Improving Timely Access to Diagnostic and Treatment Services for Lung Cancer Patients in KwaZulu-Natal, South Africa: Priority-Setting through Nominal Group Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
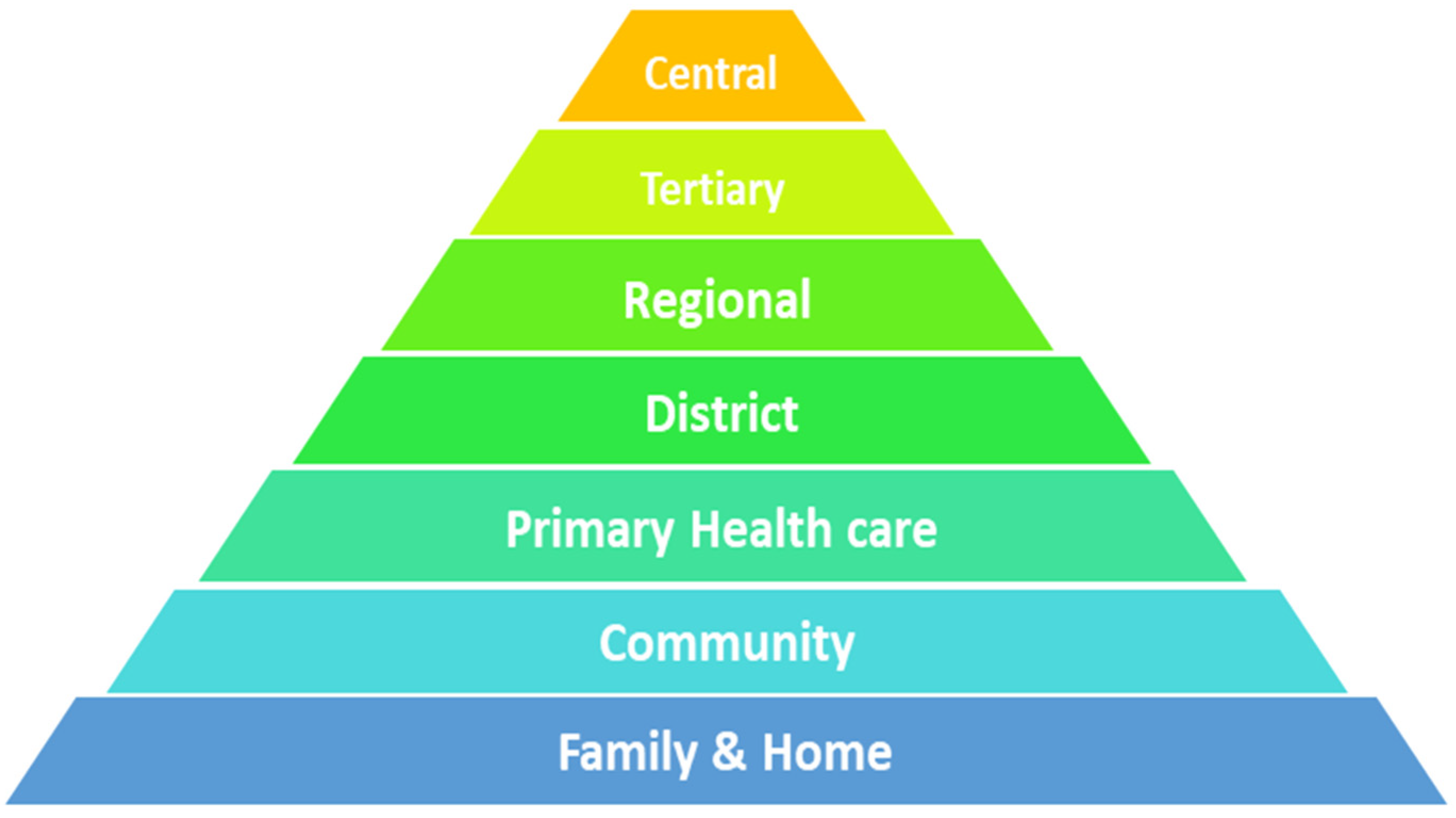
2.2. Study Setting
2.3. Study Design
2.4. Study Population and Sampling Strategy
2.5. Data Collection and Analysis
“Cancer cases, especially lung cancer, are often detected late, which in turn affects patient survivorship. In most instances, and as evidenced by the preliminary findings from the in-depth interviews the Principal Investigator presented, the late detection and survivorship challenges can be attributed to barriers related to the pathways of cancer care.
Kindly list all the barriers that you can think of, which negatively affect lung cancer patient access, diagnosis, referral and treatment in KwaZulu-Natal”.
2.6. Ethical Consideration
3. Results
3.1. Phase One
3.2. Phase Two
3.3. Phase Three
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. The Global Burden of Disease; World Health Organisation: Geneva, Switzerland, 2004. [Google Scholar]
- Fitzmaurice, C. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability- adjusted life-years for 32 cancer groups, 1990–2015: A systematic analysis for the global burden of diesease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [PubMed]
- Ferlay, J. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Fidler, M.M.; Soerjomataram, I.; Bray, F. A global view on cancer incidence and national levels of the human development index. Int. J. Cancer 2016, 139, 2436–2446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torre, L.A. Global cancer statistics, 2012. CA A Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Sylla, B.S.; Wild, C.P. A million africans a year dying from cancer by 2030: What can cancer research and control offer to the continent? Int. J. Cancer 2012, 130, 245–250. [Google Scholar] [CrossRef]
- Sun, S.; Schiller, J.H.; Gazdar, A.F. Lung cancer in never smokers—A different disease. Nat. Rev. Cancer 2007, 7, 778–790. [Google Scholar] [CrossRef]
- Dunn, J.; Garvey, G.; Valery, P.C.; Ball, D.; Fong, K.M.; Vinod, S.; O’Connell, D.L.; Chambers, S.K. Barriers to lung cancer care: Health professionals’ perspectives. Support Care Cancer 2017, 25, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Urman, A.; Josyula, S.; Rosenberg, A.; Lounsbury, D.; Rohan, T.; Hosgood, D. Burden of Lung Cancer and Associated Risk Factors in Africa by Region. J. Pulm. Respir. Med. 2016, 6, 340. [Google Scholar] [CrossRef] [Green Version]
- Global Health Metrics. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef] [Green Version]
- Bello, B.; Fadahun, O.; Kielkowski, D.; Nelson, G. Trends in lung cancer mortality in South Africa: 1995-2006. BMC Public Health 2011, 11, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradshaw, D.; Groenewald, P.; Laubscher, R.; Nannan, N.; Nojilana, B.; Norman, R.; Pieterse, D.; Schneider, M.; Bourne, D.E.; Timaeus, I.M.; et al. Initial burden of disease estimates for South Africa, 2000. S. Afr. Med. J. 2003, 93, 682–688. [Google Scholar] [PubMed]
- Cheyip, M.Y.; Nelson, G.; Ross, M.H.; Murray, J. South African platinum mine employees reduce smoking in 5 years. Tob. Control 2007, 16, 197–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coovadia, H.; Jewkes, R.; Barron, P.; Sanders, D.; McIntyre, D. The health and health system of South Africa: Historical roots of current public health challenges. Lancet 2009, 374, 817–834. [Google Scholar] [CrossRef]
- Edwards, L.B.; Greeff, L.E. Exploring grassroots feedback about cancer challenges in South Africa: A discussion of themes derived from content thematic analysis of 316 photo-narratives. Pan Afr. Med. J. 2017, 28, 173. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.B.; Greeff, L.E. Evidence-based feedback about emotional cancer challenges experienced in South Africa: A qualitative analysis of 316 photovoice interviews. Glob. Public Health 2018, 13, 1409–1421. [Google Scholar] [CrossRef]
- Department of Health. National Cancer Strategic Framework in South Africa 2017–2022; Department of Health: Pretoria, South Africa, 2017. [Google Scholar]
- Hesse, M. Cancer is South Africa’s Biggest Killer; IOL: Johannesburg, South Africa, 2018. [Google Scholar]
- Jobson, M. Structure of the health System in South Africa; Khulumani Support Group: Pretoria, South Africa, 2015. [Google Scholar]
- Joffe, M.; Ayeni, O.; Norris, S.A.; McCormack, V.A.; Ruff, P.; Das, I.; Neugut, A.I.; Jacobson, J.S.; Cubasch, H. Barriers to early presentation of breast cancer among women in Soweto, South Africa. PLoS ONE 2018, 13, e0192071. [Google Scholar] [CrossRef] [Green Version]
- Le Roux, H.A.; Urry, R.J.; Sartorius, B.; Aldous, C. Prostate Cancer at a regional hospital in South Africa: We are only seeing the tip of the iceberg. S. Afr. J. Surg. 2015, 53, 57–62. [Google Scholar]
- Made, F.; Wilson, K.; Jina, R.; Tlotleng, N.; Jack, S.; Ntlebi, V.; Kootbodien, T. Distribution of cancer mortality rates by province in South Africa. Cancer Epidemiol. 2017, 51, 56–61. [Google Scholar] [CrossRef]
- Maree, J.E.; Moshima, D.; Ngubeni, M.; Zondi, L. On being a caregiver: The experiences of South African family caregivers caring for cancer patients. Eur. J. Cancer Care 2017, 27, e12801. [Google Scholar] [CrossRef]
- Mayosi, B.M.; Flisher, A.J.; Lalloo, U.G.; Sitas, F.; Tollman, S.M.; Bradshaw, D. The burden of non-communicable diseases in South Africa. Lancet 2009, 374, 934–947. [Google Scholar] [CrossRef]
- Momberg, M.; Botha, M.H.; Van der Merwe, F.H.; Moodley, J. Women’s experiences with cervical cancer screening in a colposcopy referral clinic in Cape Town, South Africa: A qualitative analysis. BMJ Open 2017, 7, e013914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moodley, J.; Cairncross, L.; Naiker, T.; Momberg, M. Understanding pathways to breast cancer diagnosis among women in the Western Cape Province, South Africa: A qualitative study. BMJ Open 2016, 6, e009905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moodley, J.; Stefan, D.C.; Sewram, V.; Ruff, P.; Freeman, M.; Asante-Shongwe, K. An overview of cancer research in South African academic and research institutions, 2013–2014. S. Afr. Med. J. 2016, 106, 607–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moodley, J.; Walter, F.M.; Scott, S.E.; Mwaka, A.M. Towards timely diagnosis of symptomatic breast and cervical cancer in South Africa. S. Afr. Med. J. 2018, 108, 803–804. [Google Scholar] [CrossRef]
- Mukansi, M.; Smith, C.; Feldman, C. A Study of lung cancer in Johannesburg, South Africa. S. Afr. J. Infect. Dis. 2014, 29, 43–47. [Google Scholar] [CrossRef]
- Neely, A.H.; Ponshunmugam, A. A qualitative approach to examining health care access in rural South Africa. Soc. Sci. Med. 2019, 230, 214–221. [Google Scholar] [CrossRef]
- Oodit, R.L.; Ljungqvist, O.; Moodley, J. Can an Enhanced Recovery After Surgery(ERAS) programme improve colorectal cancer outcomes in South Africa? S. Afr. J. Surg. 2018, 56, 8–11. [Google Scholar] [CrossRef] [Green Version]
- Padarath, A.; King, J.; Mackie, E.L.; Casciola, J. Overview of the 2016 South African Health Review. S. Afr. Med. J. 2016, 106, 655–657. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.; Abdel Goad, E.H.; Ramklass, S.S. Waiting times for prostate cancer diagnosis in KwaZulu-Natal, South Africa. S. Afr. Med. J. 2015, 105, 484–486. [Google Scholar] [CrossRef] [Green Version]
- Somdyala, N.I. Trends in cancer incidence in rural Eastern Cape province; South Africa, 1998-2012. Int. J. Cancer 2015, 36, E470–E474. [Google Scholar] [CrossRef]
- van Rensburg, H.C. South Africa’s protracted struggle for equal distribution and equitable access-still not there. Hum. Resour. Health 2014, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- van Walbeek, C. Recent trends in smoking prevalence in South Africa--some evidence from AMPS data. S. Afr. Med. J. Suid-Afrik. Tydskr. Vir Geneeskd. 2002, 92, 468–472. [Google Scholar]
- van Zyl-Smit, R.N.; Allwood, B.; Stickells, D.; Symons, G.; Abdool-Gaffar, S.; Murphy, K.; Lalloo, U.; Vanker, A.; Dheda, K.; Richards, G. South African Tobacco Smoking Cessation Clinical Practice Guideline. S. Afr. Med. J. 2013, 103, 869–876. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.K. The African Organisation for Research and Training in Cancer and its conferences: A historical perspective and highlights of the Ninth International Conference, Durban, South Africa, 21–24 November 2013. Ecancermedicalscience 2014, 8, 396. [Google Scholar] [PubMed]
- Winkler, V.; Mangolo, N.J.; Becher, H. Lung cancer in South Africa: A forecast to 2025 based on smoking prevalence data. BMJ Open 2015, 5, e006993. [Google Scholar] [CrossRef] [Green Version]
- World Health Organisation. South Africa: Globocan 2018; World Health Organisation: Geneva, Switzerland, 2018. [Google Scholar]
- Aberg, L.; Albrecht, B.; Rudolph, T. How Health Systems Can Improve Value in Cancer Care; Health International: Harare, Zimbabwe, 2012. [Google Scholar]
- World Health Organisation. Cancer; World Health Organisation: Geniva, Switzerland, 2018. [Google Scholar]
- Bradley, E.H.; Taylor, L.A.; Cuellar, C.J. Management Matters: A Leverage Point for Health Systems Strengthening in Global Health. Int. J. Health Policy Manag. 2015, 4, 411–415. [Google Scholar] [CrossRef] [Green Version]
- Kutzin, J.; Barnum, H. How Health Insurance Affects the Delivery of Health Care in Developing Countries; IDEAS: Washington, DC, USA, 1992. [Google Scholar]
- Gaafar, R. Lung Cancer in Africa: Challenges and Perspectives. J. Thorac. Oncol. 2017, 12, S115–S116. [Google Scholar] [CrossRef]
- The National Institute For Communicable Diseases. Cancer in South Africa; The National Institute For Communicable Diseases: Johannesburg, South Africa, 2014. [Google Scholar]
- Aggarwal, A.; Lewison, G.; Idir, S.; Peters, M.; Aldige, C.; Boerckel, W.; Boyle, P.; Trimble, E.L.; Roe, P.; Sethi, T.; et al. The State of Lung Cancer Research: A Global Analysis. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 1040–1050. [Google Scholar] [CrossRef] [Green Version]
- Lyratzopoulos, G.; Wardle, J.; Rubin, G. Rethinking diagnostic delay in cancer: How difficult is the diagnosis? BMJ (Clin. Res. Ed.) 2014, 349, g7400. [Google Scholar] [CrossRef] [Green Version]
- Rubin, G.; Berendsen, A.; Crawford, S.M.; Dommett, R.; Earle, C.; Emery, J.; Fahey, T.; Grassi, L.; Grunfeld, E.; Gupta, S.; et al. The expanding role of primary care in cancer control. Lancet Oncol. 2015, 16, 1231–1272. [Google Scholar] [CrossRef] [Green Version]
- Statistics South Africa. Mid-Year Population Estimates; Statistics South Africa: Pretoria, South Africa, 17 July 2018. [Google Scholar]
- Boddy, C. The nominal group technique: An aid to brainstorming ideas in research. Qual. Mark. Res. Int. J. 2012, 15, 6–18. [Google Scholar] [CrossRef] [Green Version]
- McMillan, S.S.; King, M.; Tully, M.P. How to use the nominal group and Delphi techniques. Int. J. Clin. Pharm. 2016, 38, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Sample, J.A. Nominal Group Technique: An Alternative to Brainstorming. J. Ext. 1984, 22. [Google Scholar]
- Varga-Atkins, T. The Nominal Group Technique: A Practical Guide for Facilitators; University of Liverpool: Liverpool, UK, 2011. [Google Scholar]
- Setia, M.S. Methodology Series Module 5: Sampling Strategies. Indian J. Dermatol. 2016, 61, 505–509. [Google Scholar] [CrossRef]
- Harvey, N.; Holmes, C.A. Nominal group technique: An effective method for obtaining group consensus. Int. J. Nurs. Pract. 2012, 18, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, M.M.; Silverstein, S.C.; Quinn, M.; Waterston, L.B.; Thomas, C.A.; Benneyan, J.C.; Han, P.K.J. Timeliness of access to lung cancer diagnosis and treatment: A scoping literature review. Lung Cancer 2017, 112, 156–164. [Google Scholar] [CrossRef]
- Hlongwana, K.; Lubuzo, B.; Mlaba, P.; Zondo, S.; Ginindza, T. Multistakeholder Experiences of Providing, Receiving, and Setting Priorities for Lung Cancer Care in KwaZulu-Natal Province, South Africa. JCO Glob. Oncol. 2020, 6, 25. [Google Scholar] [CrossRef]
- Jacobs, B.; Ir, P.; Bigdeli, M.; Annear, P.L.; Van Damme, W. Addressing access barriers to health services: An analytical framework for selecting appropriate interventions in low-income Asian countries. Health Policy Plan 2012, 27, 288–300. [Google Scholar] [CrossRef]
- Lubuzo, B.; Ginindza, T.; Hlongwana, K. Exploring barriers to lung cancer patient access, diagnosis, referral and treatment in Kwazulu-Natal, South Africa: The health providers’ perspectives. Transl. Lung Cancer Res. 2019, 8, 380–391. [Google Scholar] [CrossRef]
- Lubuzo, B.; Ginindza, T.; Hlongwana, K. The barriers to initiating lung cancer care in low-and middle-income countries. Pan Afr. Med. J. 2020, 35, 38. [Google Scholar] [CrossRef] [PubMed]



| Key Issues | Respondent 1 | Respondent 2 | Respondent 3 | Respondent 4 | Respondent 5 | Respondent 6 | Respondent 7 | Points | Ranking |
|---|---|---|---|---|---|---|---|---|---|
| A-Specialized Resources | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 26 | 1st |
| B-Screening Services | 4 | 2 | 6 | - | |||||
| C-Awareness | 2 | 1 | 2 | 4 | 2 | 3 | 14 | 2nd | |
| D-Referral Guidelines | 3 | 3 | 3 | 2 | 1 | 12 | 3rd | ||
| E-Education & Training | 1 | 2 | 1 | 3 | 7 | 4th | |||
| F-Co-ordinated Care Plan | 1 | 1 | 2 | 4 | - | ||||
| G-Financial Constraints | 1 | 1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubuzo, B.; Hlongwana, K.W.; Ginindza, T.G. Improving Timely Access to Diagnostic and Treatment Services for Lung Cancer Patients in KwaZulu-Natal, South Africa: Priority-Setting through Nominal Group Techniques. Int. J. Environ. Res. Public Health 2022, 19, 1918. https://doi.org/10.3390/ijerph19041918
Lubuzo B, Hlongwana KW, Ginindza TG. Improving Timely Access to Diagnostic and Treatment Services for Lung Cancer Patients in KwaZulu-Natal, South Africa: Priority-Setting through Nominal Group Techniques. International Journal of Environmental Research and Public Health. 2022; 19(4):1918. https://doi.org/10.3390/ijerph19041918
Chicago/Turabian StyleLubuzo, Buhle, Khumbulani W. Hlongwana, and Themba G. Ginindza. 2022. "Improving Timely Access to Diagnostic and Treatment Services for Lung Cancer Patients in KwaZulu-Natal, South Africa: Priority-Setting through Nominal Group Techniques" International Journal of Environmental Research and Public Health 19, no. 4: 1918. https://doi.org/10.3390/ijerph19041918
APA StyleLubuzo, B., Hlongwana, K. W., & Ginindza, T. G. (2022). Improving Timely Access to Diagnostic and Treatment Services for Lung Cancer Patients in KwaZulu-Natal, South Africa: Priority-Setting through Nominal Group Techniques. International Journal of Environmental Research and Public Health, 19(4), 1918. https://doi.org/10.3390/ijerph19041918







