Nonlinear Dynamics of Reaction Time and Time Estimation during Repetitive Test
Abstract
:1. Introduction
2. Materials and Methods
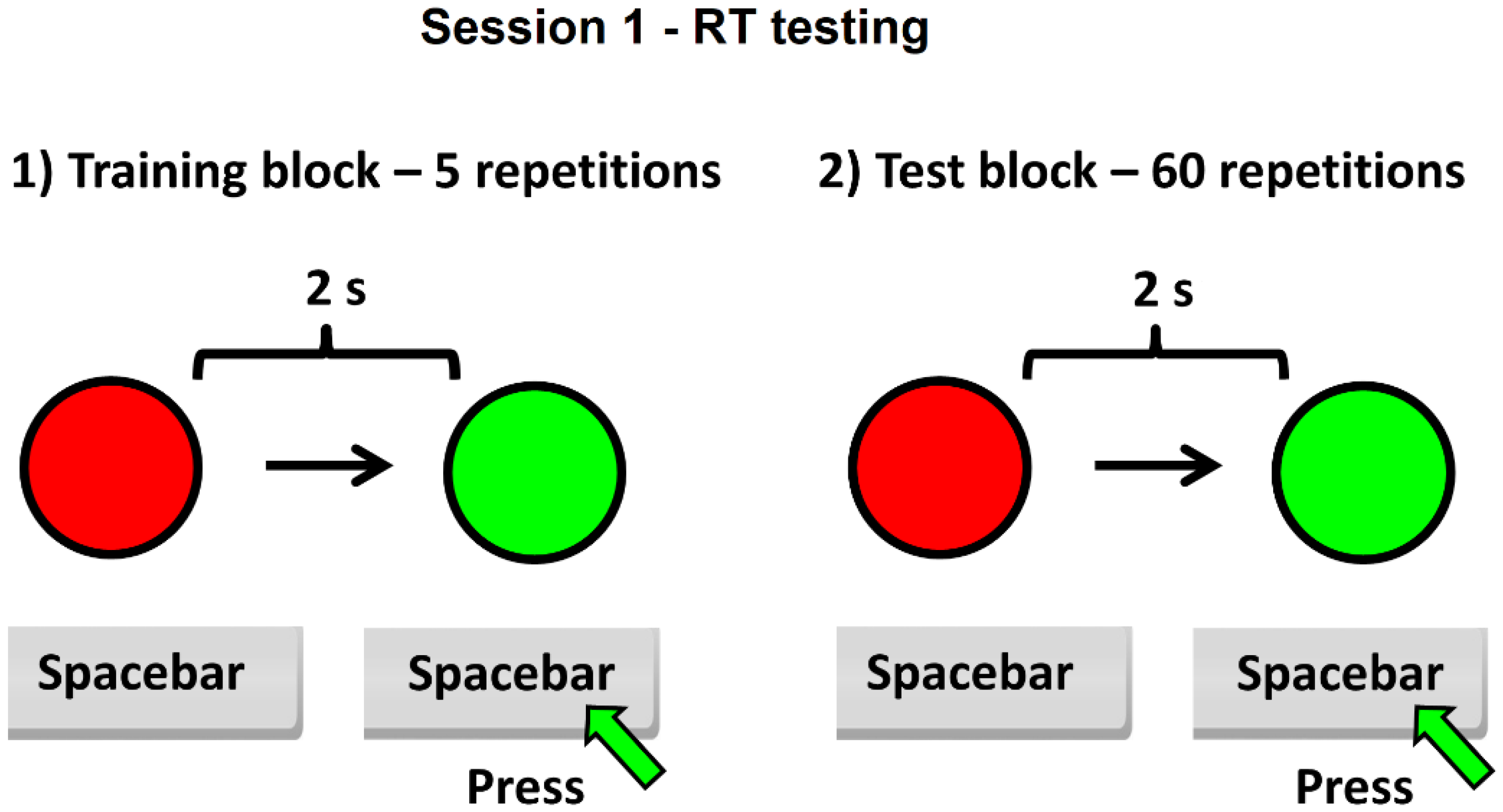
2.1. Study Design
2.2. Data Acquisition
- Sex: male—1, female—2.
- Professional status: pupil/student—1, employee—2, unemployed—3, retired—4, household—5.
- SRH: excellent—1, very good—2, good—3, satisfactory—4, poor—5.
- SRA: not at all anxious—1, slightly anxious—2, moderately anxious—3, very anxious—4, extremely anxious—5.
- Laterality (hand used for writing): right—1, left—2.
2.3. Statistical Analysis of Data
3. Results
3.1. Descriptive Statistics for the Study Group
3.2. Reliability of the RT and VRT Serial Tests
3.3. Correlation and Regression Analysis of Data
4. Discussion
4.1. The Study Implications
4.2. Limitations of the Study
4.3. Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CV | the coefficient of variation |
| RT | reaction time |
| VRT | virtual reaction time |
| SD | standard deviation |
| AFE | area of the fitting ellipse |
| WB | Weber fraction |
| SRH | self-reported health |
| SRA | self-reported anxiety |
| RRE | Relative Reproduction Error |
| R | Pearson’s coefficient of correlation |
| SE | standard error |
| F | test for overall significance for the linear model |
| p | level of statistical significance |
| β0 | the intercept coefficient |
| β1 | the regression coefficient |
| 95%LB | lower bound of the 95% confidence interval |
| 95%UB | upper bound of the 95% confidence interval |
| n | group size |
References
- Gros, A.; Giroud, M.; Bejot, Y.; Rouaud, O.; Guillemin, S.; Aboa Eboulé, C.; Manera, V.; Daumas, A.; Martin, M.L. A time estimation task as a possible measure of emotions: Difference depending on the nature of the stimulus used. Front. Behav. Neurosci. 2015, 9, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa-Fernández, L.; Miró, E.; Cano, M.; Buela-Casal, G. Age-related changes and gender differences in time estimation. Acta Psychol. 2003, 112, 221–232. [Google Scholar] [CrossRef]
- Hancock, P.A.; Rausch, R. The effects of sex, age, and interval duration on the perception of time. Acta Psychol. 2010, 133, 170–179. [Google Scholar] [CrossRef]
- Zélanti, P.S.; Droit-Volet, S. Auditory and visual differences in time perception? An investigation from a developmental perspective with neuropsychological tests. J. Exp. Child Psychol. 2012, 112, 296–311. [Google Scholar] [CrossRef]
- Noulhiane, M.; Mella, N.; Samson, S.; Ragot, R.; Pouthas, V. How emotional auditory stimuli modulate time perception. Emotion 2007, 7, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Gibbon, J. Scalar expectancy theory and Weber’s law in animal timing. Psychol. Rev. 1977, 84, 279–325. [Google Scholar] [CrossRef]
- Gibbon, J.; Church, R.M.; Meck, W.H. Scalar timing in memory. Ann. N. Y. Acad. Sci. 1984, 423, 52–77. [Google Scholar] [CrossRef]
- Church, R.M. Properties of the internal clock. Ann. N. Y. Acad. Sci. 1984, 423, 566–582. [Google Scholar] [CrossRef] [PubMed]
- Perbal-Hatif, S. A neuropsychological approach to time estimation. Dialogues Clin. Neurosci. 2012, 14, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Grondin, S. Timing and time perception: A review of recent behavioral and neuroscience findings and theoretical directions. Atten. Percept. Psychophys. 2010, 72, 561–582. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Allenmark, F.; Müller, H.J.; Shi, Z. Variation in the “coefficient of variation”: Rethinking the violation of the scalar property in time-duration judgments. Acta Psychol. 2021, 214, 103263. [Google Scholar] [CrossRef]
- Wearden, J.H.; Lejeune, H. Scalar properties in human timing: Conformity and violations. Q. J. Exp. Psychol. 2008, 61, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Grondin, S. Violation of the scalar property for time perception between 1 and 2 seconds: Evidence from interval discrimination, reproduction, and categorization. J. Exp. Psychol. Hum. Percept. Perform. 2012, 38, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Suchoon, S.; Edward, J.G. Foreperiod effect on time estimation and simple reaction time. Acta Psychol. 1977, 41, 47–59. [Google Scholar] [CrossRef]
- Herbst, S.K.; Obleser, J. Implicit variations of temporal predictability: Shaping the neural oscillatory and behavioural response. Neuropsychologia 2017, 101, 141–152. [Google Scholar] [CrossRef]
- Long, B.L.; Gillespie, A.I.; Tanaka, M.L. Mathematical model to predict drivers’ reaction speeds. J. Appl. Biomech. 2012, 28, 48–56. [Google Scholar] [CrossRef]
- Paraskevopoulou, S.E.; Coon, W.G.; Brunner, P.; Miller, K.J.; Schalk, G. Within-subject reaction time variability: Role of cortical networks and underlying neurophysiological mechanisms. Neuroimage 2021, 237, 118127. [Google Scholar] [CrossRef]
- Iconaru, E.I.; Ciucurel, M.M.; Georgescu, L.; Tudor, M.; Ciucurel, C. The Applicability of the Poincaré Plot in the Analysis of Variability of Reaction Time during Serial Testing. Int. J. Environ. Res. Public Health 2021, 18, 3706. [Google Scholar] [CrossRef]
- Satti, R.; Abid, N.U.; Bottaro, M.; De Rui, M.; Garrido, M.; Raoufy, M.R.; Montagnese, S.; Mani, A.R. The Application of the Extended Poincaré Plot in the Analysis of Physiological Variabilities. Front. Physiol. 2019, 10, 116. [Google Scholar] [CrossRef]
- Iconaru, E.I.; Ciucurel, C. Hand grip strength variability during serial testing as an entropic biomarker of aging: A Poincaré plot analysis. BMC Geriatr. 2020, 20, 12. [Google Scholar] [CrossRef]
- Crenier, L. Poincaré plot quantification for assessing glucose variability from continuous glucose monitoring systems and a new risk marker for hypoglycemia: Application to type 1 diabetes patients switching to continuous subcutaneous insulin infusion. Diabetes Technol. Ther. 2014, 16, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Guzik, P.; Piskorski, J.; Krauze, T.; Schneider, R.; Wesseling, K.H.; Wykretowicz, A.; Wysock, H. Correlations between Poincaré plot and conventional heart rate variability parameters assessed during paced breathing. J. Physiol. Sci. 2007, 57, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezaei, M.; Mohammadi, H.; Khazaie, H. EEG/EOG/EMG data from a cross sectional study on psychophysiological insomnia and normal sleep subjects. Data Brief 2017, 15, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Kovatchev, B.; Cobelli, C. Glucose variability: Timing, risk analysis, and relationship to hypoglycemia in diabetes. Diabetes Care 2016, 39, 502–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoet, G. PsyToolkit—A software package for programming psychological experiments using Linux. Behav. Res. Methods 2010, 42, 1096–1104. [Google Scholar] [CrossRef]
- Stoet, G. PsyToolkit: A novel web-based method for running online questionnaires and reaction-time experiments. Teach. Psychol. 2017, 44, 24–31. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 20.0, Released 2011; IBM Corp.: Armonk, NY, USA, 2011. [Google Scholar]
- Golińska, A.K. Poincaré plots in analysis of selected biomedical signals. Stud. Logic Gramm. Rhetor. 2013, 35, 117–127. [Google Scholar] [CrossRef]
- Karmakar, C.K.; Khandoker, A.H.; Gubbi, J.; Palaniswami, M. Complex correlation measure: A novel descriptor for Poincaré plot. Biomed. Eng. Online 2009, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Pannucci, C.J.; Wilkins, E.G. Identifying and avoiding bias in research. Plast. Reconstr. Surg. 2010, 126, 619–625. [Google Scholar] [CrossRef]
- Gao, J.; Wong-Lin, K.; Holmes, P.; Simen, P.; Cohen, J.D. Sequential effects in two-choice reaction time tasks: Decomposition and synthesis of mechanisms. Neural Comput. 2009, 21, 2407–2436. [Google Scholar] [CrossRef] [Green Version]
- Miller, J. Reaction time analysis with outlier exclusion: Bias varies with sample size. Q. J. Exp. Psychol. 1991, 43, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wong, W.; Lamoureux, E.L.; Wong, T.Y. Are linear regression techniques appropriate for analysis when the dependent (outcome) variable is not normally distributed? Investig. Opthalmol. Vis. Sci. 2012, 53, 3082. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.; Grajales, C.; Kurkiewicz, D. Assumptions of multiple regression: Correcting two misconceptions. Pract. Assess. Res. Eval. 2013, 18, 1–14. [Google Scholar]
- Schmidt, A.F.; Finan, C. Linear regression and the normality assumption. J. Clin. Epidemiol. 2018, 98, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Ernst, A.F.; Albers, C.J. Regression assumptions in clinical psychology research practice—A systematic review of common misconceptions. PeerJ 2017, 5, e3323. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Marx, I.; Rubia, K.; Reis, O.; Noreika, V. A short note on the reliability of perceptual timing tasks as commonly used in research on developmental disorders. Eur. Child. Adolesc. Psychiatry 2021, 30, 169–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, S.N.; Jindal, G.D.; Xavier, M.; Wagh, R.D.; Garje, K.S.; Nagare, G.D. Poincaré plot: A simple and powerful expression of physiological variability. MGM J. Med. Sci. 2021, 8, 435–441. [Google Scholar] [CrossRef]
- Bliudzius, A.; Puronaite, R.; Trinkunas, J.; Jakaitiene, A.; Kasiulevicius, V. Research on physical activity variability and changes of metabolic profile in patients with prediabetes using Fitbit activity trackers data. Technol. Health Care 2022, 30, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.; Kolbe, J.; Lardenoye, M.; Ellyett, K. Novel application of Poincaré analysis to detect and quantify exercise oscillatory ventilation. Physiol. Meas. 2021, 42, 04NT01. [Google Scholar] [CrossRef]
- Blomkvist, A.W.; Eika, F.; Rahbek, M.T.; Eikhof, K.D.; Hansen, M.D.; Søndergaard, M.; Ryg, J.; Andersen, S.; Jørgensen, M.G. Reference data on reaction time and aging using the Nintendo Wii Balance Board: A cross-sectional study of 354 subjects from 20 to 99 years of age. PLoS ONE 2017, 12, e0189598. [Google Scholar] [CrossRef]
- Deary, I.J.; Der, G. Reaction Time, Age, and Cognitive Ability: Longitudinal Findings from Age 16 to 63 Years in Representative Population Samples. Aging Neuropsychol. Cogn. 2005, 12, 187–215. [Google Scholar] [CrossRef]
- Talboom, J.S.; De Both, M.D.; Naymik, M.A.; Schmidt, A.M.; Lewis, C.R.; Jepsen, W.M.; Håberg, A.K.; Rundek, T.; Levin, B.E.; Hoscheidt, S.; et al. Two separate, large cohorts reveal potential modifiers of age-associated variation in visual reaction time performance. npj Aging Mech. Dis. 2021, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Taatgen, N.; Anderson, J.; Dickison, D.; van Rijn, H. Time Interval Estimation: Internal Clock or Attentional Mechanism? In Proceedings of the Annual Meeting of the Cognitive Science Society, Stresa, Italy, 21–23 July 2005; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 2005; Volume 27, pp. 2122–2127. [Google Scholar]
- Roberts, K.L.; Allen, H.A. Perception and cognition in the ageing brain: A brief review of the short- and long-term links between perceptual and cognitive decline. Front. Aging Neurosci. 2016, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, M.; Lustig, C.; Meck, W.H. Cognitive Aging and Time Perception: Roles of Bayesian Optimization and Degeneracy. Front. Aging Neurosci. 2016, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckolz, E.; Rugins, O. The relationship between estimates of foreperiod duration and simple time reaction. J. Mot. Behav. 1978, 10, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Jaśkowski, P. Simple reaction time and perception of temporal order: Dissociations and hypotheses. Percept. Mot. Skills 1996, 82, 707–730. [Google Scholar] [CrossRef]
- Simen, P. Reaction Time Analysis for Interval Timing Research. In Timing and Time Perception: Procedures, Measures, & Applications; Vatakis, A., Balcı, F., Di Luca, M., Correa, A., Eds.; Brill: Leiden, The Netherlands, 2018; pp. 165–176. [Google Scholar] [CrossRef] [Green Version]
- Roy, M.M.; Christenfeld, N.J.S. Effect of task length on remembered and predicted duration. Psychon. Bull. Rev. 2008, 15, 202–207. [Google Scholar] [CrossRef] [Green Version]
- Lewis, P.A.; Miall, R.C. The precision of temporal judgement: Milliseconds, many minutes, and beyond. Phil. Trans. R. Soc. B 2009, 364, 1897–1905. [Google Scholar] [CrossRef] [Green Version]
- Mioni, G.; Grondin, S.; Bardi, L.; Stablum, F. Understanding time perception through non-invasive brain stimulation techniques: A review of studies. Behav. Brain Res. 2020, 377, 112232. [Google Scholar] [CrossRef]
- Koch, G.; Oliveri, M.; Torriero, S.; Salerno, S.; Lo Gerfo, E.; Caltagirone, C. Repetitive TMS of cerebellum interferes with millisecond time processing. Exp. Brain Res. 2007, 179, 291–299. [Google Scholar] [CrossRef]
- Jones, C.R.G.; Rosenkranz, K.; Rothwell, J.C.; Jahanshahi, M. The right dorsolateral prefrontal cortex is essential in time reproduction: An investigation with repetitive transcranial magnetic stimulation. Exp. Brain Res. 2004, 158, 366–372. [Google Scholar] [CrossRef]
- Mioni, G.; Capizzi, M.; Vallesi, A.; Correa, Á.; Di Giacopo, R.; Stablum, F. Dissociating Explicit and Implicit Timing in Parkinson’s Disease Patients: Evidence from Bisection and Foreperiod Tasks. Front. Hum. Neurosci. 2018, 12, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltanlou, M.; Nazari, M.A.; Vahidi, P.; Nemati, P. Explicit and Implicit Timing of Short Time Intervals: Using the Same Method. Perception 2020, 49, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Kohnert, K.D.; Heinke, P.; Vogt, L.; Augstein, P.; Salzsieder, E. Applications of Variability Analysis Techniques for Continuous Glucose Monitoring Derived Time Series in Diabetic Patients. Front. Physiol. 2018, 9, 1257. [Google Scholar] [CrossRef] [PubMed]
- Grondin, S. Duration discrimination of empty and filled intervals marked by auditory and visual signals. Percept. Psychophys. 1993, 54, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Piras, F.; Coull, J.T. Implicit, Predictive Timing Draws upon the Same Scalar Representation of Time as Explicit Timing. PLoS ONE 2011, 6, e18203. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, P.; Grondin, S. Information processing limitations as revealed by temporal discrimination. Brain Cogn. 2004, 54, 198–200. [Google Scholar] [CrossRef]
- Fortin, C.; Couture, E. Short-term memory and time estimation: Beyond the 2-second “critical” value. Can. J. Exp. Psychol. 2002, 56, 120–127. [Google Scholar] [CrossRef]
- Gallego Hiroyasu, E.M.; Yotsumoto, Y. Disentangling the effects of modality, interval length and task difficulty on the accuracy and precision of older adults in a rhythmic reproduction task. PLoS ONE 2021, 16, e0248295. [Google Scholar] [CrossRef] [PubMed]
- Sierra, F.; Poeppel, D.; Tavano, A. How to minimize time distortions. PsyArXiv 2020. [Google Scholar] [CrossRef]
- Thalange, A.V.; Mergu, R.R. HRV analysis of arrhythmias using linear-nonlinear parameters. Int. J. Comput. Appl. 2010, 1, 71–77. [Google Scholar] [CrossRef]
- Packiasabapathy, S.; Prasad, V.; Rangasamy, V.; Popok, D.; Xu, X.; Novack, V.; Subramaniam, B. Cardiac surgical outcome prediction by blood pressure variability indices Poincaré plot and coefficient of variation: A retrospective study. BMC Anesthesiol. 2020, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.F.; Lynn, F.; Meade, B.D. Use of coefficient of variation in assessing variability of quantitative assays. Clin. Diagn. Lab. Immunol. 2002, 9, 1235–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schober, P.; Vetter, T.R. Linear regression in medical research. Anesth. Analg. 2021, 132, 108–109. [Google Scholar] [CrossRef]
- Batterham, P.J.; Bunce, D.; Mackinnon, A.J.; Christensen, H. Intra-individual reaction time variability and all-cause mortality over 17 years: A community-based cohort study. Age Ageing 2014, 43, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Namboodiri, V.M.; Mihalas, S.; Hussain Shuler, M.G. A temporal basis for Weber’s law in value perception. Front. Integr. Neurosci. 2014, 8, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eagle, D.M.; Baunez, C.; Hutcheson, D.M.; Lehmann, O.; Shah, A.P.; Robbins, T.W. Stop-signal reaction-time task performance: Role of prefrontal cortex and subthalamic nucleus. Cereb. Cortex 2008, 18, 178–188. [Google Scholar] [CrossRef] [Green Version]
- Verbruggen, F.; Logan, G.D. Response inhibition in the stop-signal paradigm. Trends Cogn. Sci. 2008, 12, 418–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltanifar, M.; Escobar, M.; Dupuis, A.; Schachar, R. A Bayesian mixture modelling of stop signal reaction time distributions: The second contextual solution for the problem of aftereffects of inhibition on SSRT estimations. Brain Sci. 2021, 11, 1102. [Google Scholar] [CrossRef]
- Fadeev, K.; Alikovskaia, T.; Tumyalis, A.; Smirnov, A.; Golokhvast, K. The reaction switching produces a greater bias to prepotent response than reaction inhibition. Brain Sci. 2020, 10, 188. [Google Scholar] [CrossRef] [Green Version]

| Variable | Age Years | SRH | SRA |
|---|---|---|---|
| Mean | 31.61 | 2.32 | 1.77 |
| SD | 13.56 | 0.80 | 0.79 |
| Variable | RT ms | CV % | SD1 ms | SD2 ms | AFE ms2 | SD1/SD2 |
|---|---|---|---|---|---|---|
| Mean | 263.94 | 33.04 | 80.60 | 87.76 | 28,070.77 | 0.93 |
| SD | 69.17 | 15.91 | 42.90 | 47.59 | 36,014.82 | 0.16 |
| Variable | VRT ms | CV % | RRE % | SD1 ms | SD2 ms | AFE ms2 | SD1/SD2 |
|---|---|---|---|---|---|---|---|
| Mean | 1540.80 | 15.21 | −22.96 | 170.88 | 265.34 | 191,331.54 | 0.69 |
| SD | 592.63 | 8.82 | 29.63 | 109.59 | 189.00 | 305,745.98 | 0.24 |
| Variable | Age | Sex | SRH | SRA | RT | CV RT | SD1 RT | SD2 RT | AFE RT | SD1/SD2 RT | VRT | CV VRT | RRE | SD1 VRT | SD2 VRT | AFE VRT | SD1/SD2 VRT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1.00 b | ||||||||||||||||
| Sex | −0.12 a | 1.00 a | |||||||||||||||
| SRH | 0.15 a* | 0.14 a | 1.00 a | ||||||||||||||
| SRA | −0.12 a | 0.11 a | 0.29 a* | 1.00 a | |||||||||||||
| RT | 0.49 b* | 0.13 a | 0.12 a | −0.04 a | 1.00 b | ||||||||||||
| CV RT | −0.15 b* | −0.05 a | −0.06 a | 0.14 a | −0.24 b* | 1.00 b | |||||||||||
| SD1 RT | 0.10 b | 0.03 a | 0.00 a | 0.14 a | 0.32 b* | 0.79 b* | 1.00 b | ||||||||||
| SD2 RT | 0.15 b* | 0.03 a | −0.03 a | 0.10 a | 0.46 b* | 0.71 b* | 0.92 b* | 1.00 b | |||||||||
| AFE RT | 0.19 b* | 0.03 a | −0.01 a | 0.12 a | 0.51 b* | 0.61 b* | 0.92 b* | 0.93 b* | 1.00 b | ||||||||
| SD1/SD2 RT | −0.17 b* | 0.06 a | 0.02 a | 0.16 a* | −0.31 b* | 0.24 b* | 0.24 b* | −0.10 b | 0.02 b | 1.00 b | |||||||
| VRT | −0.21 b* | −0.01 a | 0.03 a | 0.07 a | −0.09 b | 0.04 b | −0.01 b | −0.03 b | −0.06 b | 0.01 b | 1.00 b | ||||||
| CV VRT | −0.03 b | −0.15 a* | −0.04 a | 0.03 a | 0.17 b* | 0.00 b | 0.14 b | 0.13 b | 0.16 b* | 0.06 b | −0.12 b | 1.00 b | |||||
| RRE | −0.21 b* | −0.01 a | 0.03 a | 0.07 a | −0.09 b | 0.04 b | −0.01 b | −0.03 b | −0.06 b | 0.01 b | 1.00 b | −0.12 b | 1.00 b | ||||
| SD1 VRT | −0.15 b* | 0.11 a | 0.04 a | 0.06 a | 0.12 b | −0.01 b | 0.10 b | 0.10 b | 0.09 b | 0.04 b | 0.57 b* | 0.49 b* | 0.57 b* | 1.00 b | |||
| SD2 VRT | −0.14 b | 0.02 a | −0.02 a | 0.03 a | 0.11 b | 0.03 b | 0.12 b | 0.12 b | 0.11 b | 0.02 b | 0.49 b* | 0.75 b* | 0.49 b* | 0.76 b* | 1.00 b | ||
| AFE VRT | −0.14 b | 0.05 a | 0.02 a | 0.06 a | 0.11 b | 0.03 b | 0.11 b | 0.14 b | 0.11 b | −0.03 b | 0.46 b* | 0.61 b* | 0.46 b* | 0.90 b* | 0.87 b* | 1.00 b | |
| SD1/SD2 VRT | 0.06 b | 0.08 a | 0.15 a* | 0.06 a | −0.01 b | −0.08 b | −0.07 b | −0.09 | −0.08 b | 0.05 b | 0.02 b | −0.27 b* | 0.02 b* | 0.16 b* | −0.35 b* | −0.07 b | 1.00 b |
| Variable | R | R Square | Adjusted R Square | SE | F | p | β0 | SE | p | 95%LB | 95%UB | β1 | SE | p | 95%LB | 95%UB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT | 0.49 | 0.24 | 0.23 | 60.65 | 54.84 | 0.001 | 185.67 | 11.49 | 0.001 | 162.99 | 208.36 | 2.48 | 0.33 | 0.001 | 1.82 | 3.14 |
| VRT | 0.21 | 0.04 | 0.04 | 581.16 | 8.13 | 0.005 | 1829.62 | 110.15 | 0.001 | 1612.25 | 2046.98 | −9.14 | 3.20 | 0.005 | −15.46 | −2.81 |
| Variable | R | R Square | Adjusted R Square | SE | F | p | β0 | SE | p | 95%LB | 95%UB | β1 | SE | p | 95%LB | 95%UB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SD1 | 0.79 | 0.62 | 0.62 | 26.45 | 292.91 | 0.001 | 10.32 | 4.55 | 0.025 | 1.34 | 19.32 | 2.13 | 0.12 | 0.001 | 1.88 | 2.37 |
| SD2 | 0.71 | 0.51 | 0.50 | 33.55 | 182.18 | 0.001 | 17.48 | 5.78 | 0.003 | 6.08 | 28.88 | 2.13 | 0.16 | 0.001 | 1.82 | 2.44 |
| AFE | 0.61 | 0.37 | 0.36 | 28,713.49 | 103.61 | 0.001 | −17,295.44 | 4944.17 | 0.001 | −27,052.2 | −7538.71 | 1373.2 | 134.9 | 0.001 | 1106.97 | 1639.42 |
| SD1/SD2 | 0.24 | 0.06 | 0.05 | 0.16 | 10.78 | 0.001 | 0.85 | 0.03 | 0.001 | 0.79 | 0.9 | 0.002 | 0.001 | 0.001 | 0.001 | 0.004 |
| Variable | R | R Square | Adjusted R Square | SE | F | p | β0 | SE | p | 95%LB | 95%UB | β1 | SE | p | 95%LB | 95%UB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SD1 | 0.49 | 0.24 | 0.24 | 95.60 | 57.23 | 0.001 | 77.72 | 14.23 | 0.001 | 49.64 | 105.8 | 6.13 | 0.81 | 0.001 | 4.53 | 7.73 |
| SD2 | 0.75 | 0.56 | 0.56 | 125.47 | 228.15 | 0.001 | 21.21 | 18.67 | 0.001 | −15.64 | 58.06 | 16.06 | 1.06 | 0.001 | 13.96 | 18.15 |
| AFE | 0.61 | 0.37 | 0.36 | 243,925.9 | 103.23 | 0.001 | −127,906 | 36,301.7 | 0.001 | −199,543.1 | −56,268.9 | 20,995 | 2066 | 0.001 | 16,917 | 25,072.7 |
| SD1/SD2 | 0.27 | 0.07 | 0.07 | 0.23 | 14.08 | 0.001 | 0.8 | 0.03 | 0.001 | 0.74 | 0.87 | −0.007 | 0.002 | 0.001 | −0.011 | −0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iconaru, E.I.; Ciucurel, M.M.; Tudor, M.; Ciucurel, C. Nonlinear Dynamics of Reaction Time and Time Estimation during Repetitive Test. Int. J. Environ. Res. Public Health 2022, 19, 1818. https://doi.org/10.3390/ijerph19031818
Iconaru EI, Ciucurel MM, Tudor M, Ciucurel C. Nonlinear Dynamics of Reaction Time and Time Estimation during Repetitive Test. International Journal of Environmental Research and Public Health. 2022; 19(3):1818. https://doi.org/10.3390/ijerph19031818
Chicago/Turabian StyleIconaru, Elena Ioana, Manuela Mihaela Ciucurel, Mariana Tudor, and Constantin Ciucurel. 2022. "Nonlinear Dynamics of Reaction Time and Time Estimation during Repetitive Test" International Journal of Environmental Research and Public Health 19, no. 3: 1818. https://doi.org/10.3390/ijerph19031818








