Capillary Zone Electrophoresis in Tandem with Flow Cytometry in Viability Study of Various ATCC Bacterial Strains under Antibiotic Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Biological Material
2.2. Preparation of Antibiotics
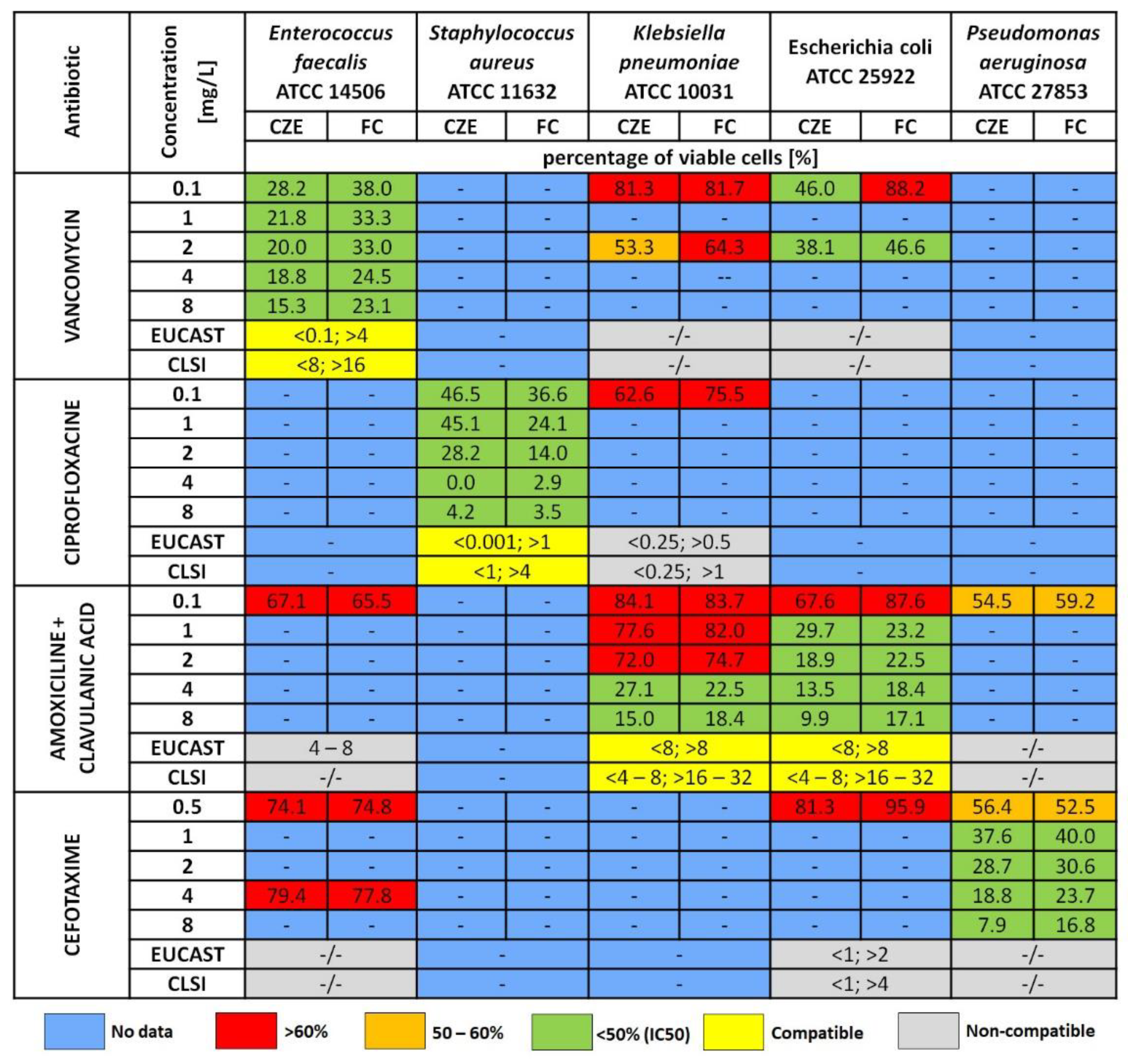
2.3. Antibiotic Susceptibility Assays (Static Assays)
2.4. Time-Dependent Assays (Dynamic Assays)
2.5. Optical Density Measurements (OD)
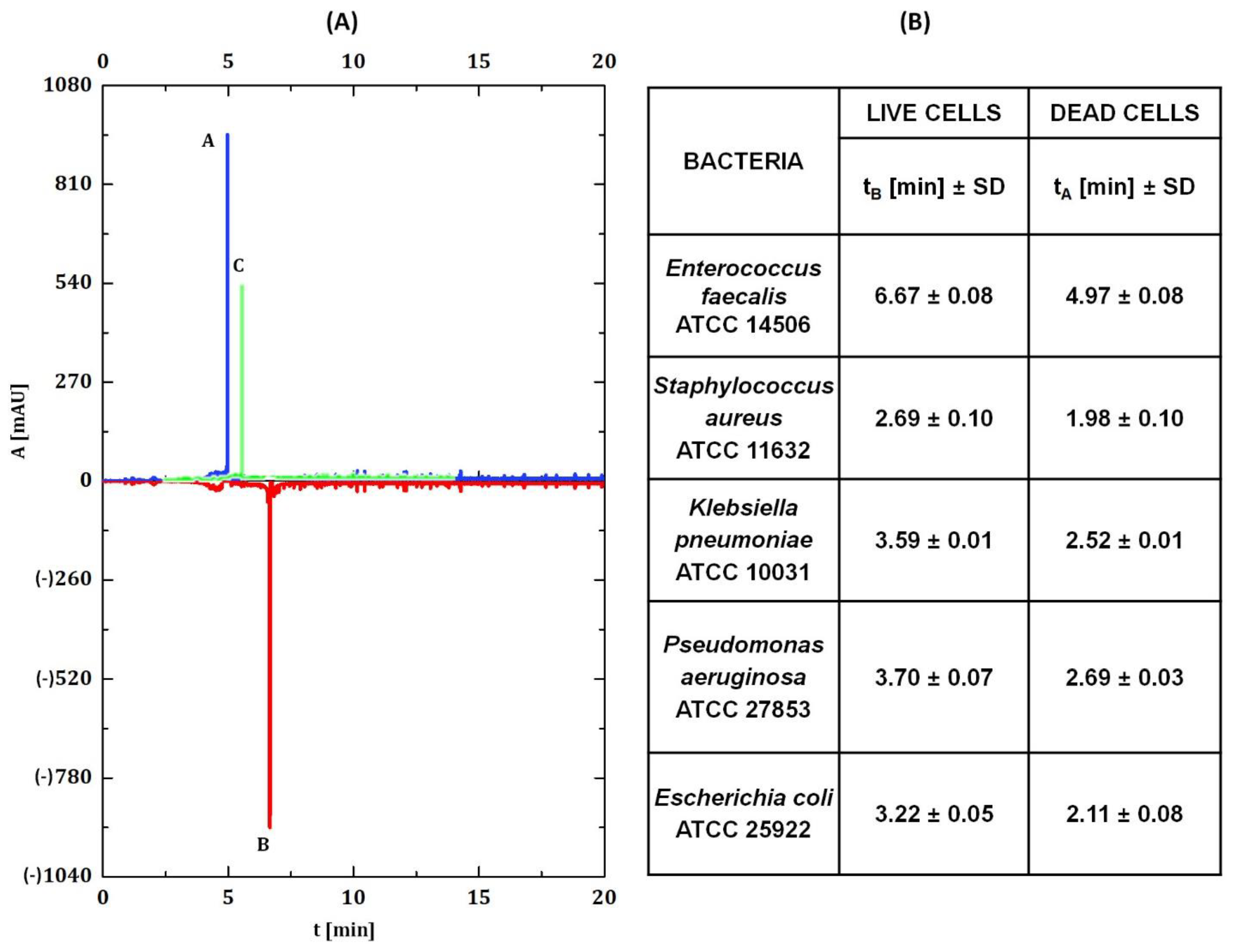
2.6. Capillary Zone Electrophoresis Measurements
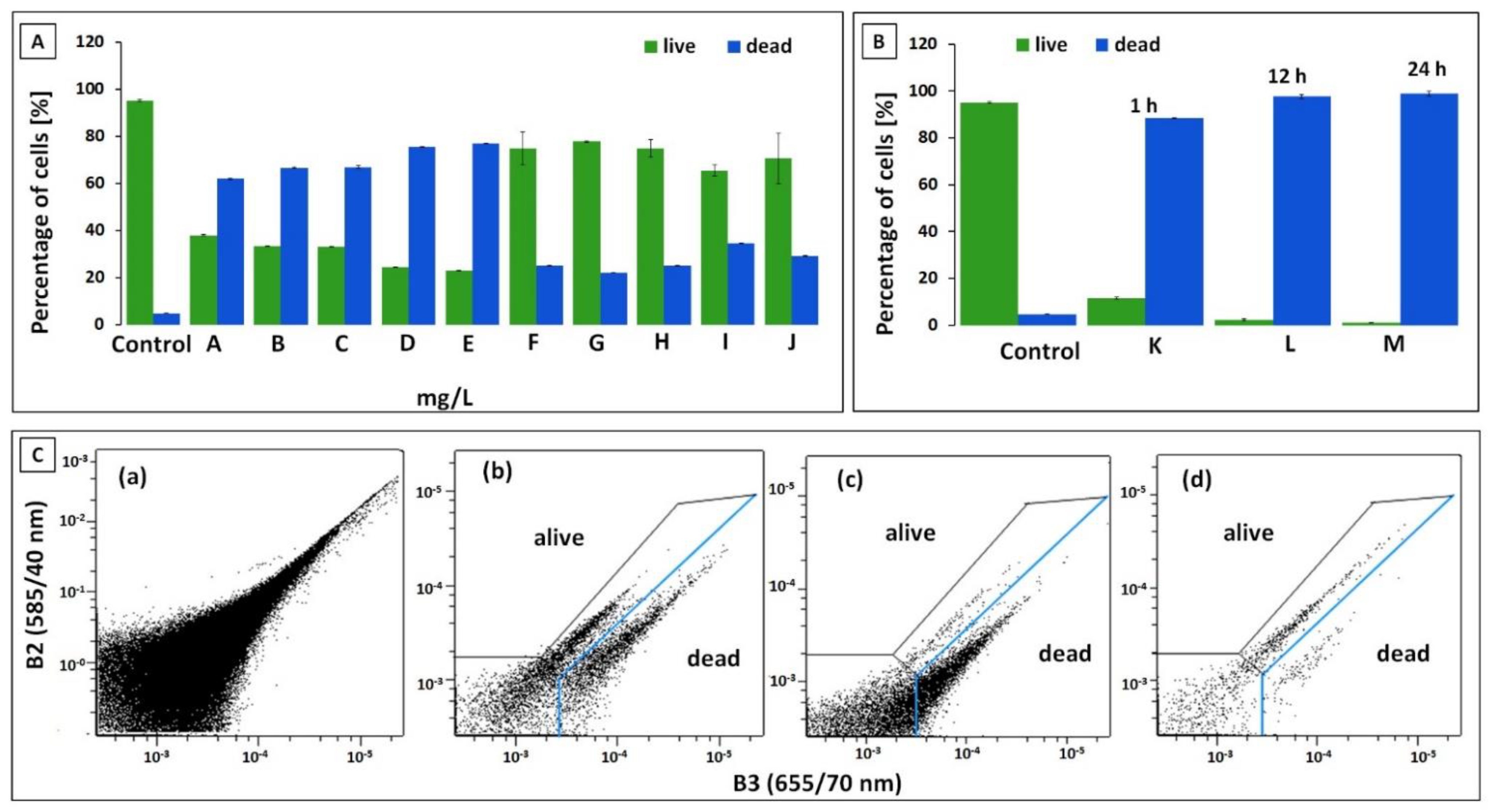
2.7. Flow Cytometric Measurements
2.8. Statistical Analyses
3. Results
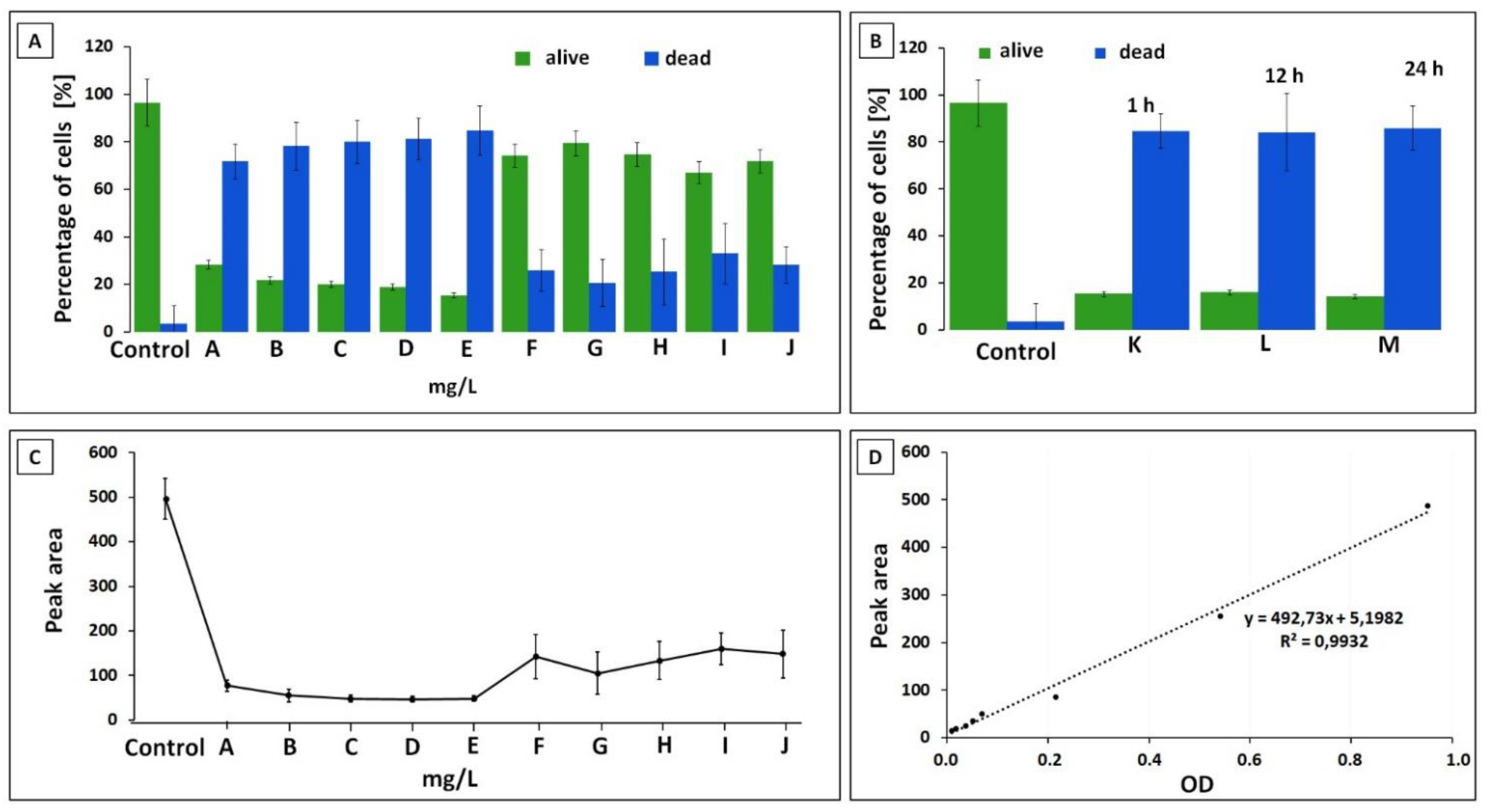
3.1. Optical Density
3.2. Capillary Zone Electrophoresis Assay
3.3. Flow Cytometry Measurement (FC)
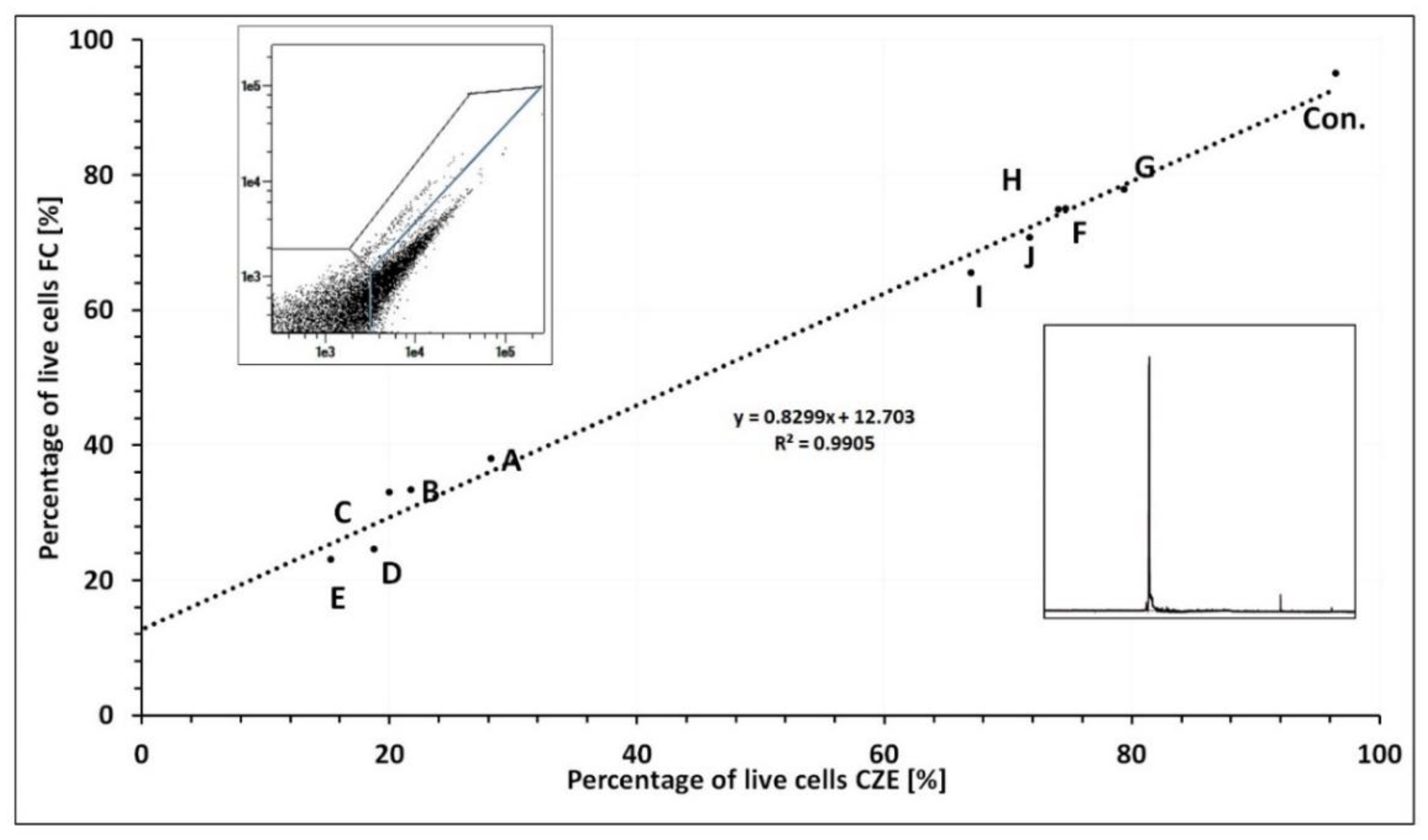
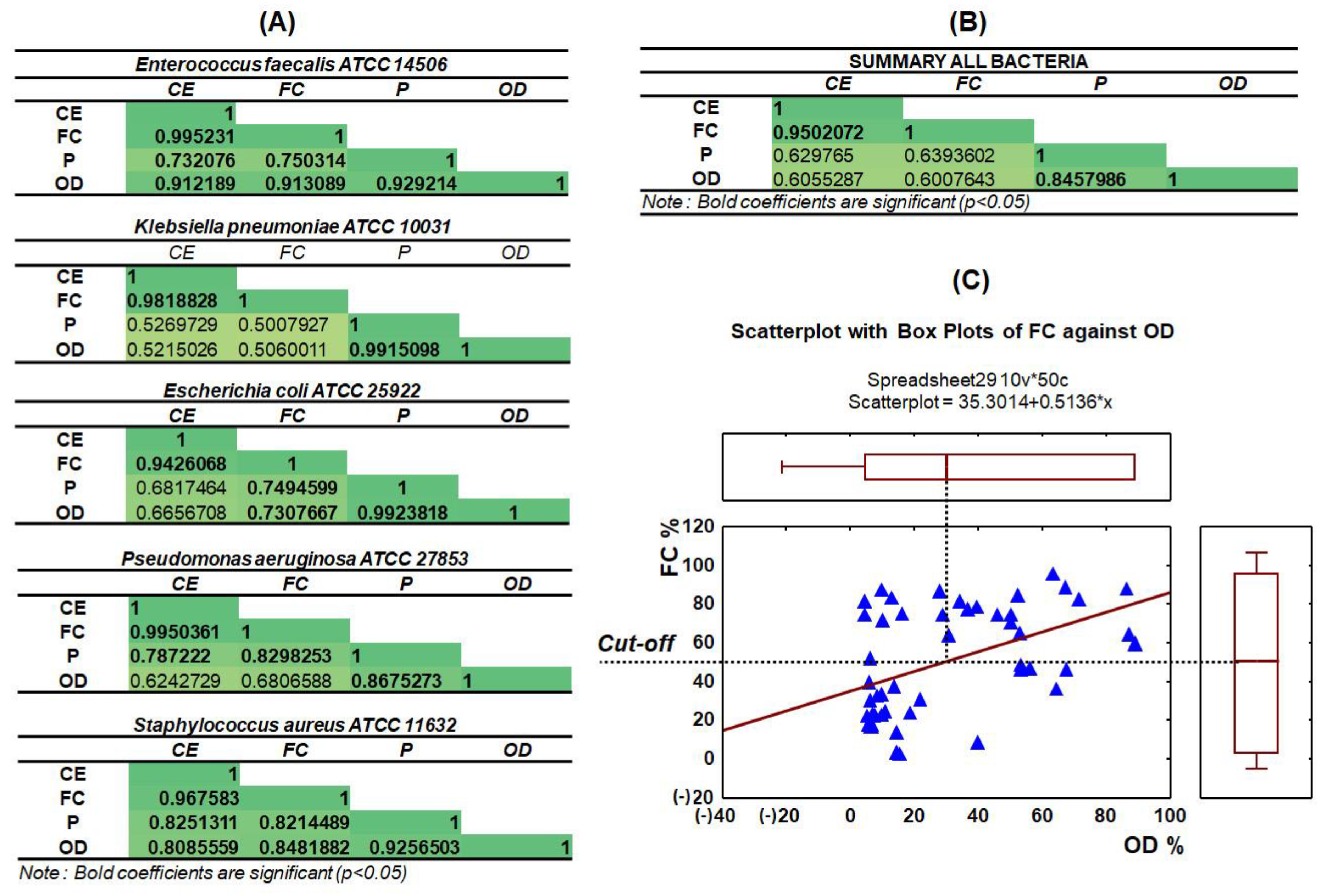
3.4. Capillary Zone Electrophoresis and Flow Cytometry Data Correlation
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, C. Enumeration of probiotic strains: Review of culture-dependent and alternative techniques to quantify viable bacteria. J. Microbiol. Methods 2014, 103, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Gopinathan, R.; Chandra, G.K.; Umapathy, S.; Saini, D.K. Rapid detection of bacterial infection and viability assessment with high specificity and sensitivity using Raman microspectroscopy. Anal. Bioanal. Chem. 2020, 412, 2505–2516. [Google Scholar] [CrossRef]
- Wilkinson, M.G. Flow cytometry as a potential method of measuring bacterial viability in probiotic products: A review. Trends Food Sci. Technol. 2018, 78, 1–10. [Google Scholar] [CrossRef]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, J.D. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol. Rev. 2010, 34, 415–425. [Google Scholar] [CrossRef] [Green Version]
- Wu, E.; Yi, J.; Liu, B.; Qiao, L. Assessment of bacterial viability by laser desorption ionization mass spectrometry for antimicrobial susceptibility testing. Talanta 2021, 233, 122535. [Google Scholar] [CrossRef]
- Sun, J.; Huang, J.; Warden, A.R.; Ding, X. Real-time detection of foodborne bacterial viability using a colorimetric bienzyme system in food and drinking water. Food Chem. 2020, 320, 126581. [Google Scholar] [CrossRef] [PubMed]
- Baatout, S.; De Boever, P.; Mergeay, M. Physiological changes induced in four bacterial strains following oxidative stress. Appl. Biochem. Microbiol. 2006, 42, 369–377. [Google Scholar] [CrossRef]
- Kennedy, D.; Cronin, U.P.; Wilkinson, M.G. Responses of Escherichia coli, Listeria monocytogenes, and Staphylococcus aureus to Simulated Food Processing Treatments, Determined Using Fluorescence-Activated Cell Sorting and Plate Counting. Appl. Environ. Microbiol. 2011, 77, 4657–4668. [Google Scholar] [CrossRef] [Green Version]
- Ou, F.; McGoverin, C.; Swift, S.; Vanholsbeeck, F. Rapid and cost-effective evaluation of bacterial viability using fluorescence spectroscopy. Anal. Bioanal. Chem. 2019, 411, 3653–3663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poreba, M.; Groborz, K.; Rut, W.; Pore, M.; Snipas, S.J.; Vizovisek, M.; Turk, B.; Kuhn, P.; Drag, M.; Salvesen, G.S. Multiplexed Probing of Proteolytic Enzymes Using Mass Cytometry-Compatible Activity-Based Probes. J. Am. Chem. Soc. 2020, 142, 16704–16715. [Google Scholar] [CrossRef]
- Leipold, M.D.; Ornatsky, O.; Baranov, V.; Whitfield, C.; Nitz, M. Development of mass cytometry methods for bacterial discrimination. Anal. Biochem. 2011, 419, 1–8. [Google Scholar] [CrossRef]
- Beal, J.; Farny, N.G.; Haddock-Angelli, T.; Selvarajah, V.; Baldwin, G.S.; Buckley-Taylor, R.; Gershater, M.; Kiga, D.; Marken, J.; Sanchania, V.; et al. Robust estimation of bacterial cell count from optical density. Commun. Biol. 2020, 3, 512. [Google Scholar] [CrossRef]
- Ebersole, R.C.; McCormick, R.M. Separation and Isolation of Viable Bacteria by Capillary Zone Electrophoresis. Nat. Biotechnol. 1993, 11, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Kłodzińska, E.; Szumski, M.; Hrynkiewicz, K.; Dziubakiewicz, E.; Jackowski, M.; Buszewski, B. Differentiation of Staphylococcus aureus strains by CE, zeta potential and coagulase gene polymorphism. Electrophoresis 2009, 30, 3086–3091. [Google Scholar] [CrossRef] [PubMed]
- Szeliga, J.; Klodzinska, E.; Jackowski, M.; Buszewski, B. The clinical use of a fast screening test based on technology of capillary zone electrophoresis (CZE) for identifcation of esche-richia coli infection in biological material. Med. Sci. Monit. 2011, 17, MT91–MT96. [Google Scholar] [CrossRef] [Green Version]
- Buszewski, B.; Szumski, M.; Kłodzińska, E.; Dahm, H. Separation of bacteria by capillary electrophoresis. J. Sep. Sci. 2003, 26, 1045–1049. [Google Scholar] [CrossRef]
- Poortinga, A.T.; Bos, R.; Norde, W.; Busscher, H.J. Electric double layer interactions in bacterial adhesion to surfaces. Surf. Sci. Rep. 2002, 47, 1–32. [Google Scholar] [CrossRef]
- Hong, Y.; Brown, D. Electrostatic behavior of the charge-regulated bacterial cell surface. Langmuir 2008, 24, 5003–5009. [Google Scholar] [CrossRef]
- Ayala-Torres, C.; Hernández, N.; Galeano, A.; Novoa-Aponte, L.; Soto, C.-Y. Zeta potential as a measure of the surface charge of mycobacterial cells. Ann. Microbiol. 2014, 64, 1189–1195. [Google Scholar] [CrossRef]
- Kłodzińska, E.; Szumski, M.; Dziubakiewicz, E.; Hrynkiewicz, K.; Skwarek, E.; Janusz, W.; Buszewski, B. Effect of zeta potential value on bacterial behavior during electrophoretic separation. Electrophoresis 2010, 31, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.J.; Armstrong, D.W. Separation, identification, and characterization of microorganisms by capillary electrophoresis. Microbiol. Mol. Biol. Rev. 2003, 67, 38–51. [Google Scholar] [CrossRef] [Green Version]
- Buszewski, B.; Kłodzińska, E. Rapid microbiological diagnostics in medicine using electromigration techniques. TrAC Trends Anal. Chem. 2016, 78, 95–108. [Google Scholar] [CrossRef]
- Petr, J.; Maier, V. Analysis of microorganisms by capillary electrophoresis. TrAC Trends Anal. Chem. 2012, 31, 9–22. [Google Scholar] [CrossRef]
- Dziubakiewicz, E.; Buszewski, B. Capillary electrophoresis of microbial aggregates. Electrophoresis 2014, 35, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, A.; Pomastowski, P.; Zloch, M.; Railean-Plugaru, V.; Król, A.; Rafińska, K.; Szultka-Młyńska, M.; Buszewski, B. The influence of different pH on the electrophoretic behaviour of Saccharomyces cerevisiae modified by calcium ions. Sci. Rep. 2018, 8, 7261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crispo, F.; Capece, A.; Guerrieri, A.; Romano, P. Capillary zone electrophoresis as alternative tool for rapid identification and quantification of viable Saccharomyces cerevisiae cells. LWT 2016, 68, 506–513. [Google Scholar] [CrossRef]
- Bernatova, S.; Samek, O.; Pilát, Z.; Šerý, M.; Ježek, J.; Jákl, P.; Šiler, M.; Krzyzánek, V.; Zemánek, P.; Holá, V.; et al. Following the mechanisms of bacteriostatic versus bactericidal action using raman spectroscopy. Molecules 2013, 18, 13188–13199. [Google Scholar] [CrossRef]
- Weber, D.J.; Tolkoff-Rubin, N.E.; Rubin, R.H. Amoxicillin and potassium clavulanate: An antibiotic combination mechanism of action, pharmacokinetics, antimicrobial spectrum, clinical efficacy and adverse effects. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1984, 4, 122–133. [Google Scholar] [CrossRef]
- Lee, T.; Pang, S.; Abraham, S.; Coombs, G.W. Antimicrobial-resistant CC17 Enterococcus faecium: The past, the present and the future. J. Glob. Antimicrob. Resist. 2019, 16, 36–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campoli-Richards, D.M.; Monk, J.P.; Price, A.; Benfield, P.; Todd, P.A.; Ward, A. Ciprofloxacin. Drugs 1988, 35, 373–447. [Google Scholar] [CrossRef] [PubMed]
- Nodzo, S.R.; Boyle, K.K.; Frisch, N.B. Nationwide organism susceptibility patterns to common preoperative prophylactic antibiotics: What are we covering? J. Arthroplast. 2019, 34, S302–S306. [Google Scholar] [CrossRef] [PubMed]
- Leitsch, D. A review on metronidazole: An old warhorse in antimicrobial chemotherapy. Parasitology 2019, 146, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Greulich, P.; Doležal, J.; Scott, M.; Evans, M.R.; Allen, R.J. Predicting the dynamics of bacterial growth inhibition by ribosome-targeting antibiotics. Phys. Biol. 2017, 14, 065005. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupczyk, W.; Maślak, E.; Railean-Plugaru, V.; Pomastowski, P.; Jackowski, M.; Buszewski, B. Capillary Zone Electrophoresis in Tandem with Flow Cytometry in Viability Study of Various ATCC Bacterial Strains under Antibiotic Treatment. Int. J. Environ. Res. Public Health 2022, 19, 1833. https://doi.org/10.3390/ijerph19031833
Kupczyk W, Maślak E, Railean-Plugaru V, Pomastowski P, Jackowski M, Buszewski B. Capillary Zone Electrophoresis in Tandem with Flow Cytometry in Viability Study of Various ATCC Bacterial Strains under Antibiotic Treatment. International Journal of Environmental Research and Public Health. 2022; 19(3):1833. https://doi.org/10.3390/ijerph19031833
Chicago/Turabian StyleKupczyk, Wojciech, Ewelina Maślak, Viorica Railean-Plugaru, Paweł Pomastowski, Marek Jackowski, and Bogusław Buszewski. 2022. "Capillary Zone Electrophoresis in Tandem with Flow Cytometry in Viability Study of Various ATCC Bacterial Strains under Antibiotic Treatment" International Journal of Environmental Research and Public Health 19, no. 3: 1833. https://doi.org/10.3390/ijerph19031833






