A Clue on the Skin: A Systematic Review on Immunohistochemical Analyses of the Ligature Mark
Abstract
:1. Introduction
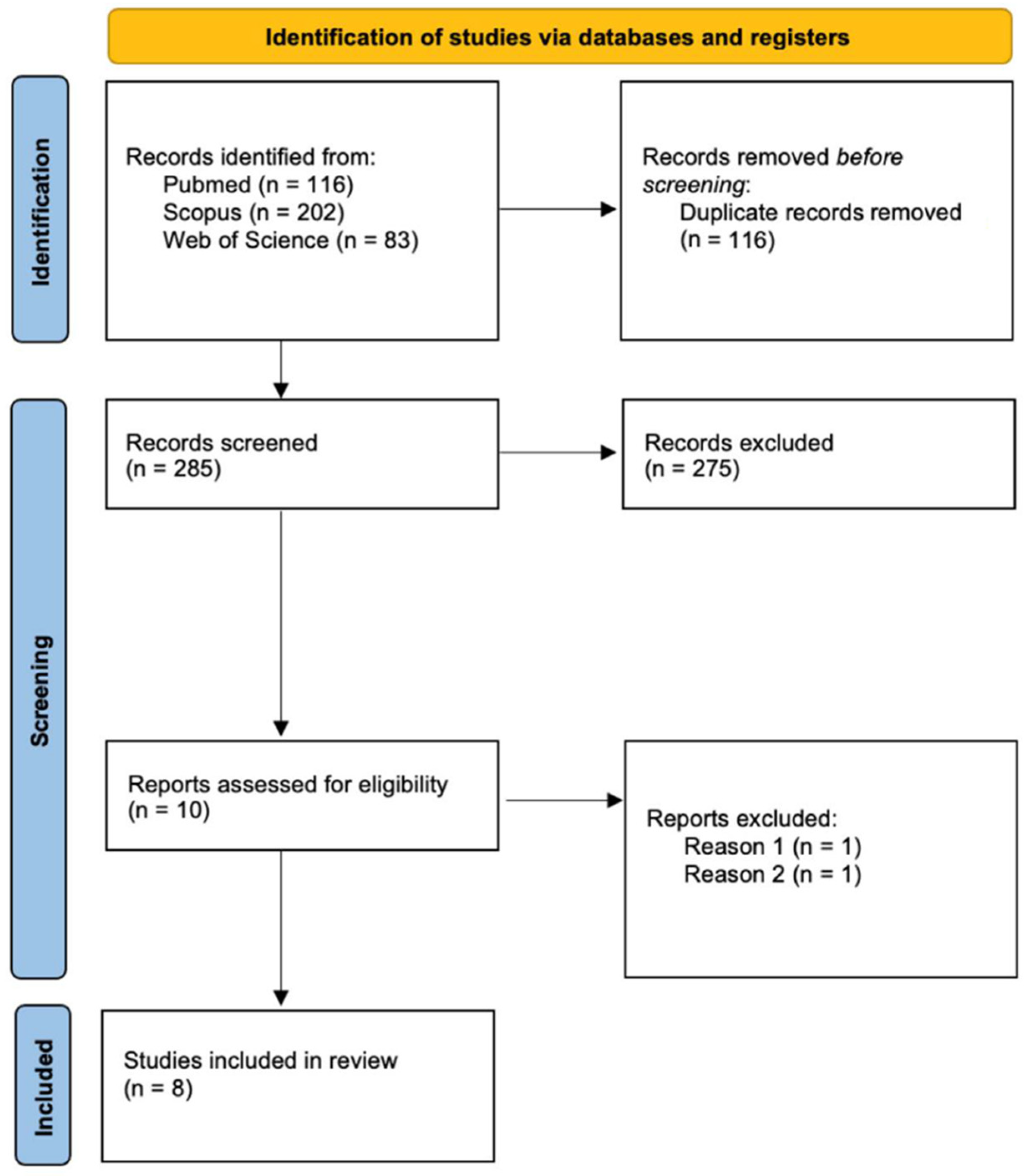
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Di Maio, V.J.M.; Di Maio, D.J. Forensic Pathology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Ishida, Y.; Kuninaka, Y.; Nosaka, M.; Shimada, E.; Hata, S.; Yamamoto, H.; Hashizume, Y.; Kimura, A.; Furukawa, F.; Kondo, T. Forensic application of epidermal AQP3 expression to determination of wound vitality in human compressed neck skin. Int. J. Leg. Med. 2018, 132, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Dettmeyer, R.B.; Verhoff, M.A.; Schutz, H.F. Forensic Medicine; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Legaz, I.; Pérez-Cárceles, M.P.; Gimenez, M.; Martínez-Díaz, F.; Osuna, E.; Luna, A. Immunohistochemistry as a tool to characterize human skin wounds of hanging marks. Rom. J. Leg. Med. 2018, 26, 354–358. [Google Scholar]
- Langlois, N.E.; Gresham, G.A. The ageing of bruises: A review and study of the colour changes with time. Forensic Sci. Int. 1991, 50, 227–238. [Google Scholar] [CrossRef]
- Focardi, M.; Puliti, E.; Grifoni, R.; Palandri, M.; Bugelli, V.; Pinchi, V.; Norelli, G.A.; Bacci, S. Immunohistochemical localization of Langerhans cells as a tool for vitality in hanging mark wounds: A pilot study. Aust. J. Forensic Sci. 2020, 52, 393–405. [Google Scholar] [CrossRef]
- Madea, B.; Doberentz, E.; Jackowski, C. Vital reactions—An updated overview. Forensic Sci. Int. 2019, 305, 110029. [Google Scholar] [CrossRef]
- Sauvageau, A.; Racette, S. Agonal sequences in a filmed suicidal hanging: Analysis of respiratory and movement responses to asphyxia by hanging. J. Forensic Sci. 2007, 52, 957–959. [Google Scholar] [CrossRef]
- Cecchi, R. Estimating wound age: Looking into the future. Int. J. Leg. Med. 2010, 124, 523–536. [Google Scholar] [CrossRef]
- Dettmeyer, R.B. Forensic Histopathology; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Campobasso, C.P.; Colonna, M.F.; Zotti, F.; Sblano, S.; Dell’Erba, A.S. An immunohistochemical study of pulmonary surfactant apoprotein A (SP-A) in forensic autopsy materials. Rom. J. Leg. Med. 2012, 20, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sblano, S.; Campobasso, C.P.; Zotti, F.; Arpaio, A.; Di Vella, G.; Colonna, M.F. Beta-APP immunoreactivity as diagnostic tool of diffuse axonal injury (DAI). Rom. J. Leg. Med. 2012, 20, 89–94. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Focardi, M.; Bugelli, V.; Venturini, M.; Bianchi, I.; Defraia, B.; Pinchi, V.; Bacci, S. Increased expression of iNOS by Langerhans cells in hanging marks. Aust. J. Forensic Sci. 2020. [Google Scholar] [CrossRef]
- Maiese, A.; De Matteis, A.; Bolino, G.; Turillazzi, E.; Frati, P.; Fineschi, V. Hypo-Expression of Flice-Inhibitory Protein and Activation of the Caspase-8 Apoptotic Pathways in the Death-Inducing Signaling Complex Due to Ischemia Induced by the Compression of the Asphyxiogenic Tool on the Skin in Hanging Cases. Diagnostics 2020, 10, 938. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Fabbri, M.; D’Errico, S.; Di Paolo, M.; Frati, P.; Gaudio, R.M.; La Russa, R.; Maiese, A.; Marti, M.; Pinchi, E.; et al. Regulation of miRNAs as new tool for cutaneous vitality lesions demonstration in ligature marks in deaths by hanging. Sci. Rep. 2019, 9, 20011. [Google Scholar] [CrossRef] [PubMed]
- Turillazzi, E.; Vacchiano, G.; Luna-Maldonado, A.; Neri, M.; Pomara, C.; Rabozzi, R.; Riezzo, I.; Fineschi, V. Tryptase, CD-15 and IL-15 as reliable markers for the determination of soft and hard ligature marks vitality. Histol. Histopathol. 2010, 25, 1539–1546. [Google Scholar] [CrossRef]
- Mansueto, G.; Di Napoli, M.; Mascolo, P.; Carfora, A.; Zangani, P.; Della Pietra, B.; Campobasso, C.P. Electrocution Stigmas in Organ Damage: The Pathological Marks. Diagnostics 2021, 11, 682. [Google Scholar] [CrossRef]
- Buonomo, R.; Giacco, F.; Vasaturo, A.; Caserta, S.; Guido, S.; Pagliara, V.; Garbi, C.; Mansueto, G.; Cassese, A.; Perruolo, G.; et al. PED/PEA-15 controls fibroblast motility and wound closure by ERK1/2-dependent mechanisms. J. Cell. Physiol. 2012, 227, 2106–2116. [Google Scholar] [CrossRef] [Green Version]
- Mansueto, G.; Costa, D.; Capasso, E.; Varavallo, F.; Brunitto, G.; Caserta, R.; Esposito, S.; Niola, M.; Sardu, C.; Marfella, R.; et al. The dating of thrombus organization in cases of pulmonary embolism: An autopsy study. BMC Cardiovasc. Disord. 2019, 19, 250. [Google Scholar] [CrossRef]
- Sen, C.K.; Roy, S. OxymiRs in cutaneous development, wound repair and regeneration. Semin. Cell Dev. Biol. 2012, 23, 971–980. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, J.; Sen, C.K. MicroRNA and Wound Healing. Adv. Exp. Med. Biol. 2015, 888, 291–305. [Google Scholar] [CrossRef] [Green Version]
- Betz, P. Immunohistochemical parameters for the age estimation of human skin wounds. Am. J. Forensic Med. Pathol. 1995, 16, 203–209. [Google Scholar] [CrossRef]
- Cao, C.; Wan, S.; Jiang, Q.; Amaral, A.; Lu, S.; Hu, G.; Bi, Z.; Kouttab, N.; Chu, W.; Wan, Y. All-trans retinoic acid attenuates ultraviolet radiation-induced down-regulation of aquaporin-3 and water permeability in human keratinocytes. J. Cell. Physiol. 2008, 215, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Legaz Pérez, I.; Falcón, M.; Gimenez, M.; Diaz, F.M.; Pérez-Cárceles, M.D.; Osuna, E.; Nuno-Vieira, D.; Luna, A. Diagnosis of Vitality in Skin Wounds in the Ligature Marks Resulting from Suicide Hanging. Am. J. Forensic Med. Pathol. 2017, 38, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Grellner, W.; Madea, B. Demands on scientific studies: Vitality of wounds and wound age estimation. Forensic Sci. Int. 2007, 165, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Casse, J.M.; Martrille, L.; Vignaud, J.M.; Gauchotte, G. Skin wounds vitality markers in forensic pathology: An updated review. Med. Sci. Law 2016, 56, 128–137. [Google Scholar] [CrossRef] [PubMed]


| Reference | Ligature Marks (Skin Sample n.) | Control Group (Skin Sample n.) | H&E | IH/IF (Abs) | Results |
|---|---|---|---|---|---|
| Focardi M. et al., 2021 [14] | 10 cases of suicidal hanging | 10 samples taken 20 cm below the ligature marks; 10 vital lesions (surgical wounds, abrasions, and lacerations of the knee or ankles); 10 samples of post-mortem wounds | √ | CD1a, iNOS, MHC-II, UEA, Spm250. | The inflammatory infiltrate is greater in vital wounds and in hanging ligature than in controls and post-mortem injuries. Dendritic cells in the spinous or granular layers of the epidermis in hanging marks were similar to vital wounds. Langerhans cells express iNOS more in the hanging grooves than other groups of lesions; while the percentage of these cells that do not express the enzyme is similar to the hanging marks and vital wounds, it is lower in the controls and higher in post-mortal lesions. The keratinocytes’ expression of iNOS is similar in all groups of lesions. Mast cells express iNOS in vital wounds in very few cellular types, but in hanging furrows, the expression of iNOS characterizes a large fraction of the Mast cells population. |
| Maiese et al., 2020 [15] | 21 cases of suicidal hanging | 13 skin samples taken from victims of opioid overdoses, car accidents, sudden cardiac death, and post-mortem suspension of bodies | √ | FLIP (ab8421), Tryptase, CD15. | Flattening of the epidermal layers with liquid-filled vesicles; mild leukocyte reactions. Intracytoplasmic depletion of FLIP in the epidermal layers from hanging. |
| Focardi M. et al., 2020 [6] | 20 cases of suicidal hanging (10 from superficial layers and 10 from deep layers of the skin) | 10 samples of vital wounds, abrasions and lacerations 10 samples of post-mortem wounds | √ | MHC-II, CD1a | Different expression of MHC class II from dendritic cells, Langerhans cells, and macrophages in the skin 20 cm far from the wound; in the vital wound; in the ligature mark, and in post-mortem lesion. Dendritic cells CD1a+ is mostly present in vital lesions and hanging marks. |
| Neri M. et al., 2019 [16] | 36 ligature marks | 28 samples of the not-injured skin of the neck | √ | Tryptase, IL-15, andCD15. | CD15 (neutrophils), tryptase (mast cells), and IL-15 are strongly positive in the marginal zones above and below the hanging marks. IL-15 positivity is in the dermis and around the vessels. CD15 and tryptase are intensely positive in the dermal connective tissue. CD15 and IL-15 are co-expressed and, there is increased expression of miR125a-5p and miR125b-5p. |
| Ishida Y. et al., 2018 [2] | 35 cases of hanging; 21 cases of ligature strangulation | Samples of not-injured skin from the same corpse | n.d. | AQP1 and AQP3 | The AQP3 expression on keratinocytes is enhanced in ligature marks. |
| Legaz I. et al., 2018 [4] | 15 cases of suicidal hanging | 15 samples of not-injured skin from the same corpses | n.d. | Fibronectin, Cathepsin D, and P-selectin | Fibronectin is strongly positive in the derma of ligature marks. Cathepsin D expression is increased in the basal layer of the epidermis in ligature marks. P-selectin expression is decreased in the basal layer of the epidermis in ligature marks. |
| Turillazzi E. et al., 2010 [17] | 49 cases of suicidal hanging | 14 samples of not-injured skin of the neck; 7 samples of post-mortem hanging | √ | Tryptase, Fibronectin, TNFα, IL-6, IL-8, IL-10, IL-15, IL-1ß, MCP-1, CD45, CD4, CD3, CD8, CD68, CD20, and CD15. | CD15, tryptase, and IL-15 are strongly positive in the dermal of marginal zones above and below the hanging marks. IL-15 is located around the dermal vessels and is diffusely sparse in depth. CD15 and tryptase positivity is in dermal connective tissue. CD15 and IL- 15 are co-expressed. |
| Marker | Role, Characteristics and Cell Type Expression |
|---|---|
| CD1A | It is a membrane glycoprotein related to the MHC that mediates the presentation of antigens to T cells. It is expressed by thymocytes, dendritic cells, and Langerhans cells. |
| iNOS | It is an isozyme synthase involved in the immune response by binding calmodulin and producing NO (nitric oxide). It is induced in different cell types of the cardiovascular system like endothelial cells and in immune cells. |
| FLIP | It is an anti-apoptotic protein produced by different cell types. It regulates the activity of caspase-8 and modulates the apoptotic signal mediated by TRAIL-R1/R2 (death receptors), TNFR1 (TNF receptor), and Toll-like receptors. |
| Tryptase | It is a serine protease present in the granules of the cytoplasm of mast cells; it is implicated in hypersensitivity reactions and anaphylaxis. |
| AQP1/AQP3 | The aquaporins (AQPs) are a family of channel proteins involved in the passage of water through the cells. They are predominantly expressed by the epithelium of the proximal renal tubules, erythrocytes (AQPs), and visceral pleura epithelium (AQP1) and by the epithelium of the trachea, bronchi, and nasopharynx (AQP3). |
| Fibronectin | It is a glycoprotein of the extracellular matrix (connective tissue) that plays a fundamental role in the repair of tissue damage. It is produced by many cell types. |
| Cathepsin D | It is a lysosomal aspartyl protease which degrades fibronectin and laminin. It is present in cell lysosomes and involved in the pathogenesis of several diseases such as breast cancer and possibly Alzheimer disease. |
| P-selectin | It is a membrane protein that acts as a cell adhesion molecule on the surfaces of endothelial cells and activated platelets. |
| IL-15 | It is a cytokine mainly produced by macrophages. It promotes cell survival of natural killer (NK) lymphocytes and activates the differentiation of NK lymphocytes. |
| CD15 | It is an adhesion molecule produced by granulocytes implicated in phagocytosis and chemotaxis. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansueto, G.; Feola, A.; Zangani, P.; Porzio, A.; Carfora, A.; Campobasso, C.P. A Clue on the Skin: A Systematic Review on Immunohistochemical Analyses of the Ligature Mark. Int. J. Environ. Res. Public Health 2022, 19, 2035. https://doi.org/10.3390/ijerph19042035
Mansueto G, Feola A, Zangani P, Porzio A, Carfora A, Campobasso CP. A Clue on the Skin: A Systematic Review on Immunohistochemical Analyses of the Ligature Mark. International Journal of Environmental Research and Public Health. 2022; 19(4):2035. https://doi.org/10.3390/ijerph19042035
Chicago/Turabian StyleMansueto, Gelsomina, Alessandro Feola, Pierluca Zangani, Antonietta Porzio, Anna Carfora, and Carlo Pietro Campobasso. 2022. "A Clue on the Skin: A Systematic Review on Immunohistochemical Analyses of the Ligature Mark" International Journal of Environmental Research and Public Health 19, no. 4: 2035. https://doi.org/10.3390/ijerph19042035








