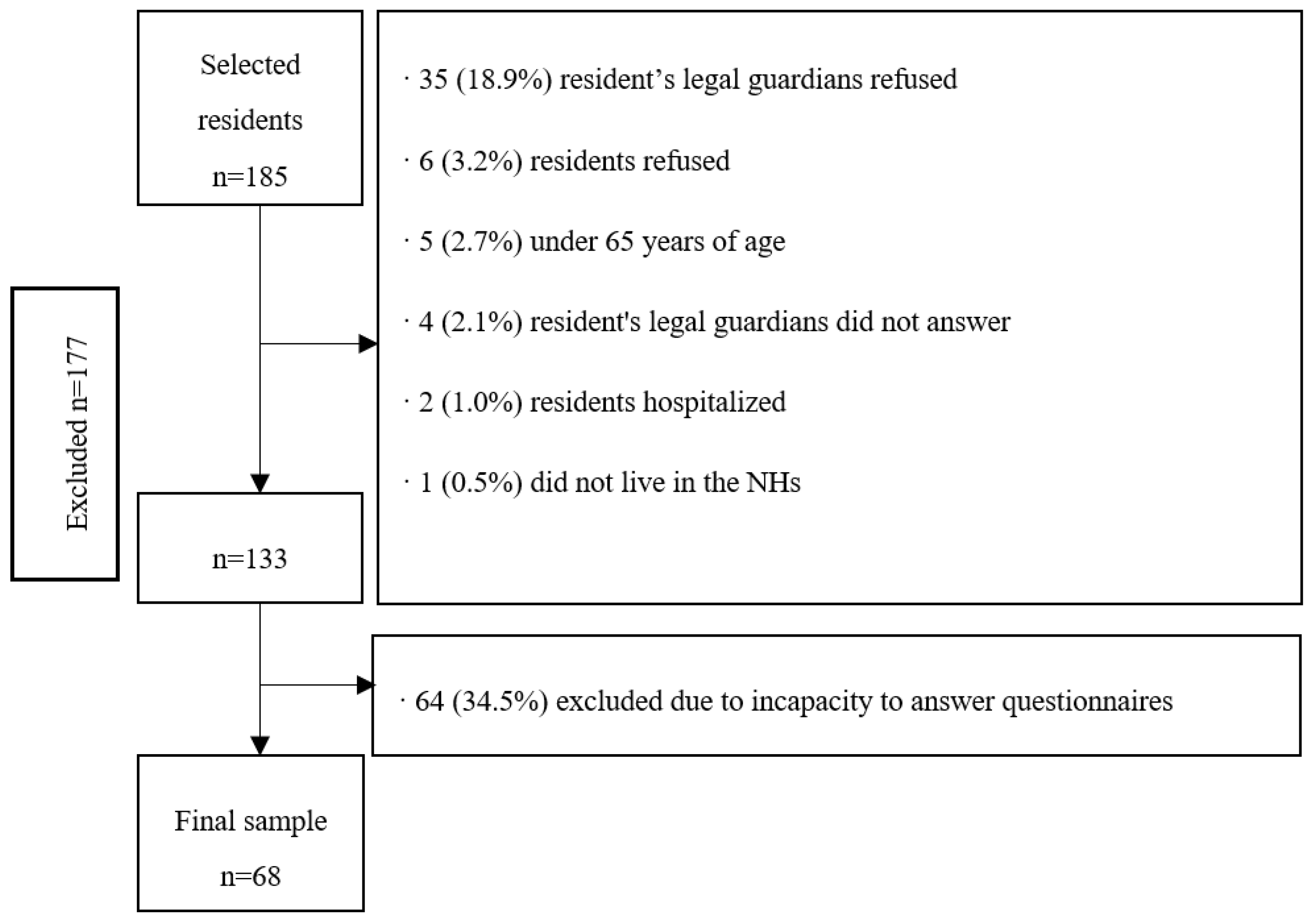
The final sample consisted of 68 residents with a mean age of 83.6 (SD = 7.6) years, mostly women (80.9%), and with an average duration living in the NHs of 29.1 (SD 29.0) months. Fifteen people (22.0%) lived in a private NH and 53 (78.0%) in a subsidized NH. From the 68 residents, 66 (97.0%) had descendants, with a mean number of 1.6 (SD 1.5) descendants, and of the 55 women, 47 (69.1%) had given birth.
Figure 1 shows the flow chart of the excluded participants of the study.
Regarding the presence of diagnosed medical conditions, all 68 (100.0%) residents were diagnosed with at least one chronic condition, with a mean number of 5.1 (SD 2.3) diagnosed medical conditions. More specifically: 45 (66.2%) presented arterial hypertension, 30 (44.1%) cardiac disease, 29 (42.6%) dementia, 23 (33.8%) diabetes, 23 (33.8%) dyslipidaemia, 22 (32.4%) kidney failure, 20 (29.4%) diagnosed depression, 14 (20.6%) lung disease, 12 (17.6%) mental disease, 12 (17.6%) Parkinson’s disease, 12 (17.6%) stroke, 12 (17.6%) circulatory disease, 11 (16.2%) digestive disease, 11 (16.2%) hypothyroidism, 10 (14.7%) cancer, 10 (14.7%) diagnosed osteoporosis, 7 (10.3%) anaemia, 7 (10.3%) arthrosis, 6 (8.8%) vertigo, 5 (7.4%) diagnosed anxiety, 5 (7.4%) chronic pain, 5 (7.4%) visual deficit, 3 (4.4%) epilepsy, 3 (3.4%) hiatal hernia, 3 (4.4%) low blood pressure, 2 (2.9%) hyperparathyroidism, 1 (1.5%) hyperthyroidism, 1 (1.5%) ataxia, and 1 (1.5%) sleep disorders. Concerning pelvic health, three (4.4%) individuals had prostatic hyperplasia and two (2.9%) diagnosed vaginal prolapse.
It was found that 48 (70.5%) residents had cognitive decline, 62 (91.1%) presented functional dependency, 62 (91.1%) decreased functional capacity and frailty, 50 (73.5%) had risk of sarcopenia, 3 (48.5%) had risk of malnutrition, 58 (85.2%) residents presented depressive symptomatology, and 46 (67.6%) presented loneliness. All residents were using medication, with an average of 8.3 (SD 10.4) medications per day, 52 (76.5%) residents were taking five medications or more and 42 (61.8%) were at risk of anticholinergic adverse effects. It was identified that 30 (44.1%) residents had fallen at least one time in the last year, 17 (25.0%) had episodes of delirium, 9 (13.2%) had lost weight in the last 12 months, 8 (11.8%) had skin lesions, and 2 (2.9%) had leg ulcers. Also, in the last 12 months, five (7.4%) were hospitalized and four (5.9%) had a bone fracture.
Table 1 shows socio-demographic and health-related information.
Concerning SB and the waking time movement behaviour (WTMB) of the residents, their average upright time was 2.2 (SD 1.8) hours, sitting time was 8.8 (SD 1.6) hours, and the average duration of SB bouts was 44.6 (SD 50.9) minutes. Also, 43 (63.2%) residents followed a physical exercise program led by NH staff at least once per week. Most of the residents who participated in the physical exercise programs had UI.
Table 2 shows more information about SB and WTMB.
Regarding pelvic health, 2 (2.9%) residents had a permanent catheter, 4 (5.9%) residents had experienced urinary tract infection in the previous 30 days, 60 (88.2%) residents took medication that decreased their micturition, 54 (79.4%) residents took medications that produce an increase in micturition, and 21 (30.9%) took laxatives. The total average fluid consumption was 1864.1 (SD 804.7) millilitres (mL): 12.19 (SD 56.61) mL of cold drinks with caffeine, 228.13 (SD 316.83) mL of hot drinks with caffeine, 1396.48 (SD 633.73) mL of non-caffeine cold drinks, 121.70 (SD 206.93) mL of non-caffeine hot drinks, and 2.34 (SD 13.88) mL of alcohol. Nocturia (1+ waking at night to urinate) was reported in 21 (30.8%) residents by the proxy and 31 (45.5%) residents self-reported nocturia with the IPSS questionnaire.
According to the MDS, 44 residents presented with UI, i.e., prevalence of 66.1% (CI: 95%, 53.6–77.2). Among these, 33 (48.5%) had UI for more than a year, 22 (32.4%) had urinary leakage day and night, and 31 (45.6%) had large amounts of urinary leakage. From the 26 (38.2%) that were following a toileting program, only 8.8% totally improved their continence with the program. Besides, 12 residents had FI, i.e., prevalence of 17.6% (CI: 95%, 10.3–28.3). From these 12 residents, 11 (16.1%) presented double incontinence. Twenty-three residents (33.8%) followed a toileting program, 12 (17.6%) had constipation, and 5 (7.4%) diarrhoea. The average of faecal evacuations was 1.3 (SD 0.8) per day. In the total sample, the average number of diapers per resident was 2.2 (SD 2.0) per day, and within the incontinent group, 3.2 (SD 1.8).
Table 3 shows UI and FI-related variables, according to the MDS.
According to the ICIQ-SF, 35 (51.4%) residents self-reported UI at least once a week. Among these, 17 (25.0%) before reaching a toilet, 19 (27.9%) when he/she coughs/sneezes, 23 (33.8%) whilst asleep, 13 (19.1%) during physical activity/exercise, 10 (14.7%) after urination while already dressed, 7 (10.2%) for no obvious reason, and 6 (8.8%) all the time. Regarding the frequency of urinary leakage, 11 (16.1%) subjects had leakages at least one time per week and 24 (35.2%) had daily losses. The urine leakage was low in 13 (19.1%) subjects, moderate in 11 (16.1%), and large in 12 (17.6%) residents. According to the MDS questionnaire, 7 (10.2%) incontinent residents reported no urine leakage or refused to answer. The impact of UI on QoL was low (score 0–3) in 22 (56.4%) subjects, moderate (4–6) in 8 (20.5%), and high (7–10) in 9 (23.0%) residents.
In the information gathered from the IPSS, bladder symptoms reported at least once in the last 30 days were: urgency: 37 (54.4%), increased frequency: 36 (52.9%), incomplete emptying: 20 (29.4%), intermittency: 18 (26.4%), weak stream: 16 (23.5%), and straining: 15 (22.0%). For the QoL associated with prostatic symptoms, 10 (14.7%) were delighted, 7 (10.3%) pleased, 16 (23.5%) mostly satisfied, 10 (14.7%) mixed, 12 (17.6%) not satisfied, 6 (8.8%) unhappy, and 4 (5.9%) terrible.
Table 4 shows the classification of bladder symptoms and UI according to the ICIQ-SF and IPSS.
Table 5 shows the results of the bivariate analysis, and
Table 6 and
Table 7 show the group comparisons of the quantitative variables grouped by the dependent variable, and the presence of UI (yes/no) according to the proxy respondent, by Section H item 3a of the MDS questionnaire, with a
p value equal to or under 0.200. The two groups were: continent (
n = 23, mean age 83.00, SD = 7.7) and incontinent group (
n = 45, mean age 84.04, SD = 7.7). The variables that were significantly associated with UI were anxiety, physical performance, cognitive status, frailty, and FI.
Regarding the comparison between the continent and the incontinent groups, the differences between groups were statistically significant in the variables of QoL, ADL limitations, mobility, handgrip strength, and all the SB and WTMB variables (see
Table 6 and
Table 7).
Table 6.
Association between urinary incontinence (UI) and parametric variables with the independent samples t-test among 68 residents living in five nursing homes (NHs) from Osona, Spain (2020).
Table 6.
Association between urinary incontinence (UI) and parametric variables with the independent samples t-test among 68 residents living in five nursing homes (NHs) from Osona, Spain (2020).
| UI |
|---|
| | Yes | No | | | |
|---|
| | Mean | SD | Mean | SD | Mean Difference | t | p |
|---|
| Waking duration (h) | 10.76 | 1.55 | 11.90 | 0.71 | 1.13 | 3.58 | <0.001 * |
| Absolute time sitting in events <30 min | 1.72 | 1.55 | 2.57 | 1.14 | 0.85 | 2.01 | 0.050 |
| % time sitting in events <30 min | 14.09 | 10.93 | 20.60 | 7.89 | 7.35 | 2.03 | 0.048 * |
| % time sitting in events between 30–60 min | 14.09 | 10.93 | 20.60 | 7.89 | 6.50 | 2.42 | 0.019 * |
| Number of events between 30–60 min bouts | 2.21 | 1.88 | 3.58 | 1.35 | 1.37 | 2.97 | 0.005 * |
| Number of events between 30–60 min bouts per hour | 0.20 | 0.15 | 0.29 | 0.10 | 0.08 | 2.09 | 0.041 * |
| Absolute time spent in events >60 min | 5.67 | 3.34 | 3.62 | 2.21 | −2.04 | −2.60 | 0.012 * |
| Right hand Handgrip | 12.56 | 6.18 | 18.67 | 8.16 | 6.10 | 2.77 | 0.005 * |
| Left hand Handgrip | 11.21 | 5.11 | 18.12 | 7.16 | 6.91 | 3.61 | 0.001 * |
| Dominant hand Handgrip | 12.57 | 6.03 | 18.35 | 8.13 | 5.77 | 2.98 | 0.014 * |
Table 7.
Association between urinary incontinence (UI) and non-parametric variables through the Mann–Whitney U test among 68 residents living in five nursing homes (NHs) from Osona, Spain (2020).
Table 7.
Association between urinary incontinence (UI) and non-parametric variables through the Mann–Whitney U test among 68 residents living in five nursing homes (NHs) from Osona, Spain (2020).
| UI |
|---|
| | Yes | No | | |
|---|
| | Mean Rank | Sum of Ranks | Mean Rank | Sum of Ranks | U | p |
|---|
| Spanish Index EuroQoL 5D-5L | 29.55 | 1300.00 | 42.52 | 978.00 | 310.00 | 0.010 * |
| Barthel | 28.21 | 1269.50 | 46.80 | 1076.50 | 234.50 | <0.001 * |
| Rivermead Mobility Index | 27.64 | 1244.00 | 47.91 | 1102.00 | 209.00 | <0.001 * |
| Absolute time spent walking (h) | 22.29 | 758.00 | 33.41 | 568.00 | 163.00 | 0.012 * |
| % of waking time walking | 22.29 | 758.00 | 33.41 | 568.00 | 163.00 | 0.012 * |
| Absolute time spent standing (h) | 22.26 | 757.00 | 33.47 | 569.00 | 162.00 | 0.011 * |
| % of waking time standing | 22.00 | 748.00 | 34.00 | 578.00 | 153.00 | 0.007 * |
| Absolute time spent upright (h) | 22.18 | 754.00 | 33.65 | 572.00 | 159.00 | 0.009 * |
| % of waking time upright | 22.06 | 750.00 | 33.88 | 576.00 | 155.00 | 0.007 * |
| Sit to stand transitions | 22.76 | 774.00 | 32.47 | 552.00 | 179.00 | 0.028 * |
| Sit to stand transitions per hour awake | 3.09 | 785.00 | 31.82 | 514.00 | 190.00 | 0.048 * |
| % of waking time sitting | 29.94 | 1018.00 | 18.12 | 308.00 | 155.00 | 0.007 * |
| Number of <30 min bouts | 23.04 | 783.00 | 31.91 | 542.00 | 188.50 | 0.045 * |
| Number of <30 min bouts per hour | 23.00 | 782.00 | 32.00 | 544.00 | 187.00 | 0.042 * |
| Absolute time sitting in events between 30–60 min | 22.09 | 751.00 | 33.82 | 575.00 | 156.00 | 0.008 * |
| % of time sitting in events >60 min | 29.00 | 986.00 | 20.00 | 340.00 | 187.00 | 0.042 * |
| Average duration of sedentary behaviour bouts in min | 28.91 | 983.00 | 20.18 | 343.00 | 190.00 | 0.048 * |
4. Discussion
This study aimed to verify the prevalence of UI and its associated factors in a sample of NH residents from Osona (Spain), as well as report information on other pelvic health issues such as FI and bladder symptoms. The findings indicate that the prevalence of UI was high, approximately 66%. Physical health issues (physical performance, frailty, FI, ADL limitations, mobility, SB and dominant hand handgrip strength, and psycho-cognitive issues (anxiety, cognitive state, and quality of life) were significantly associated with UI.
When comparing other studies in NH residents with capacity to answer questionnaires, the prevalence of UI is higher than the one found by Jerez-Roig et al. (2015) among Brazilian NH residents, with a prevalence of 42% [
65], but slightly lower than the Jachan et al. (2019) study, with a prevalence of 70% in German NH residents [
13]. In these studies, the MDS was used to assess the UI, except Jachan et al. who used the ICIQ-SF. Regarding other information on pelvic health given by the MDS, we found a prevalence of FI of 35% and 30% for double incontinence (faecal and urinary). An existing systematic review found a median prevalence for FI of 42.8% and 65% for double incontinence [
66]. Our prevalence results are lower for double incontinence, however, regarding FI, the results are between the ranges they found.
Regarding LUTS, the most prevalent ones were storage symptoms, i.e., urgency, increased frequency, and nocturia, followed by voiding symptoms, intermittency, straining, and weak stream; post micturition symptoms [incomplete emptying], more common in men, were less frequent. In line with our results, a study conducted in community-dwelling older adults from Korea also found that storage symptoms were more prevalent than voiding symptoms and much more prevalent than post-micturition symptoms [
8]. For nocturia, residents tended to self-report its presence more often with the IPSS than did the NH staff using the MDS. These findings might be partially explained due to NH staff not recording residents’ voiding patterns and residents not having a voiding diary routine. Since every resident’s room has its own private bathroom, particularly for the residents with preserved autonomy, the NH staff might not be aware of who is getting up at night to urinate. This is a possible hypothesis that could explain why the residents reported higher nocturia than the NH staff [
67,
68].
The prevalence of the different types of UI differs between the self-reported IPSS and the NH staff report. According to the self-reported ICIQ-SF, the most prevalent type of UI was nocturnal, followed by stress, urgency, dribbling post-micturition, and indeterminate and continuous UI. Nevertheless, NH staff reported that the most prevalent type of UI was urgency, followed by UI due to cognitive decline; functional, indeterminate UI; stress; and finally, effort UI. These differences between self-reported and NH staff answers could partially be explained by the difficulties of the NH staff in classifying the UI types due to a lack of knowledge on pelvic health disorders. It may also be due to the culture of secrecy and profound sense of shame felt by those that suffer from UI, which makes it very difficult to talk about and seek help from NH staff or health professionals, because residents may feel uncomfortable, embarrassed, or ashamed, as previous studies have reported [
69,
70,
71,
72,
73]. This sense of shame and secrecy from people with UI is well reported in previous studies and profoundly affects their QoL in the domains of dignity, autonomy, and mood [
19,
71,
74].
Incontinent residents had worse self-reported QoL than continent residents. UI can occur rapidly and in large volumes, which severely affects normal social participation among affected people [
18]. It also increases the risk of isolation and decreases satisfaction with life. As a result, a decrease in QoL and functional independence of the resident can be observed, which may lead to greater frailty. There are different risk factors that could cause this decline in QoL, including sex, age, dementia and mobility, and the embarrassment of leaking urine and being wet can make residents feel a loss of personal dignity [
74]. DuBeau et al. (2007) also showed that frail, functionally, and cognitively disabled residents with UI experienced a decrease in QoL [
22].
Our study results also show that approximately three quarters of the sample who self-reported UI answered that UI had a low-moderate impact on their QoL, a lower proportion compared to previous studies. A review in Brazilian NH residents found that the impact of UI in QoL was higher than in our study, with approximately 57% reporting low-moderate impact [
75]. Similarly, Jachan et al. (2019) found that in 51% of German NH residents, incontinence had a low-moderate impact on their QoL, according to ICIQ-SF [
13].
Regarding LUTS and their impact on QoL, our results showed that most residents have good QoL. To our knowledge, there is no evidence of the impact of LUTS on QoL in NH residents. The evidence in community-dwelling older adults shows that prostatic symptoms and their severity increases with age, and also their higher frequency of poor QoL [
76,
77,
78]. However, our results did not coincide with these previous statements, although they were in line with the results of the studies done by Adegun et al. (2016) and Ojewola et al. (2016) who found that even mild symptomatology could be associated with poor QoL, whereas some severe symptomatology could be associated with good QoL [
77,
78].
Regarding SB and WTMB, the incontinent group showed statistically significant differences, with lower periods of time spent standing, walking, and in an upright position; lower duration of time spent sitting in bouts of <30 min and bouts between 30 and 60 min; as well as higher time spent sitting in bouts of >60 min, than the continent group. Regarding SB, our results showed that all residents spent 80% of their waking time sitting or lying. Our results were slightly lower compared with Reid et al. (2013) in Australian NH residents, where they found an average of 85% of waking hours sitting or lying, or much lower than Chan et al. (2016) results in Canadian NH residents, where they found 95.1% of waking time spent sedentary. In all the studies, the gold standard ActivPAL monitor was used over 7 consecutive days, except Chan et al. who only used the device over 3 days [
79,
80]. Researchers still do not identify a threshold for how many consecutive minutes of sitting are needed before health risks are increased, but previous evidence suggests that sitting for as little as 20 consecutive minutes affects cardiometabolic health [
81]. In line with the evidence of our study results, previous studies found statistical significance between UI and almost 20% longer duration of sedentary bouts in community-dwelling older women [
34,
39]. These findings could reinforce the hypothesis that the main risks of SB are the duration of the sitting bouts rather than the total time spent sitting. Finally, evidence suggests that SB is a risk factor for UI in older adults. Hence, future interventions to increase physical activity and break long SB bouts could be beneficial in preventing these symptoms [
15,
33,
82].
Regarding factors associated with UI, our results show that incontinent residents had greater decline in their physical health and in their psycho-cognitive health than the continent individuals. On psycho-cognitive health, UI as a geriatric syndrome, has been strongly associated with cognitive decline and higher anxiety and depression levels in previous studies [
18,
22]. Regarding physical health, researchers over the years have found a strong association between UI and ADL limitations, frailty, physical performance, and overall physical condition loss [
12,
14,
21,
25,
65,
83,
84]. However, there is no research done in NH populations to explain the results between low handgrip strength in incontinent residents. However, Bag Soytas et al. (2021) found a positive correlation between low handgrip strength and quantitatively measured weak pelvic floor muscle strength in adult women, a characteristic related to multiple causes in the pathophysiology of UI in women [
85,
86]. This could be one of multiple possible explanations, however, more research is needed to explain it in different populations and in both sexes. In addition, fluid intake, mobility, and diuretic treatment may also influence diuresis and, therefore, UI. In our study sample, neither medication nor total fluid intake were found to be significantly associated with UI. It could be interesting for further research to examine effects of these variables on UI in a longitudinal study [
19].
The main limitation of this study was the small sample size, due to the COVID-19 outbreak that interrupted the data collection in NHs in March 2020 and reduced the number of participants initially estimated for the project. Consequently, a multivariate analysis of the significant variables could not be performed. In addition, since individuals without capacity to answer questionnaires were excluded from our study sample, the results cannot be generalized to residents with cognitive decline. Despite this, our sample size has sufficient power to analyze the association between UI and the average duration of sedentary bouts, since the differences between groups were high. Studies with a larger sample size, including confounders in multivariate analysis, are required to specifically analyze the role of SB in pelvic health. Also, the cross-sectional design of this study prevents establishing any cause–effect relationships between the variables; longitudinal designs are necessary to analyze these pathways. On the other hand, this study is the first that investigated the association between UI and SB in NH residents (considering both sexes), using the gold standard device ActivPAL to accurately measure SB. To our knowledge, this is also the first study describing LUTS and analyzing the association between UI and handgrip strength in institutionalized older adults. These results may be useful for the creation of new strategies to prevent UI and the SB effects on the NH population.