Froth-Flotation Separation as an Alternative for the Treatment of Soil Enriched with Fluorine Derived from Mica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Characterization
2.2. Micro Flotation Tests
2.2.1. Determination of Operational Parameters Using Pure Minerals
2.2.2. Determination of Operational Parameters Using Mica Minerals Collected from the Sample
2.2.3. Surface Characterization Using FT-IR
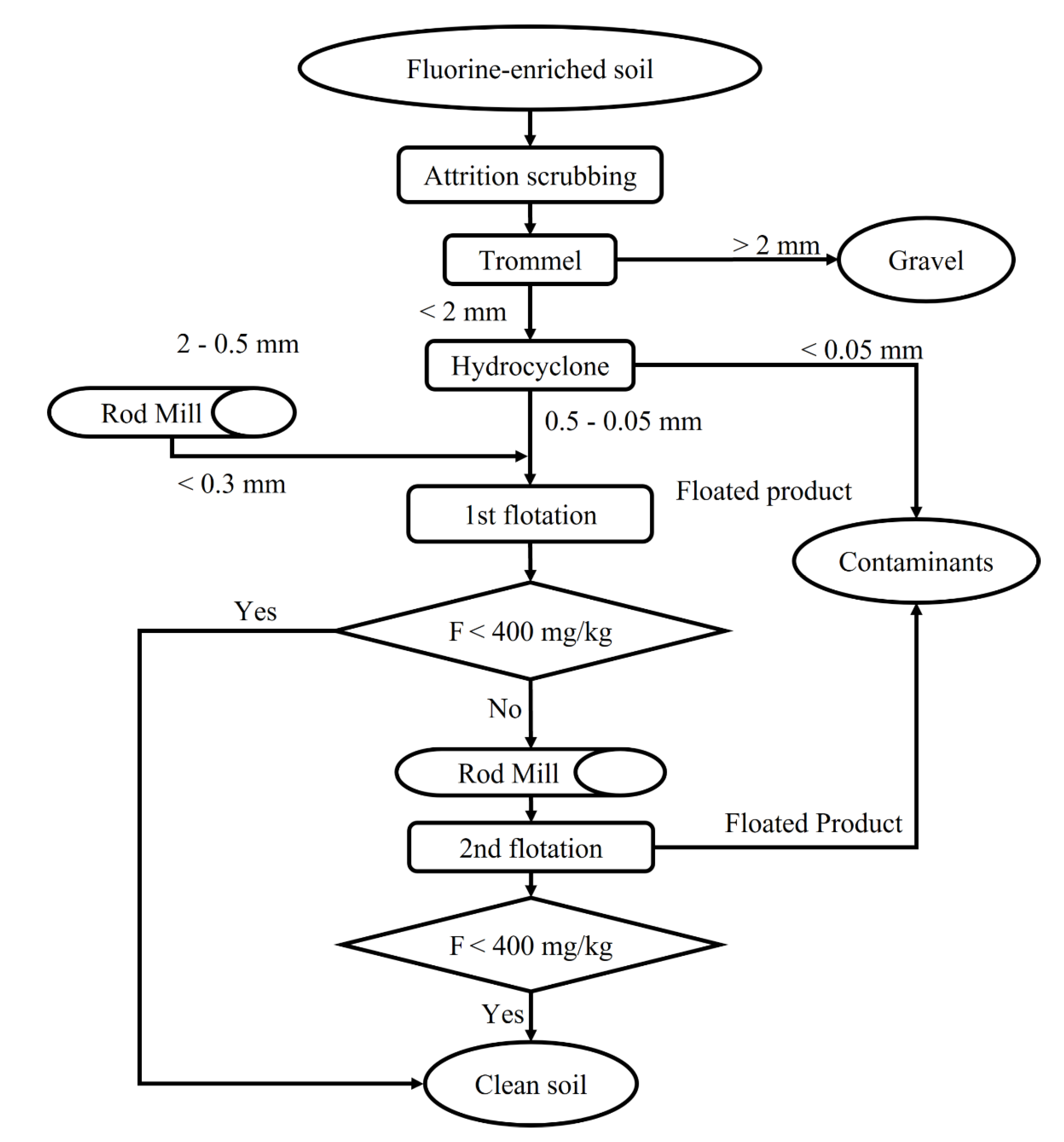
2.3. Bench Flotation Tests on the Soil Sample
2.3.1. Optimization of the Soil Sample Mechanical Preparation
2.3.2. Determination of Collector Dosage
2.3.3. Multi-Stage Flotation Process
3. Results and Discussion
3.1. Distribution of F Concentration in Soil
3.2. Micro Flotation Tests
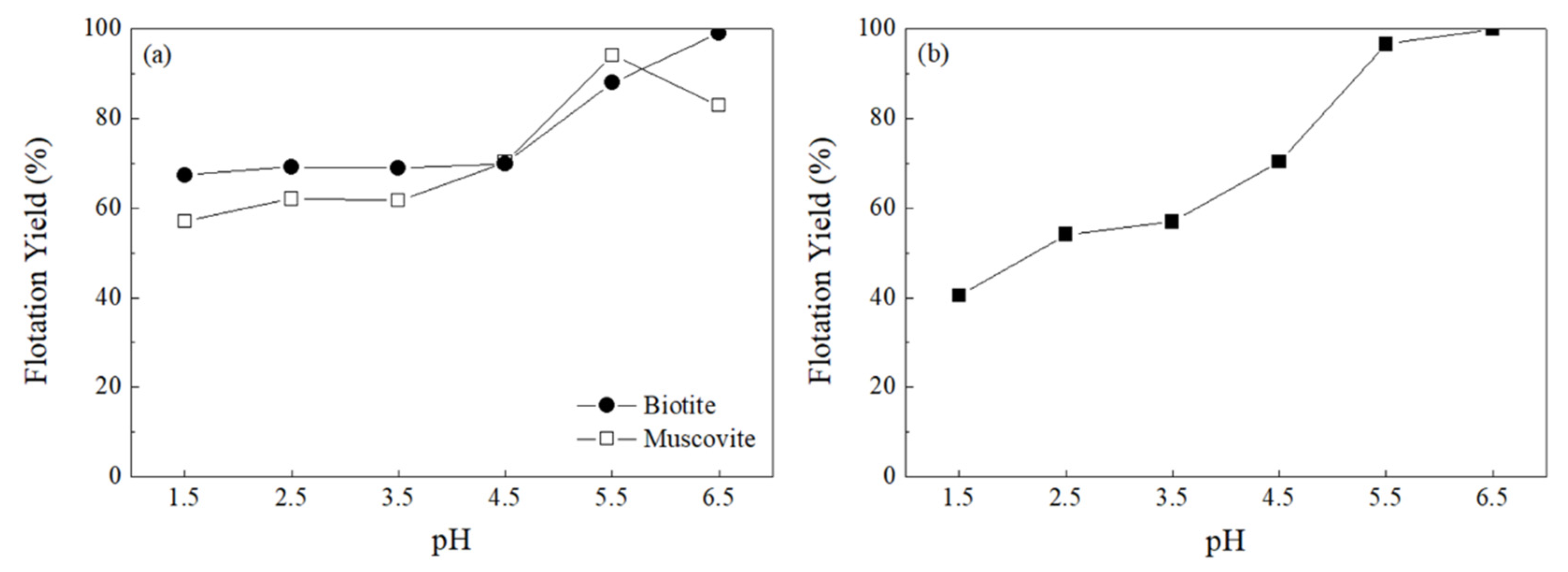
3.2.1. pH Optimization
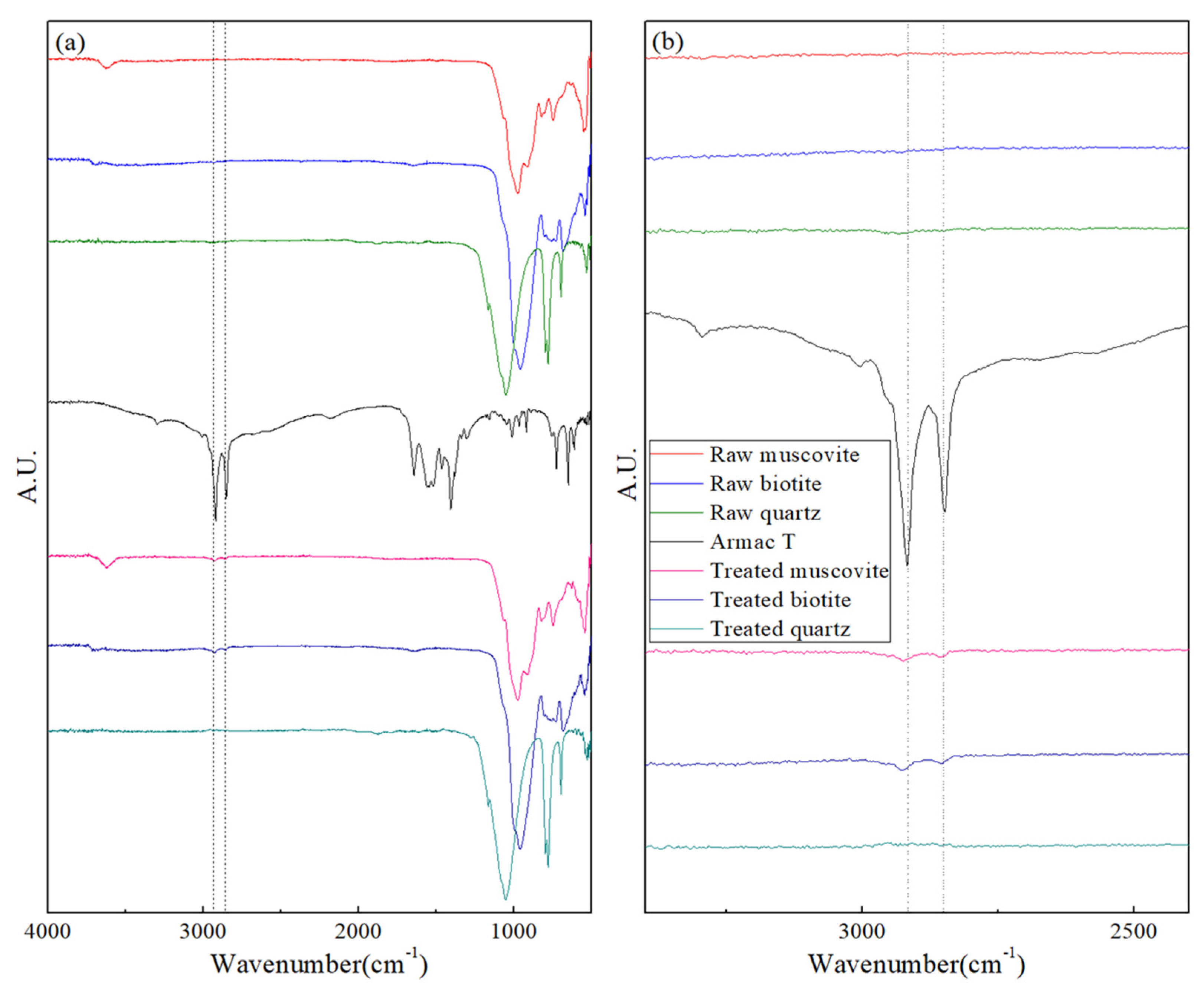
3.2.2. Surface Characterization Using FT-IR
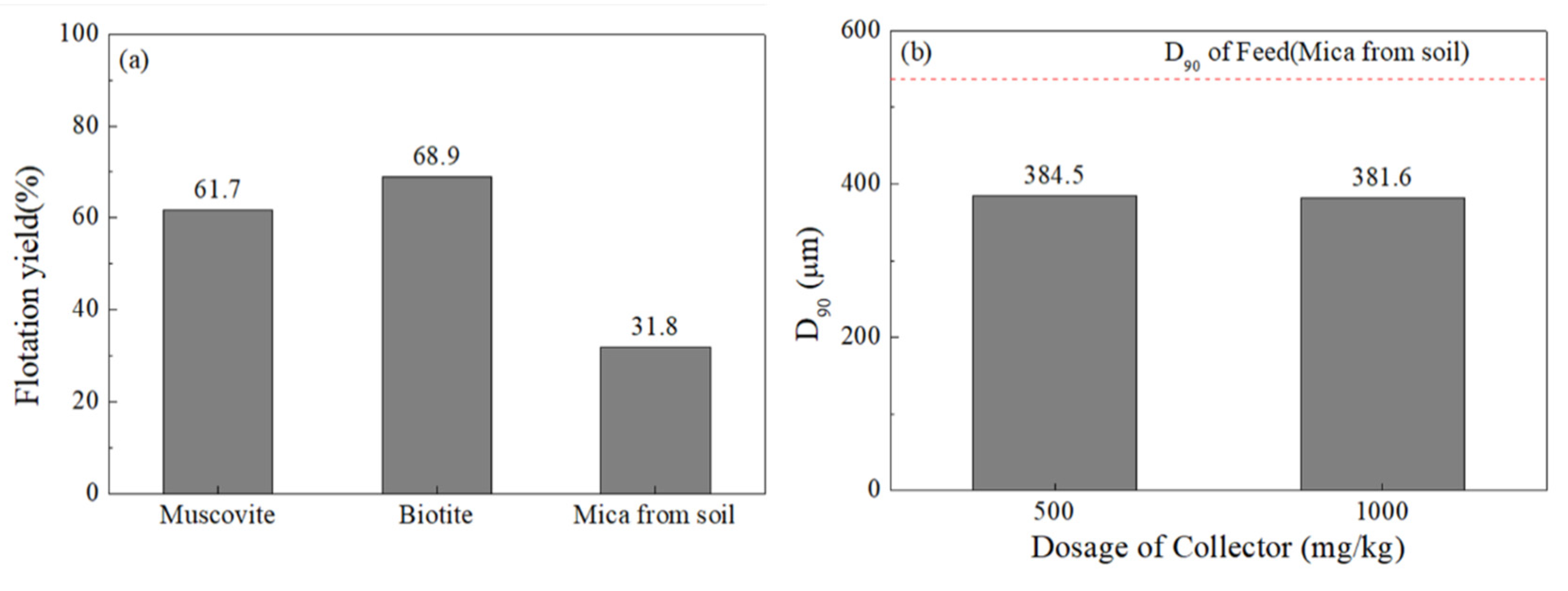
3.2.3. Particle Size Optimization
3.3. Bench Flotation Tests
3.3.1. Optimal Procedures for Soil Sample Mechanical Preparation
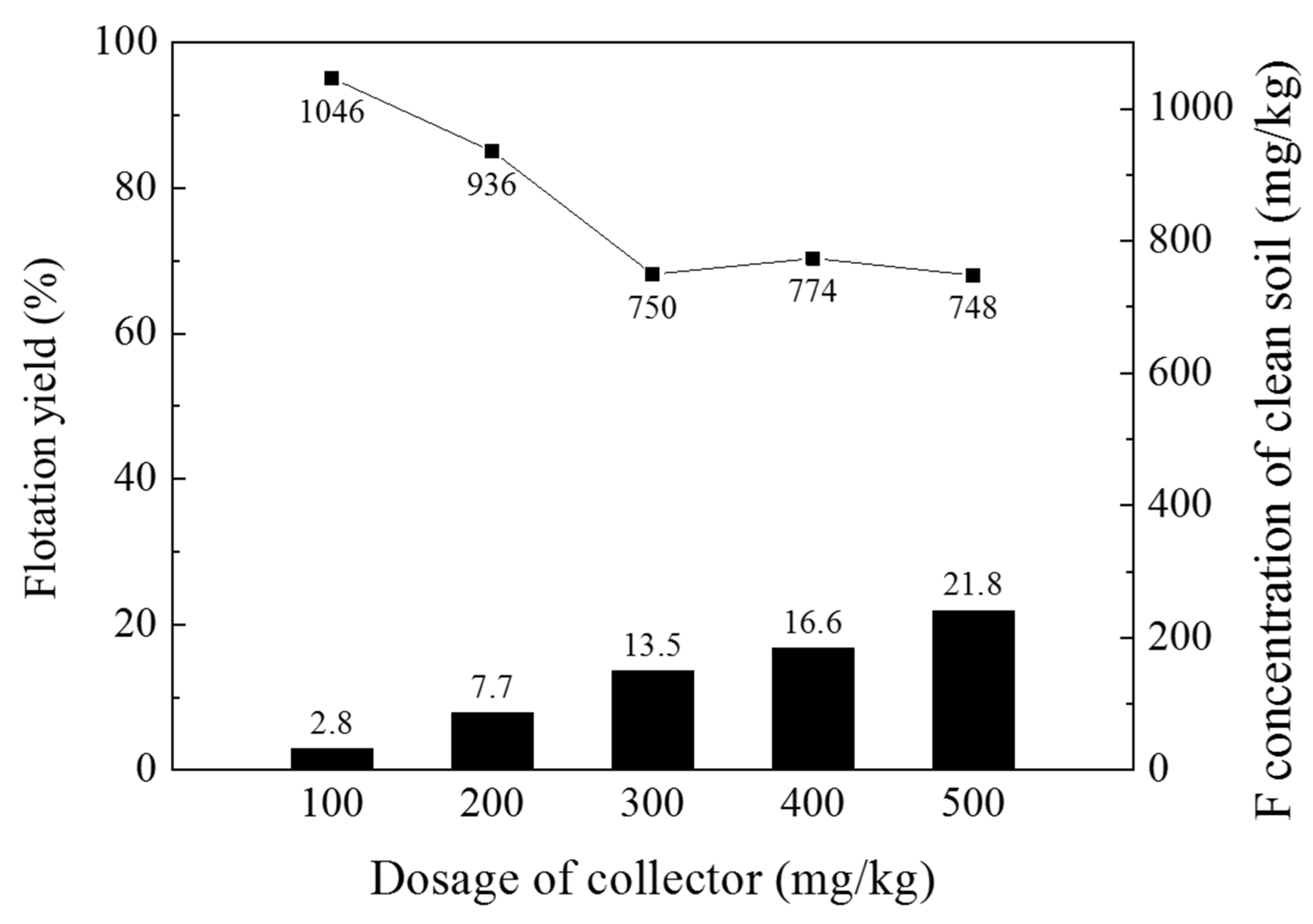
3.3.2. Determination of Collector Dosage
3.3.3. Application of Multi-Stage Flotation Processes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koga, K.T.; Rose-Koga, E.F. Fluorine in the Earth and the solar system, where does it come from and can it be found? Comptes Rendus Chim. 2018, 21, 749–756. [Google Scholar] [CrossRef]
- McDonogh, W.F.; Sun, S.-S. The composition of the earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Czerwinski, E.; Nowak, J.; Dabrowska, D.; Skolarczyk, A.; Kita, B.; Ksiezyk, M. Bone and joint pathology in fluoride-exposed workers. Arch. Environ. Health 1988, 43, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P. Classical syndromes in occupational medicine occupational fluorosis through 50 years: Clinical and epidemiological experiences. Am. J. Ind. Med. 1982, 3, 227–236. [Google Scholar] [CrossRef]
- Hodge, H.C.; Smith, F.A. Occupational fluoride exposure. J. Occup. Med. 1977, 19, 12–39. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Preventing Disease through Healthy Environments: Inadequate or Excess Fluoride: A Major Public Health Concern; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Luo, K.; Ren, D.; Xu, L.; Dai, S.; Cao, D.; Feng, F.; Tan, J.A. Fluorine content and distribution pattern in Chinese coals. Int. J. Coal. Geol. 2004, 57, 143–149. [Google Scholar] [CrossRef]
- Na, J.-Y.; Woo, K.-H.; Yoon, S.-Y.; Cho, S.-Y.; Song, I.-U.; Kim, J.-A.; Kim, J.-S. Acute symptoms after a community hydrogen fluoride spill. Ann. Occup. Environ. Med. 2013, 25, 17. [Google Scholar] [CrossRef] [Green Version]
- Park, S.B. Alert over South Korea toxic leaks: Government moves to tighten oversight after string of hydrogen fluoride accidents. Nat. News 2013, 494, 15–17. [Google Scholar] [CrossRef]
- Deshmukh, A.N.; Wadaskar, P.M.; Malpe, D.B. Fluorine in environment: A review. Gondwana Geol. Mag. 1995, 9, 1–20. [Google Scholar]
- Fuge, R.; Andrews, M.J. Fluorine in the UK environment. Environ. Geochem. Health 1988, 10, 96–104. [Google Scholar] [CrossRef]
- Handa, B.K. Geochemistry and genesis of fluoride-containing ground waters in India. Groundwater 1975, 13, 275–281. [Google Scholar] [CrossRef]
- Pickering, W.F. The mobility of soluble fluoride in soils. Environ. Pollut. Ser. B 1985, 9, 281–308. [Google Scholar] [CrossRef]
- Rao, N.S.; Devadas, D.J. Fluoride incidence in groundwater in an area of peninsular India. Environ. Geol. 2003, 45, 243–251. [Google Scholar]
- Robinson, W.O.; Edgington, G. Fluorine in soils. Soil Sci. 1946, 61, 341–354. [Google Scholar] [CrossRef]
- Chae, G.-T.; Yun, S.-T.; Mayer, B.; Kim, K.-H.; Kim, S.-Y.; Kwon, J.-S.; Kim, K.; Koh, Y.-K. Fluorine geochemistry in bedrock groundwater of South Korea. Sci. Total Environ. 2007, 385, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Choo, C.-O.; Kim, J.-T.; Chung, I.-M.; Kim, N.-W.; Jeong, G.-C. Geochemical aspects of groundwater in granite area and the origin of fluoride with emphasis on the water-rock interaction. J. Eng. Geol. 2008, 18, 103–115. [Google Scholar]
- Ahn, Y.; Pandi, K.; Cho, D.-W.; Choi, J. Feasibility of soil washing agents to removal fluoride and risk assessment of fluoride-contaminated soils. J. Soils Sediments 2021, 21, 2770–2777. [Google Scholar] [CrossRef]
- Baek, D.-J.; Kim, Y.-E.; Jung, M.-Y.; Yoon, H.-O.; An, J. Feasibility of a chemical washing method for treating fluorine-contaminated soil of a natural origin derived from mica. Minerals 2021, 11, 134. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jeon, C.-S.; Baek, K.; Ko, S.-H.; Yang, J.-S. Electrokinetic remediation of fluorine-contaminated soil: Conditioning of anolyte. J. Hazard. Mater. 2009, 161, 565–596. [Google Scholar] [CrossRef]
- Moon, D.H.; Jo, R.; Koutsospyros, A.; Cheong, K.H.; Park, J.-H. Soil washing of fluorine contaminated soil using various washing solutions. Bull. Environ. Contam. Toxicol. 2015, 94, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Zhu, S.; Wei, X. Effects of electrolyte on the removal of fluorine from red mud by electrokinetic remediation. Environ. Technol. 2021, 42, 2313–2324. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, S.; Wei, X.; Xie, Y. Electrodialytic remediation of fluorine contaminated soil using 2-electrolysis compartment. Arch. Agron. Soil Sci. 2019, 65, 886–896. [Google Scholar] [CrossRef]
- Wen, D.; Fu, R.; Li, Q. Removal of inorganic contaminants in soil by electrokinetic remediation technologies: A review. J. Hazard. Mater. 2021, 401, 123345. [Google Scholar] [CrossRef]
- Jung, M.Y.; Kim, S.H.; An, Y.H.; Lee, Y.J.; Lee, C.H.; Kim, J.W.; Jeon, C.S. Remediation technologies and characteristics of contaminated soil in the vicinity of Sang-Dong mine. J. Korean Soc. Miner. Energy Resour. Eng. 2015, 52, 31–41. [Google Scholar] [CrossRef]
- Park, J.H.; Park, J.H.; Yang, S.C.; Kim, J.N.; Yu, S.H.; Jung, M.Y. The properties and remediation technologies of contaminated soil in the vicinity of Daebong Mine. J. Korean Soc. Miner. Energy Resour. Eng. 2017, 54, 637–645. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Spottiswood, D.J.; Kelly, E.G. Introduction to Mineral Processing; John Wiley & Sons Inc.: New York, NY, USA, 1982; p. 235. [Google Scholar]
- Urum, K.; Pekdemir, T.; Ross, D.; Grigson, S. Crude oil contaminated soil washing in air sparging assisted stirred tank reactor using biosurfactants. Chemosphere 2005, 60, 334–343. [Google Scholar] [CrossRef]
- Zhang, L.; Somasundaran, P.; Ososkov, V.; Chou, C.C. Flotation of hydrophobic contaminants from soil. Colloids Surf. A 2001, 117, 235–246. [Google Scholar] [CrossRef]
- McQuaker, N.R.; Gurney, M. Determination of total fluoride in soil and vegetation using an alkali fusion-selective ion electrode technique. Anal. Chem. 1977, 49, 53–56. [Google Scholar] [CrossRef]
- Förstner, U.; Salomons, W. Trace metal analysis of polluted sediments, Part 1. Assessment of sources and intensities. Environ. Technol. Lett. 1980, 1, 494–505. [Google Scholar] [CrossRef]
- Haque, M.A.; Subramanian, V.; Gibbs, R.J. Copper, lead and Zinc pollution of soil environment. Crit. Rev. Environ. Sci. Technol. 1982, 12, 13–68. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Hu, Y.-H.; Xu, L.-H. Adsorption mechanism of mixed anionic/cationic collectors in Muscovite–Quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Xu, L.; Wu, H.; Dong, F.; Wang, L.; Wang, Z.; Xiao, J. Flotation and adsorption of mixed cationic/anionic collectors on muscovite mica. Miner. Eng. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Hu, Y.; Wei, S.; Hao, J.; Miller, J.D.; Fa, K. The anomalous behavior of kaolinite flotation with dodecyl amine collector as explained from crystal structure considerations. Int. J. Miner. Process. 2005, 76, 163–172. [Google Scholar] [CrossRef]
- Özün, S.; Atalay, M.; Demirci, Ş. Study of adsorption characteristics of long chain alkyl amine and petroleum sulfonate on silicates by electrokinetic potential, microflotation, FTIR and AFM analyses. Part. Sci. Technol. 2017, 37, 492–503. [Google Scholar] [CrossRef]
- Gaudin, A.M. Principles of Mineral Dressing; McGraw Hill Book Co.: New York, NY, USA, 1939. [Google Scholar]
- Vallejos, P.; Yianatos, J.; Vinnett, L.; Bergh, L. Characterization of the industrial flotation process based on size-liberation relationships. Miner. Eng. 2018, 121, 189–195. [Google Scholar] [CrossRef]
- Kirjavainen, V.M. Review and analysis of factors controlling the mechanical flotation of gangue minerals. Int. J. Miner. Process. 1995, 46, 21–34. [Google Scholar] [CrossRef]
- Arnold, B.J.; Aplan, F.F. The effect of clay slimes on coal flotation, part I: The nature of the clay. Int. J. Miner. Process. 1986, 17, 225–242. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, L.; Cao, M.; Liu, Q. Slime coatings in froth flotation: A review. Miner. Eng. 1986, 114, 26–36. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice; Volume 3: Flotation of Industrial Minerals; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
| Particle Size (mm) | Weight Composition (%) | Total F Concentration (mg/kg) |
|---|---|---|
| <2 | 100 | 1197.7 ± 105.0 |
| 0.5–2.0 | 26.4 ± 1.6 | 1126.3 ± 272.4 |
| 0.15–0.5 | 28.0 ± 1.3 | 1036.3 ± 34.0 |
| 0.075–0.15 | 8.4 ± 0.5 | 1564.3 ± 159.5 |
| <0.075 | 37.2 ± 1.9 | 1594.3 ± 42.3 |
| Sample Classification | Flotation Yield (%) | F Concentration in Treated Soil (mg/kg) |
|---|---|---|
| After performing first flotation process | 24 | 715 |
| After performing second flotation process without additional milling | 7 | 637 |
| After performing second flotation process with additional milling to residue of first flotation process | 28.3 | 355 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.; Jung, M.Y.; Lee, H.; An, J. Froth-Flotation Separation as an Alternative for the Treatment of Soil Enriched with Fluorine Derived from Mica. Int. J. Environ. Res. Public Health 2022, 19, 1775. https://doi.org/10.3390/ijerph19031775
Cho J, Jung MY, Lee H, An J. Froth-Flotation Separation as an Alternative for the Treatment of Soil Enriched with Fluorine Derived from Mica. International Journal of Environmental Research and Public Health. 2022; 19(3):1775. https://doi.org/10.3390/ijerph19031775
Chicago/Turabian StyleCho, Jeonghwan, Moon Young Jung, Hwan Lee, and Jinsung An. 2022. "Froth-Flotation Separation as an Alternative for the Treatment of Soil Enriched with Fluorine Derived from Mica" International Journal of Environmental Research and Public Health 19, no. 3: 1775. https://doi.org/10.3390/ijerph19031775
APA StyleCho, J., Jung, M. Y., Lee, H., & An, J. (2022). Froth-Flotation Separation as an Alternative for the Treatment of Soil Enriched with Fluorine Derived from Mica. International Journal of Environmental Research and Public Health, 19(3), 1775. https://doi.org/10.3390/ijerph19031775






