Benthic Biofilm Bacterial Communities and Their Linkage with Water-Soluble Organic Matter in Effluent Receivers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site, Experimental Design, and Water Characteristics
2.2. Biofilm Harvesting and Water-Soluble Organic Matter (WSOM) Extraction
2.3. Spectral Analyses of Biofilm WSOM
2.4. Microbial Community Analysis
2.4.1. DNA Extraction and PCR Amplification
2.4.2. Sequence Analyses and Functional Prediction
2.5. Statistical Analyses
3. Results
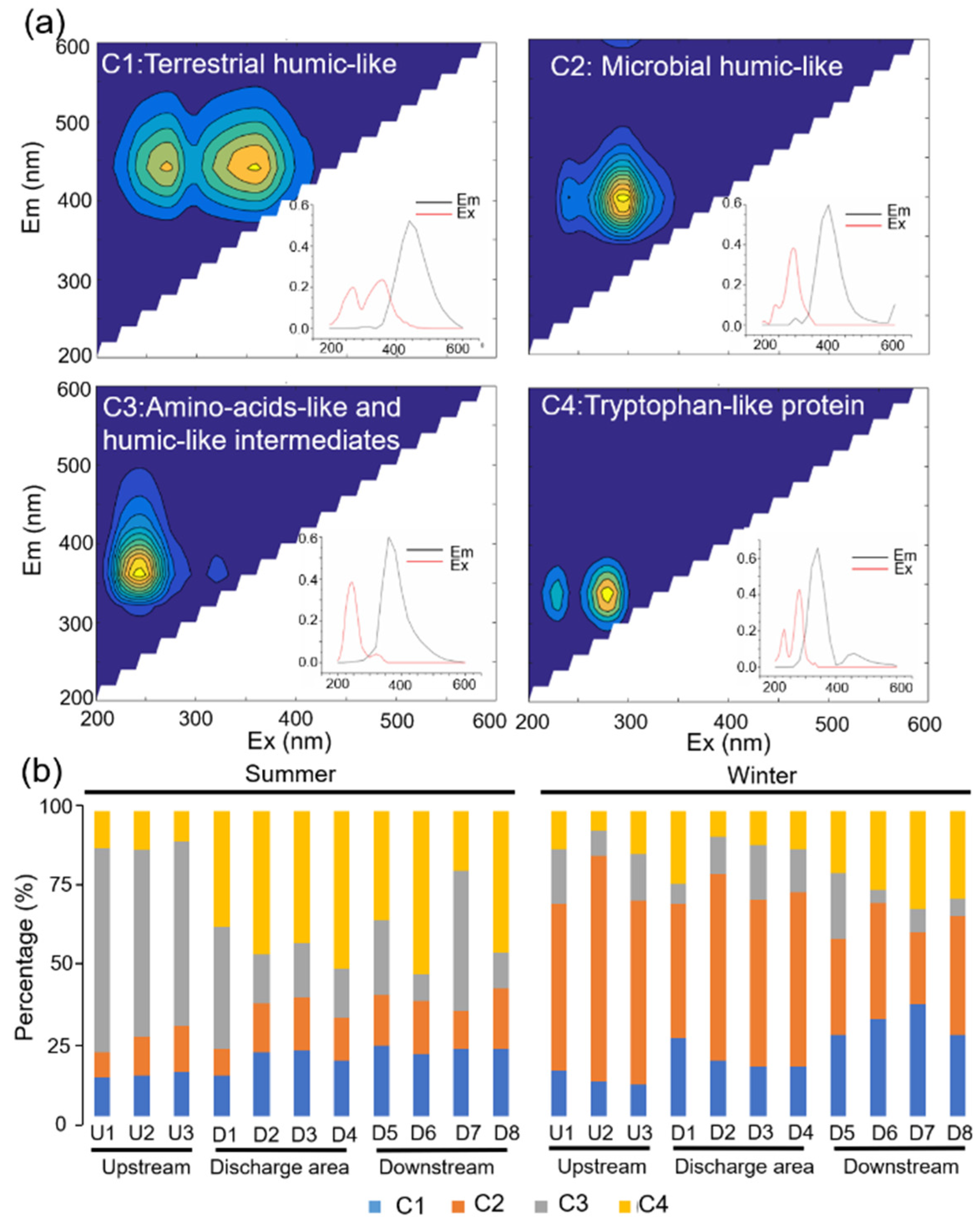
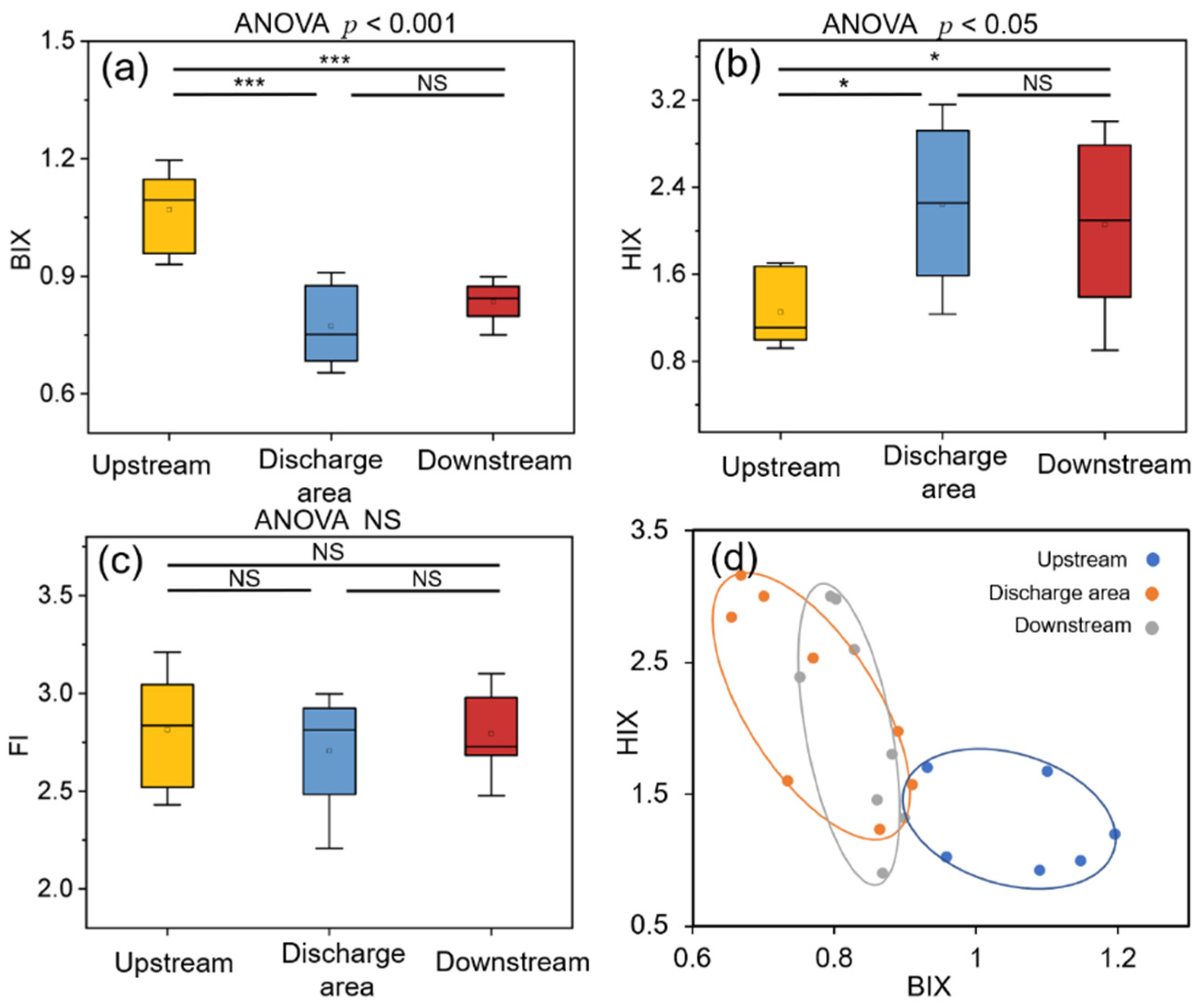
3.1. Spectral Characteristics of Biofilm WSOM
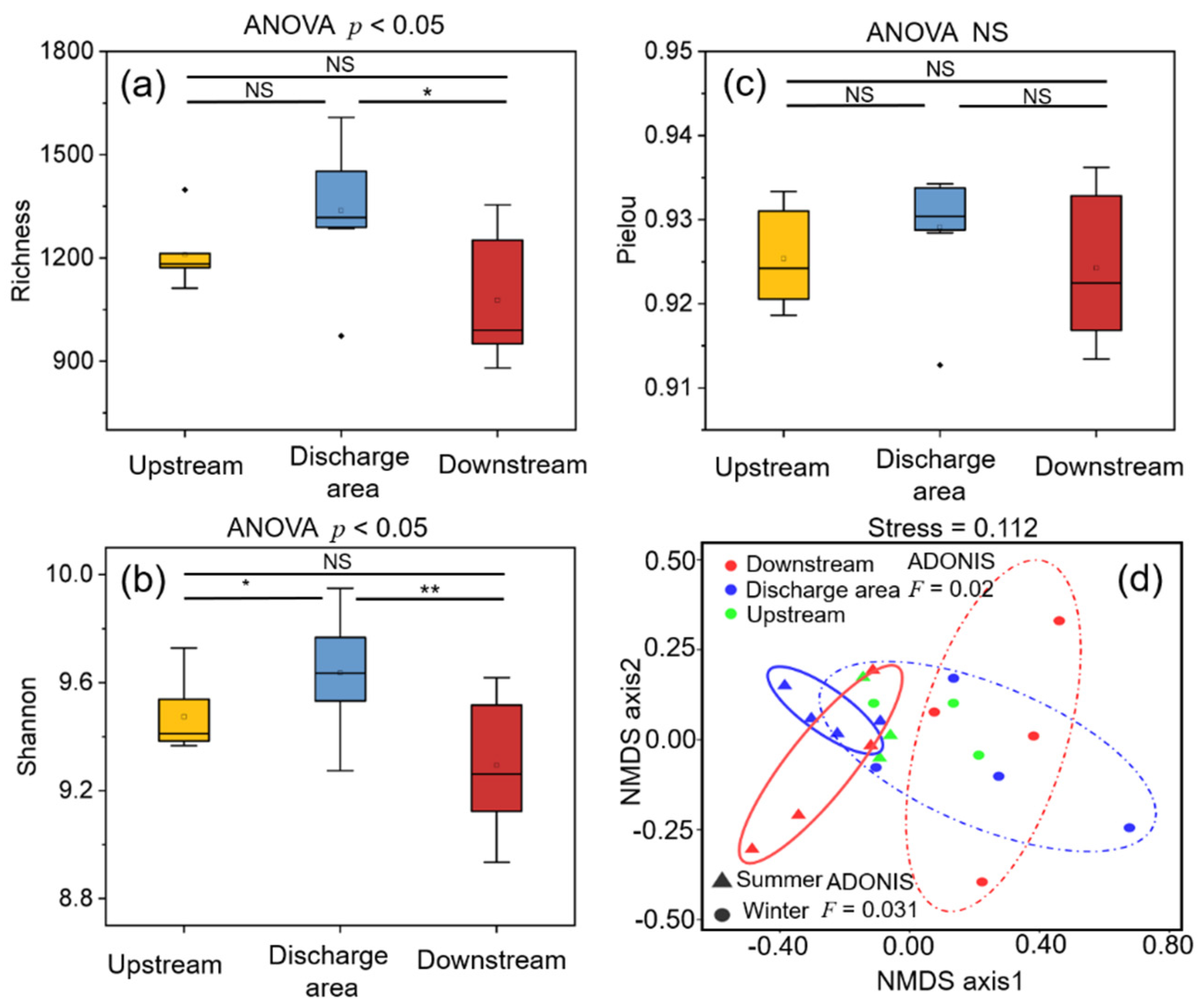
3.2. Dynamics, Diversity and Assembly Mechanisms of Bacterial Communities
3.3. Functional Prediction of Bacterial Communities
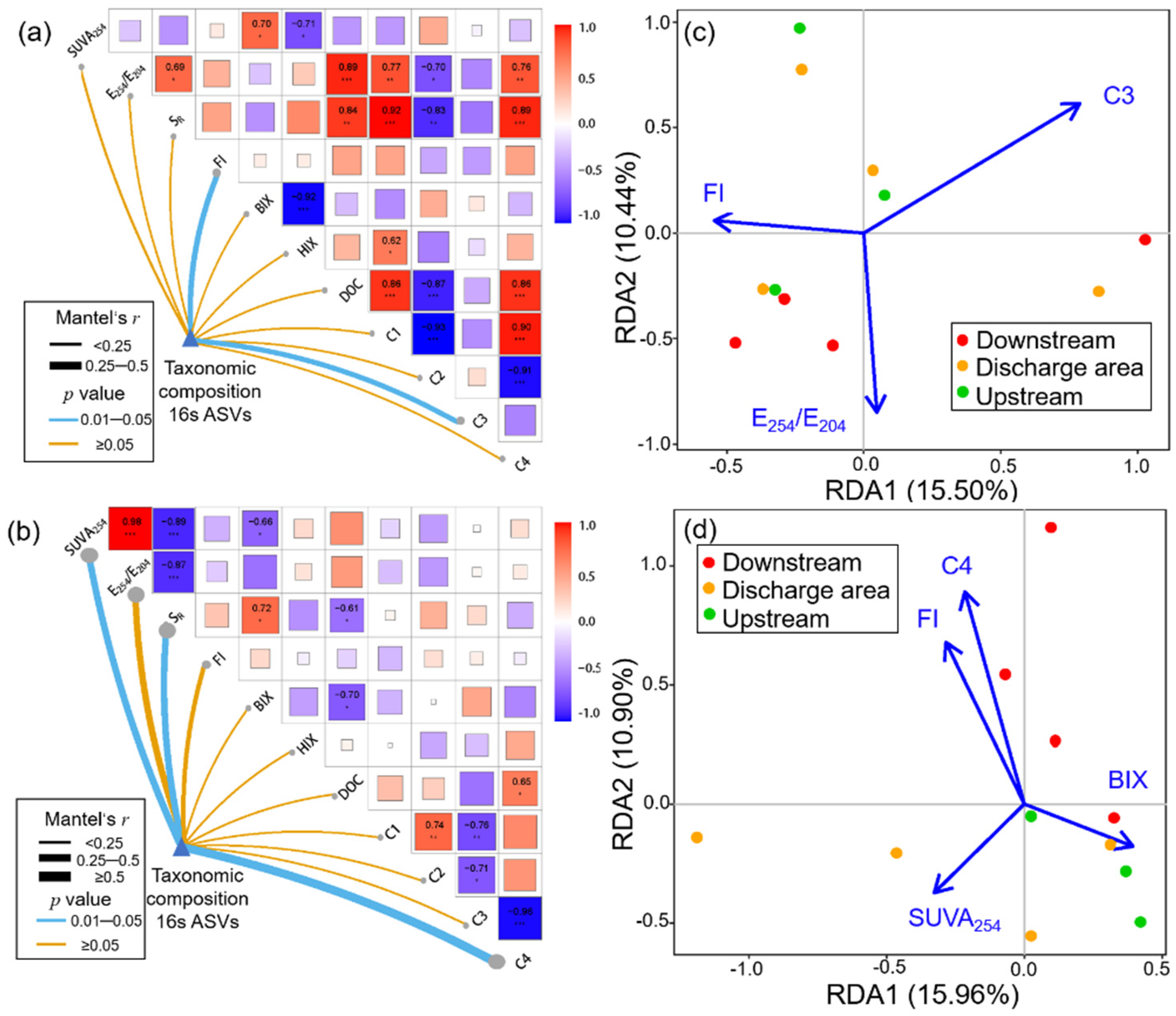
3.4. Key WSOM Parameters Affecting Bacterial Community Composition
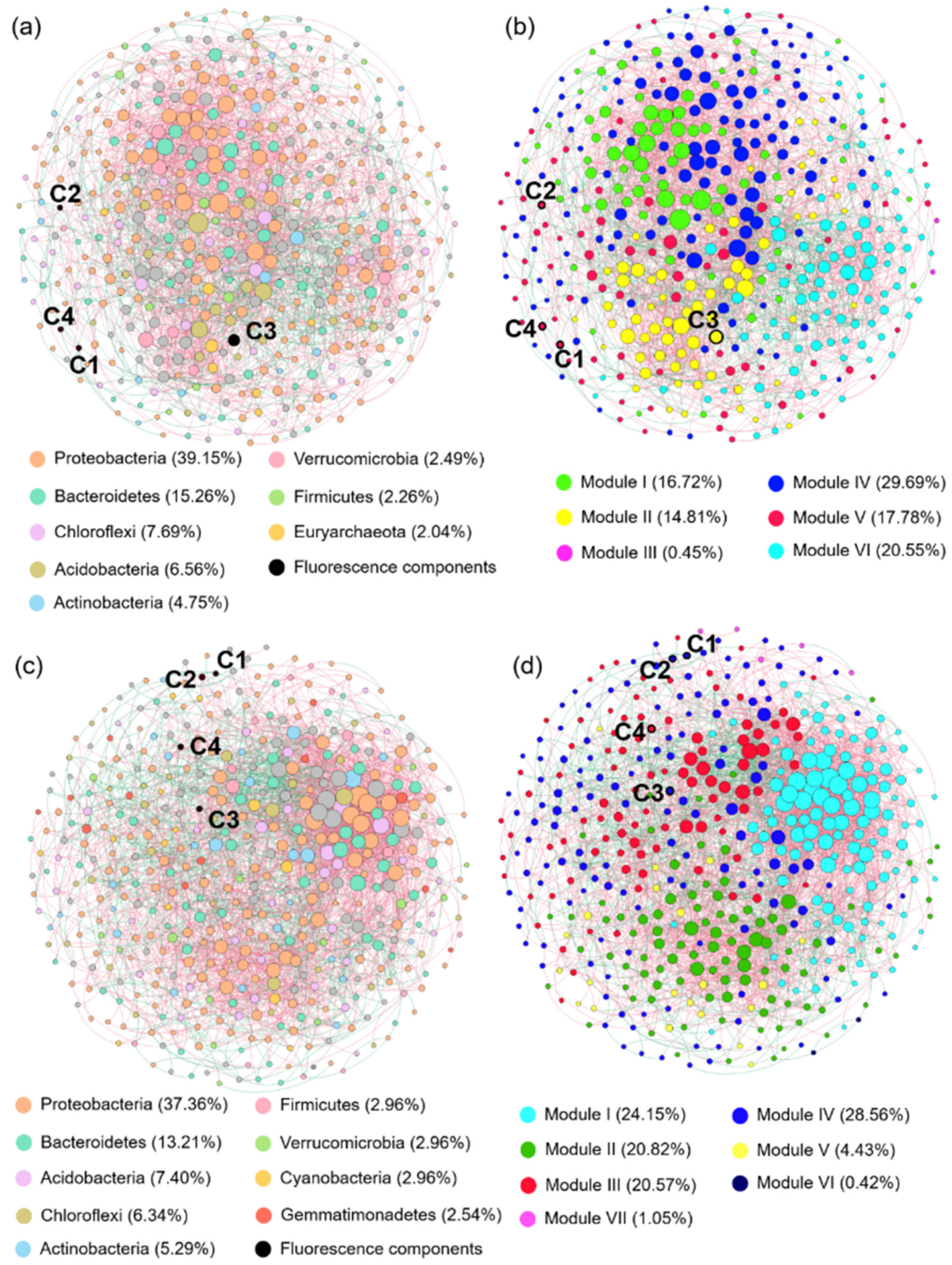
3.5. Co-Occurrence Network Analysis
4. Discussion
4.1. Effluent Discharge Alters the Nature of Biofilm WSOM
4.2. Response of Biofilm Bacterial Communities to Effluent Discharge
4.3. Variations in Metabolic Functions of Bacterial Communities
4.4. Roles of Bacteria in Shaping Biofilm WSOM
4.5. Co-Occurrence Patterns Relate to Seasonal and Spatial Variation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamjunke, N.; Hertkorn, N.; Harir, M.; Schmitt-Kopplin, P.; Griebler, C.; Brauns, M.; von Tumpling, W.; Weitere, M.; Herzsprung, P. Molecular change of dissolved organic matter and patterns of bacterial activity in a stream along a land-use gradient. Water Res. 2019, 164, 114919. [Google Scholar] [CrossRef] [PubMed]
- Burdon, F.J.; Bai, Y.; Reyes, M.; Tamminen, M.; Staudacher, P.; Mangold, S.; Singer, H.; Rasanen, K.; Joss, A.; Tiegs, S.D.; et al. Stream microbial communities and ecosystem functioning show complex responses to multiple stressors in wastewater. Glob. Chang. Biol. 2020, 26, 6363–6382. [Google Scholar] [CrossRef] [PubMed]
- Waiser, M.J.; Tumber, V.; Holm, J. Effluent-dominated streams. Part 1: Presence and effects of excess nitrogen and phosphorus in Wascana Creek, Saskatchewan, Canada. Environ. Toxicol. Chem. 2011, 30, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Freixa, A.; Perujo, N.; Langenheder, S.; Romani, A.M. River biofilms adapted to anthropogenic disturbances are more resistant to WWTP inputs. FEMS Microbiol. Ecol. 2020, 96, 152. [Google Scholar] [CrossRef] [PubMed]
- Drury, B.; Rosi-Marshall, E.; Kelly, J.J. Wastewater treatment effluent reduces the abundance and diversity of benthic bacterial communities in urban and suburban rivers. Appl. Environ. Microbiol. 2013, 79, 1897–1905. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Liu, S.; Li, G.; Zhang, H.; Xi, B.; Tian, Z.; Zhang, Y.; He, X. Municipal wastewater effluent influences dissolved organic matter quality and microbial community composition in an urbanized stream. Sci. Total. Environ. 2020, 705, 135952. [Google Scholar] [CrossRef]
- Pereda, O.; Solagaistua, L.; Atristain, M.; de Guzman, I.; Larranaga, A.; von Schiller, D.; Elosegi, A. Impact of wastewater effluent pollution on stream functioning: A whole-ecosystem manipulation experiment. Environ. Pollut. 2020, 258, 113719. [Google Scholar] [CrossRef]
- Romero, F.; Acuna, V.; Font, C.; Freixa, A.; Sabater, S. Effects of multiple stressors on river biofilms depend on the time scale. Sci. Rep. 2019, 9, 15810. [Google Scholar] [CrossRef] [Green Version]
- Osorio, V.; Proia, L.; Ricart, M.; Perez, S.; Ginebreda, A.; Cortina, J.L.; Sabater, S.; Barcelo, D. Hydrological variation modulates pharmaceutical levels and biofilm responses in a Mediterranean river. Sci. Total. Environ. 2014, 472, 1052–1061. [Google Scholar] [CrossRef]
- Sabater-Liesa, L.; Montemurro, N.; Font, C.; Ginebreda, A.; Gonzalez-Trujillo, J.D.; Mingorance, N.; Perez, S.; Barcelo, D. The response patterns of stream biofilms to urban sewage change with exposure time and dilution. Sci. Total. Environ. 2019, 674, 401–411. [Google Scholar] [CrossRef]
- Pereda, O.; von Schiller, D.; Garcia-Baquero, G.; Mor, J.R.; Acuna, V.; Sabater, S.; Elosegi, A. Combined effects of urban pollution and hydrological stress on ecosystem functions of Mediterranean streams. Sci. Total. Environ. 2021, 753, 141971. [Google Scholar] [CrossRef] [PubMed]
- Battin, T.J.; Besemer, K.; Bengtsson, M.M.; Romani, A.M.; Packmann, A.I. The ecology and biogeochemistry of stream biofilms. Nat. Rev. Microbiol. 2016, 14, 251–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besemer, K. Biodiversity, community structure and function of biofilms in stream ecosystems. Res. Microbiol. 2015, 166, 774–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risse-Buhl, U.; Anlanger, C.; Kalla, K.; Neu, T.R.; Noss, C.; Lorke, A.; Weitere, M. The role of hydrodynamics in shaping the composition and architecture of epilithic biofilms in fluvial ecosystems. Water Res. 2017, 127, 211–222. [Google Scholar] [CrossRef]
- Sabater, S.; Guasch, H.; Ricart, M.; Romani, A.; Vidal, G.; Klunder, C.; Schmitt-Jansen, M. Monitoring the effect of chemicals on biological communities. The biofilm as an interface. Anal. Bioanal. Chem. 2007, 387, 1425–1434. [Google Scholar] [CrossRef]
- Pu, Y.; Ngan, W.Y.; Yao, Y.; Habimana, O. Could benthic biofilm analyses be used as a reliable proxy for freshwater environmental health? Environ. Pollut. 2019, 252 (Pt A), 440–449. [Google Scholar] [CrossRef]
- Hobbs, W.O.; Collyard, S.A.; Larson, C.; Carey, A.J.; O’Neill, S.M. Toxic Burdens of Freshwater Biofilms and Use as a Source Tracking Tool in Rivers and Streams. Environ. Sci. Technol. 2019, 53, 11102–11111. [Google Scholar] [CrossRef]
- Tlili, A.; Corcoll, N.; Arrhenius, Å.; Backhaus, T.; Hollender, J.; Creusot, N.; Wagner, B.; Behra, R. Tolerance Patterns in Stream Biofilms Link Complex Chemical Pollution to Ecological Impacts. Environ. Sci. Technol. 2020, 54, 10745–10753. [Google Scholar] [CrossRef]
- Qiu, L.; Cui, H.; Wu, J.; Wang, B.; Zhao, Y.; Li, J.; Jia, L.; Wei, Z. Snowmelt-driven changes in dissolved organic matter and bacterioplankton communities in the Heilongjiang watershed of China. Sci. Total. Environ. 2016, 556, 242–251. [Google Scholar] [CrossRef]
- Kamjunke, N.; Herzsprung, P.; Neu, T.R. Quality of dissolved organic matter affects planktonic but not biofilm bacterial production in streams. Sci. Total. Environ. 2015, 506–507, 353–360. [Google Scholar] [CrossRef]
- Logue, J.B.; Stedmon, C.A.; Kellerman, A.M.; Nielsen, N.J.; Andersson, A.F.; Laudon, H.; Lindstrom, E.S.; Kritzberg, E.S. Experimental insights into the importance of aquatic bacterial community composition to the degradation of dissolved organic matter. ISME J. 2016, 10, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.P.; Brandao, L.P.M.; Brighenti, L.S.; Tonetta, D.; Reis, M.P.; Staehr, P.A.; Asmala, E.; Amado, A.M.; Barbosa, F.A.R.; Bezerra-Neto, J.F.; et al. Linking shifts in bacterial community with changes in dissolved organic matter pool in a tropical lake. Sci. Total. Environ. 2019, 672, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fang, W.; Li, X.; Lu, W.; Li, J. Strong linkages between dissolved organic matter and the aquatic bacterial community in an urban river. Water Res. 2020, 184, 116089. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Li, Y.; Li, Y.; Hu, Q.; Wang, C.D.; Hu, J.; Zhang, W.; Wang, L.; Zhang, C.; Zhang, H. New insights into the vertical distribution and microbial degradation of microplastics in urban river sediments. Water Res. 2021, 188, 116449. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chu, S.S.; He, D.; Wu, D.M.; Mo, Q.F.; Zeng, S.C. Sewage sludge application alters the composition and co-occurrence pattern of the soil bacterial community in southern China forestlands. Appl. Soil Ecol. 2021, 157, 103744. [Google Scholar] [CrossRef]
- Zhou, H.; Gao, Y.; Jia, X.H.; Wang, M.M.; Ding, J.J.; Cheng, L.; Bao, F.; Wu, B. Network analysis reveals the strengthening of microbial interaction in biological soil crust development in the Mu Us Sandy Land, northwestern China. Soil Biol. Biochem. 2020, 144, 107782. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, J.; Wu, Q.; Ai, Y.; Huang, Y.; Wei, W.; Sun, S.; Weng, Q. Co-occurrence pattern and function prediction of bacterial community in Karst cave. BMC Microbiol. 2020, 20, 137. [Google Scholar] [CrossRef]
- Fang, D.; Hao, L.; Cao, Z.; Huang, X.L.; Qin, M.S.; Hu, J.C.; Liu, Y.Q.; Sun, G. Combined effects of urbanization and climate change on watershed evapotranspiration at multiple spatial scales. J. Hydrol. 2020, 587, 124869. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Wang, J.; Meier, S.; Soininen, J.; Casamayor, E.O.; Pan, F.; Tang, X.; Yang, X.; Zhang, Y.; Wu, Q.; Zhou, J.; et al. Regional and global elevational patterns of microbial species richness and evenness. Ecography 2017, 40, 393–402. [Google Scholar] [CrossRef]
- Chantigny, M.H.; Harrison-Kirk, T.; Curtin, D.; Beare, M. Temperature and duration of extraction affect the biochemical composition of soil water-extractable organic matter. Soil Biol. Biochem. 2014, 75, 161–166. [Google Scholar] [CrossRef]
- Huang, M.; Chai, L.; Jiang, D.; Zhang, M.; Jia, W.; Huang, Y. Spatial Patterns of Soil Fungal Communities Are Driven by Dissolved Organic Matter (DOM) Quality in Semi-Arid Regions. Microb Ecol. 2021, 82, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr-Meth. 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Author Correction: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 1091. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Global Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Konopka, A.E.; Fredrickson, J.K. Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J. 2012, 6, 1653–1664. [Google Scholar] [CrossRef] [Green Version]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Chen, X.; Kennedy, D.W.; Murray, C.J.; Rockhold, M.L.; Konopka, A. Quantifying community assembly processes and identifying features that impose them. ISME J. 2013, 7, 2069–2079. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Luo, F.; He, Z.; Tu, Q.; Zhi, X. Functional molecular ecological networks. MBio 2010, 1, 10. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Jiang, Y.H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinformatics 2012, 13, 113. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Bai, C.; Cai, J.; Dai, J.; Shao, K.; Tang, X.; Gao, G. Co-occurrence network reveals the higher fragmentation of the bacterial community in Kaidu River than its tributaries in Northwestern China. Microbes Environ. 2018, 33, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kothawala, D.N.; von Wachenfeldt, E.; Koehler, B.; Tranvik, L.J. Selective loss and preservation of lake water dissolved organic matter fluorescence during long-term dark incubations. Sci. Total Environ. 2012, 433, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Osburn, C.L.; Wigdahl, C.R.; Fritz, S.C.; Saros, J.E. Dissolved organic matter composition and photoreactivity in prairie lakes of the U.S. Great Plains. Limnol. Oceanogr. 2011, 56, 2371–2390. [Google Scholar] [CrossRef] [Green Version]
- Retelletti Brogi, S.; Jung, J.Y.; Ha, S.Y.; Hur, J. Seasonal differences in dissolved organic matter properties and sources in an Arctic fjord: Implications for future conditions. Sci. Total Environ. 2019, 694, 133740. [Google Scholar] [CrossRef]
- Jørgensen, L.; Stedmon, C.A.; Kragh, T.; Markager, S.; Middelboe, M.; Søndergaard, M. Global trends in the fluorescence characteristics and distribution of marine dissolved organic matter. Marine Chemistry 2011, 126, 139–148. [Google Scholar] [CrossRef]
- Hambly, A.C.; Arvin, E.; Pedersen, L.F.; Pedersen, P.B.; Seredynska-Sobecka, B.; Stedmon, C.A. Characterising organic matter in recirculating aquaculture systems with fluorescence EEM spectroscopy. Water Res. 2015, 83, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Weisenhorn, P.; Gilbert, J.A.; Chu, H. Wheat rhizosphere harbors a less complex and more stable microbial co-occurrence pattern than bulk soil. Soil Biol. Biochem. 2018, 125, 251–260. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y. Generation and characterization of DOM in wastewater treatment processes. Chemosphere 2018, 201, 96–109. [Google Scholar] [CrossRef]
- Simsek, H.; Wadhawan, T.; Khan, E. Overlapping photodegradable and biodegradable organic nitrogen in wastewater effluents. Environ. Sci. Technol. 2013, 47, 7163–7170. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Geng, J.; Li, S.; Yu, Q.; Xu, K.; Ren, H. Distribution and removal of fluorescent dissolved organic matter in 15 municipal wastewater treatment plants in China. Chemosphere 2020, 251, 126375. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Yang, C.; Wang, Q.; Jiang, D. Variations of DOM quantity and compositions along WWTPs-river-lake continuum: Implications for watershed environmental management. Chemosphere 2019, 218, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, T.; Zhang, J.; Qin, Y.; Ly, Q.V.; Asif, M.B.; Zhang, X.; Zhang, Z. Seasonal occurrence of N-nitrosamines and their association with dissolved organic matter in full-scale drinking water systems: Determination by LC-MS and EEM-PARAFAC. Water Res. 2020, 183, 116096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fang, W.; Li, X.; Gao, G.; Jiang, J. Linking bacterial community shifts with changes in the dissolved organic matter pool in a eutrophic lake. Sci. Total Environ. 2020, 719, 137387. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Z.; Raju, M.N.; Cai, X.; Meng, F. Selective elimination of chromophoric and fluorescent dissolved organic matter in a full-scale municipal wastewater treatment plant. J. Environ. Sci. 2017, 57, 150–161. [Google Scholar] [CrossRef]
- Perujo, N.; Freixa, A.; Vivas, Z.; Gallegos, A.M.; Butturini, A.; Romaní, A.M. Fluvial biofilms from upper and lower river reaches respond differently to wastewater treatment plant inputs. Hydrobiologia 2015, 765, 169–183. [Google Scholar] [CrossRef]
- Pascual-Benito, M.; Balleste, E.; Monleon-Getino, T.; Urmeneta, J.; Blanch, A.R.; Garcia-Aljaro, C.; Lucena, F. Impact of treated sewage effluent on the bacterial community composition in an intermittent mediterranean stream. Environ. Pollut. 2020, 266 Pt 1, 115254. [Google Scholar] [CrossRef]
- Zhang, W.; Lei, M.; Li, Y.; Wang, P.; Wang, C.; Gao, Y.; Wu, H.; Xu, C.; Niu, L.; Wang, L.; et al. Determination of vertical and horizontal assemblage drivers of bacterial community in a heavily polluted urban river. Water Res. 2019, 161, 98–107. [Google Scholar] [CrossRef]
- Brislawn, C.J.; Graham, E.B.; Dana, K.; Ihardt, P.; Fansler, S.J.; Chrisler, W.B.; Cliff, J.B.; Stegen, J.C.; Moran, J.J.; Bernstein, H.C. Forfeiting the priority effect: Turnover defines biofilm community succession. ISME J. 2019, 13, 1865–1877. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Zhang, S.; Meng, F. Large-sized planktonic bioaggregates possess high biofilm formation potentials: Bacterial succession and assembly in the biofilm metacommunity. Water Res. 2020, 170, 115307. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Zhang, W.; Lin, L.; Wang, L.; Niu, L.; Zhang, H.; Wang, P.; Wang, C. Response of bacterial community in composition and function to the various DOM at river confluences in the urban area. Water Res. 2020, 169, 115293. [Google Scholar] [CrossRef]
- Neis, E.P.; Dejong, C.H.; Rensen, S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera, J.M.; Garcia, P.E.; Pedrozo, F.L.; Queimalinos, C.P. Dynamics of the dissolved organic matter in a stream-lake system within an extremely acid to neutral pH range: Agrio-Caviahue watershed. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2020, 235, 118278. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, Y.; Tang, X.; Zhang, Y.; Jang, K.S.; Szekely, A.J.; Jeppesen, E. Resource aromaticity affects bacterial community successions in response to different sources of dissolved organic matter. Water Res. 2021, 190, 116776. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Lee, T.H.; Chen, Y.L.; Wang, Y.S.; Wang, P.H.; Yu, C.P.; Chu, K.H.; Chiang, Y.R. Metabolites Involved in Aerobic Degradation of the A and B Rings of Estrogen. Appl. Environ. Microb. 2019, 85. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Cao, C.; Wang, Y.H.; Yu, K.; Liu, C.; He, C.; Shi, Q.; Wang, J.J. Chemodiversity of water-extractable organic matter in sediment columns of a polluted urban river in South China. Sci. Total Environ. 2021, 777, 146127. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, W.; Li, Y.; Zhang, C.; Wang, L.; Niu, L.; Zhang, H. Microbial community shift via black carbon: Insight into biological nitrogen removal from microbial assemblage and functional patterns. Environ. Res. 2021, 192, 110266. [Google Scholar] [CrossRef]
- Morrien, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; de Hollander, M.; Soto, R.L.; Bouffaud, M.L.; Buee, M.; Dimmers, W.; et al. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef]
- Yang, W.; Jing, X.; Guan, Y.; Zhai, C.; Wang, T.; Shi, D.; Sun, W.; Gu, S. Response of Fungal Communities and Co-occurrence Network Patterns to Compost Amendment in Black Soil of Northeast China. Front. Microbiol. 2019, 10, 1562. [Google Scholar] [CrossRef]
- Zhu, P.; Li, Y.; Gao, Y.; Yin, M.; Wu, Y.; Liu, L.; Du, N.; Liu, J.; Yu, X.; Wang, L. Insight into the effect of nitrogen-rich substrates on the community structure and the co-occurrence network of thermophiles during lignocellulose-based composting. Bioresour. Technol. 2021, 319, 124111. [Google Scholar] [CrossRef]
- Newton, R.J.; Jones, S.E.; Eiler, A.; McMahon, K.D.; Bertilsson, S. A guide to the natural history of freshwater lake bacteria. Microbiol. Mol. Biol. Rev. 2011, 75, 14–49. [Google Scholar] [CrossRef] [Green Version]
- Kirchman, D.L. The ecology of Cytophaga-Flavobacteria in aquatic environments. FEMS Microbiol. Ecol. 2002, 39, 91–100. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, H.; Liu, W.; Huang, L.; Huang, J.; Wang, B.; Dong, H.; Chu, R.K.; Tolic, N. Potential utilization of terrestrially derived dissolved organic matter by aquatic microbial communities in saline lakes. ISME J. 2020, 14, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- Schellenberger, S.; Kolb, S.; Drake, H.L. Metabolic responses of novel cellulolytic and saccharolytic agricultural soil Bacteria to oxygen. Environ. Microbiol. 2009, 12, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Joung, Y.; Kim, H.; Kang, H.; Lee, B.I.; Ahn, T.S.; Joh, K. Lacihabitans soyangensis gen. nov.; sp. nov.; a new member of the family Cytophagaceae, isolated from a freshwater reservoir. Int. J. Syst. Evol. Microbiol. 2014, 64 Pt 9, 3188–3194. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Luo, F.; He, Z.; Yang, Y. Phylogenetic molecular ecological network of soil microbial communities in response to elevated CO2. mBio 2011, 2, e00122-11. [Google Scholar] [CrossRef] [Green Version]
- Dedysh, S.N.; Haupt, E.S.; Dunfield, P.F. Emended description of the family Beijerinckiaceae and transfer of the genera Chelatococcus and Camelimonas to the family Chelatococcaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 3177–3182. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The Genus Aeromonas: Taxonomy, Pathogenicity, and Infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zepp, R.G.; Sheldon, W.M.; Moran, M.A. Dissolved organic fluorophores in southeastern US coastal waters: Correction method for eliminating Rayleigh and Raman scattering peaks in excitation–emission matrices. Mar. Chem. 2004, 89, 15–36. [Google Scholar]
- Lee, M.H.; Lee, Y.K.; Derrien, M.; Choi, K.; Shin, K.H.; Jang, K.S.; Hur, J. Evaluating the contributions of different organic matter sources to urban river water during a storm event via optical indices and molecular composition. Water Res. 2019, 165, 115006. [Google Scholar]
- Ye, Q.H.; Wang, Y.H.; Zhang, Z.T.; Huang, W.L.; Li, L.P.; Li, J.T.; Liu, J.S.; Zheng, Y.; Mo, J.M.; Zhang, W.; et al. Dissolved organic matter characteristics in soils of tropical legume and non-legume tree plantations. Soil Biol. Biochem. 2020, 148, 107880. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, Y.; Li, Y.; Zhang, W.; Zhang, H.; Niu, L.; Habibul, N. Benthic Biofilm Bacterial Communities and Their Linkage with Water-Soluble Organic Matter in Effluent Receivers. Int. J. Environ. Res. Public Health 2022, 19, 1994. https://doi.org/10.3390/ijerph19041994
Wang L, Wang Y, Li Y, Zhang W, Zhang H, Niu L, Habibul N. Benthic Biofilm Bacterial Communities and Their Linkage with Water-Soluble Organic Matter in Effluent Receivers. International Journal of Environmental Research and Public Health. 2022; 19(4):1994. https://doi.org/10.3390/ijerph19041994
Chicago/Turabian StyleWang, Longfei, Yutao Wang, Yi Li, Wenlong Zhang, Huanjun Zhang, Lihua Niu, and Nuzahat Habibul. 2022. "Benthic Biofilm Bacterial Communities and Their Linkage with Water-Soluble Organic Matter in Effluent Receivers" International Journal of Environmental Research and Public Health 19, no. 4: 1994. https://doi.org/10.3390/ijerph19041994
APA StyleWang, L., Wang, Y., Li, Y., Zhang, W., Zhang, H., Niu, L., & Habibul, N. (2022). Benthic Biofilm Bacterial Communities and Their Linkage with Water-Soluble Organic Matter in Effluent Receivers. International Journal of Environmental Research and Public Health, 19(4), 1994. https://doi.org/10.3390/ijerph19041994




