Experimental and Numerical Study of the Laminar Burning Velocity and Pollutant Emissions of the Mixture Gas of Methane and Carbon Dioxide
Abstract
:1. Introduction
2. Materials and Method
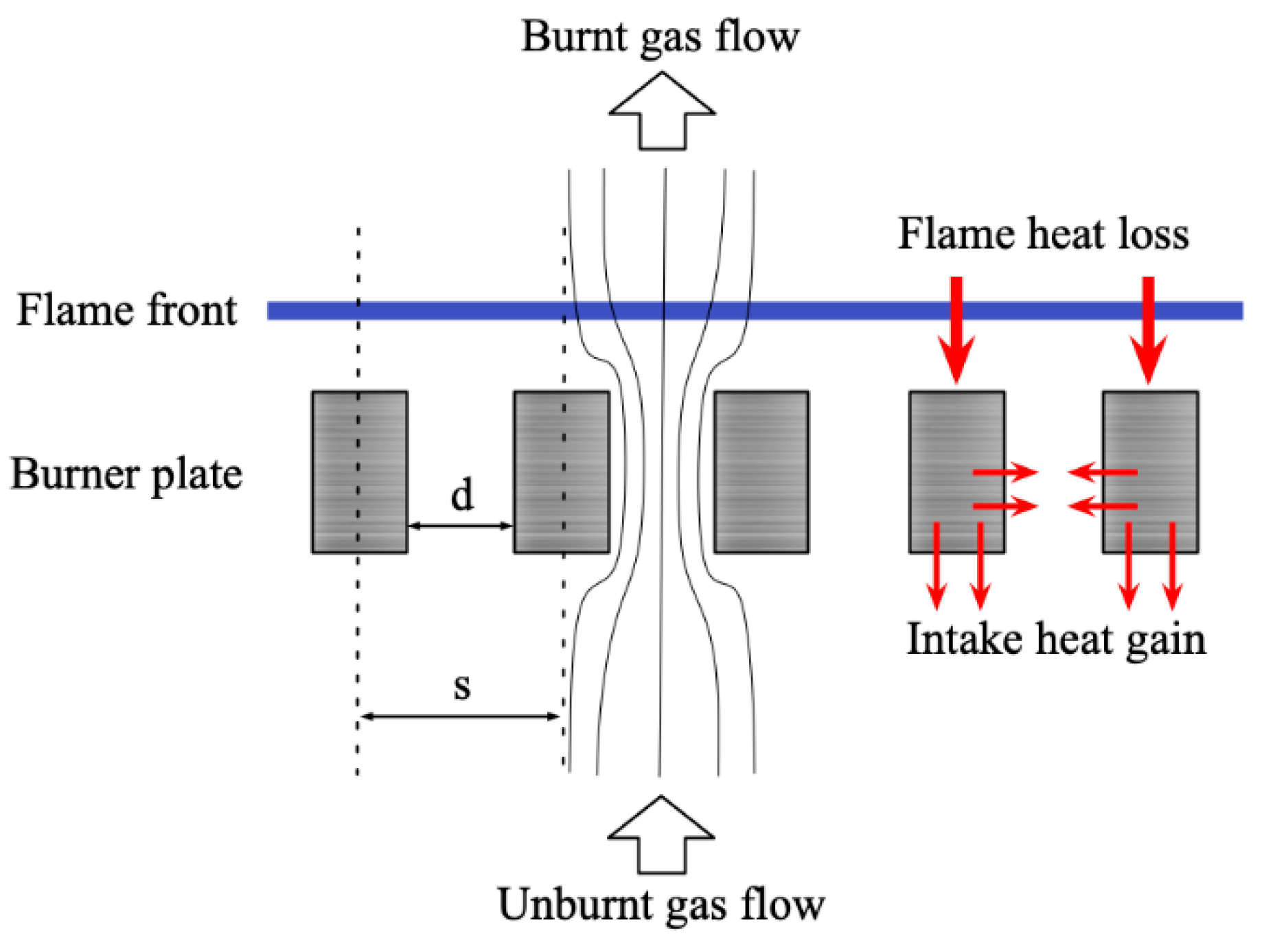
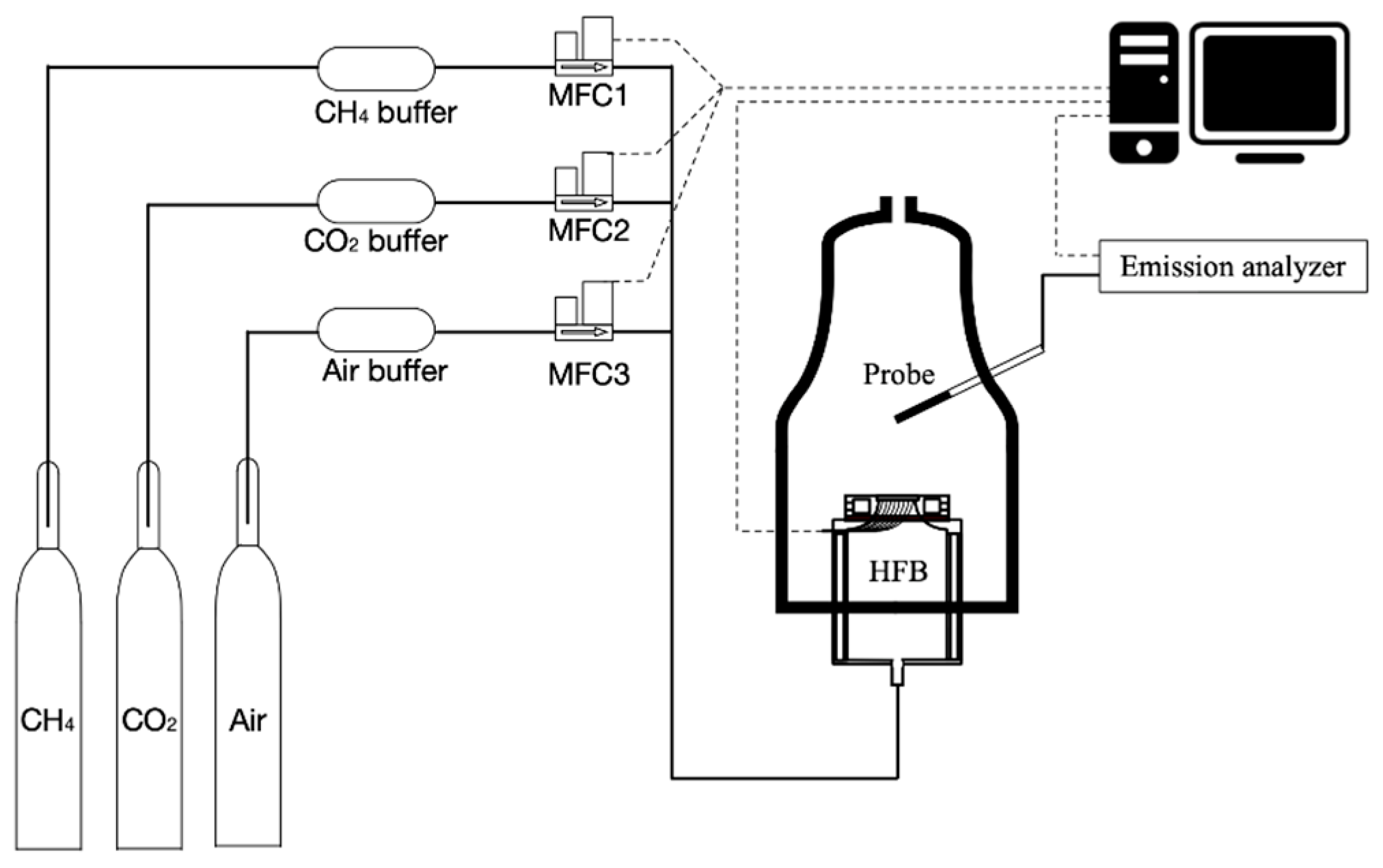
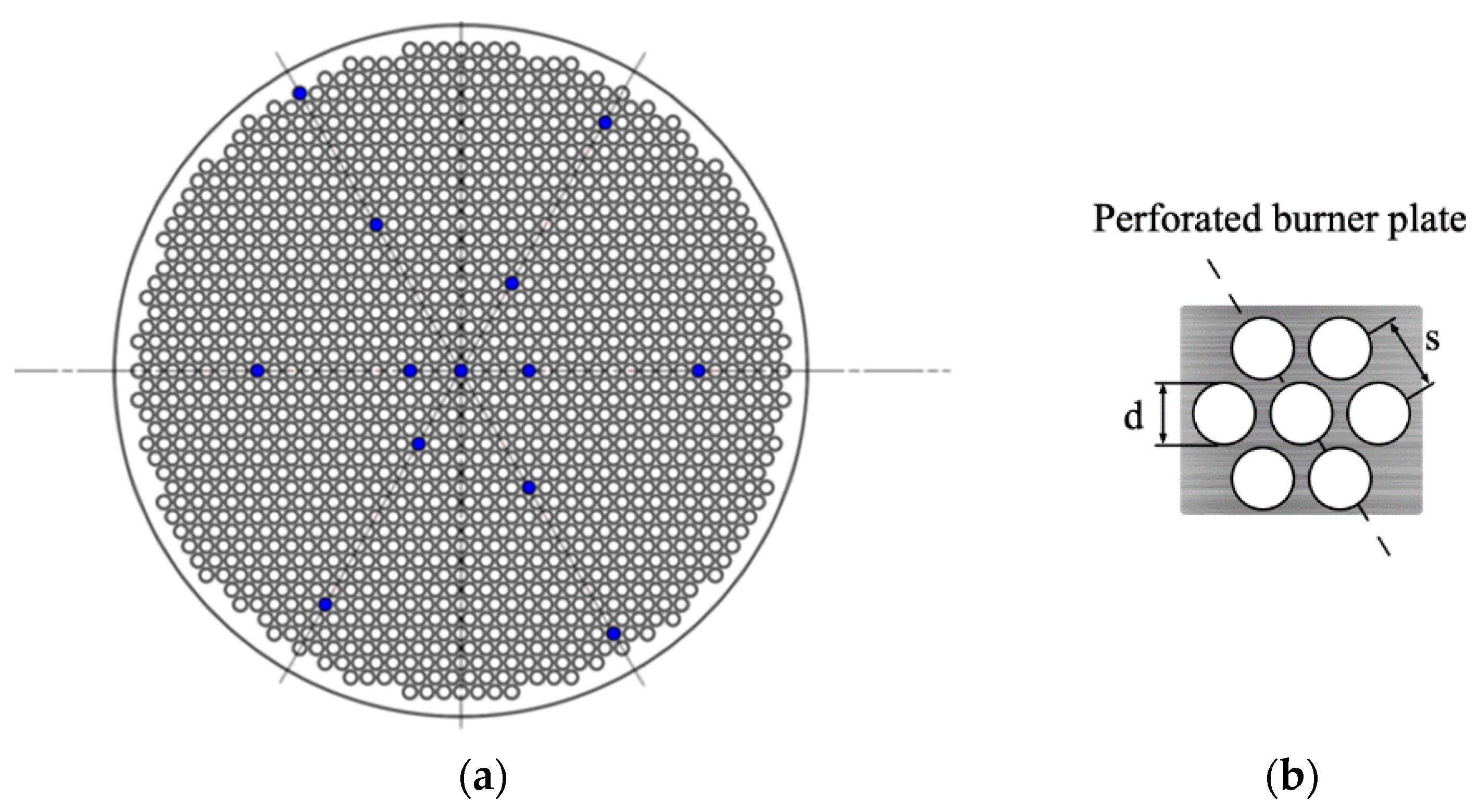
2.1. Heat Flux Burner
2.2. The Principle of the Experiment
2.3. Experimental Design
2.4. Error Analysis
2.5. One-Dimensional Flame Simulation
3. Results and Discussion
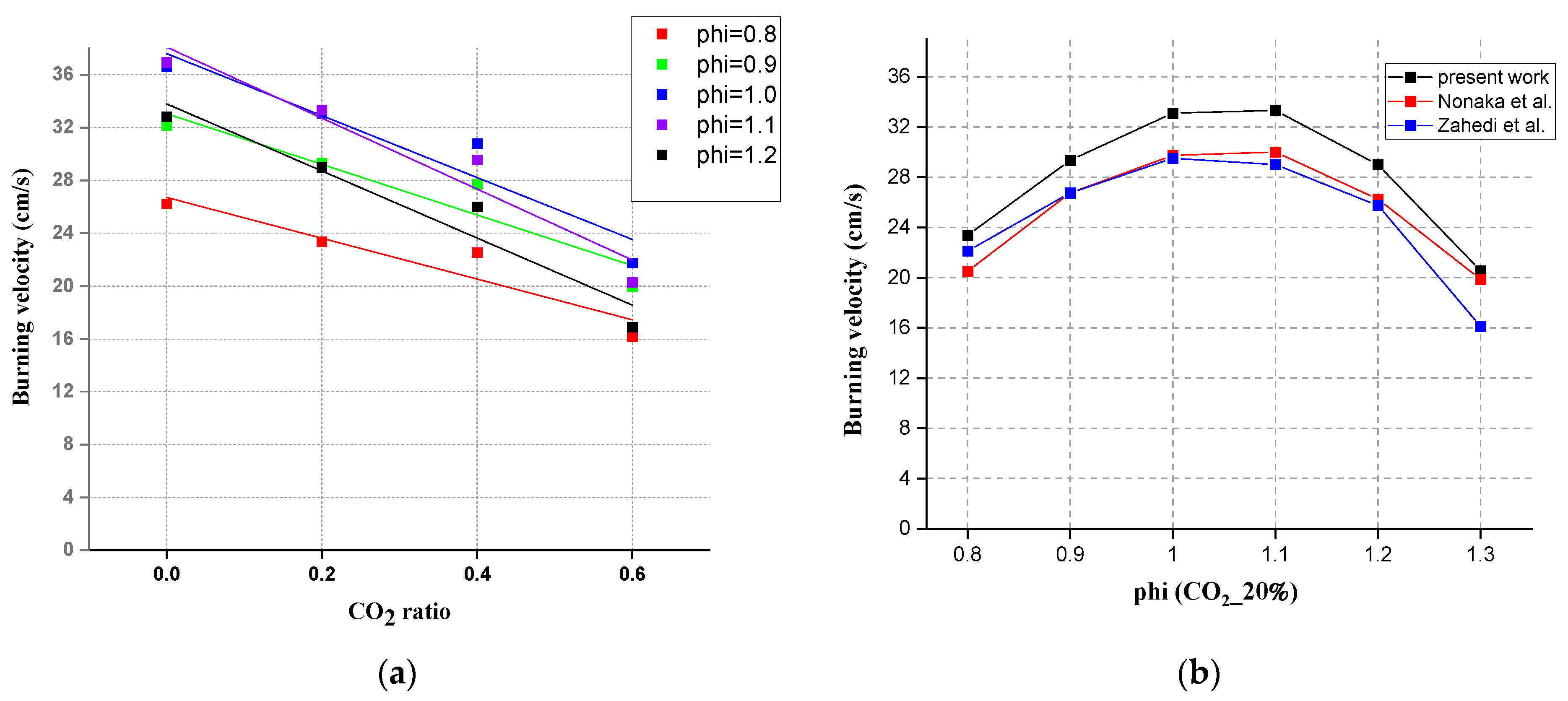
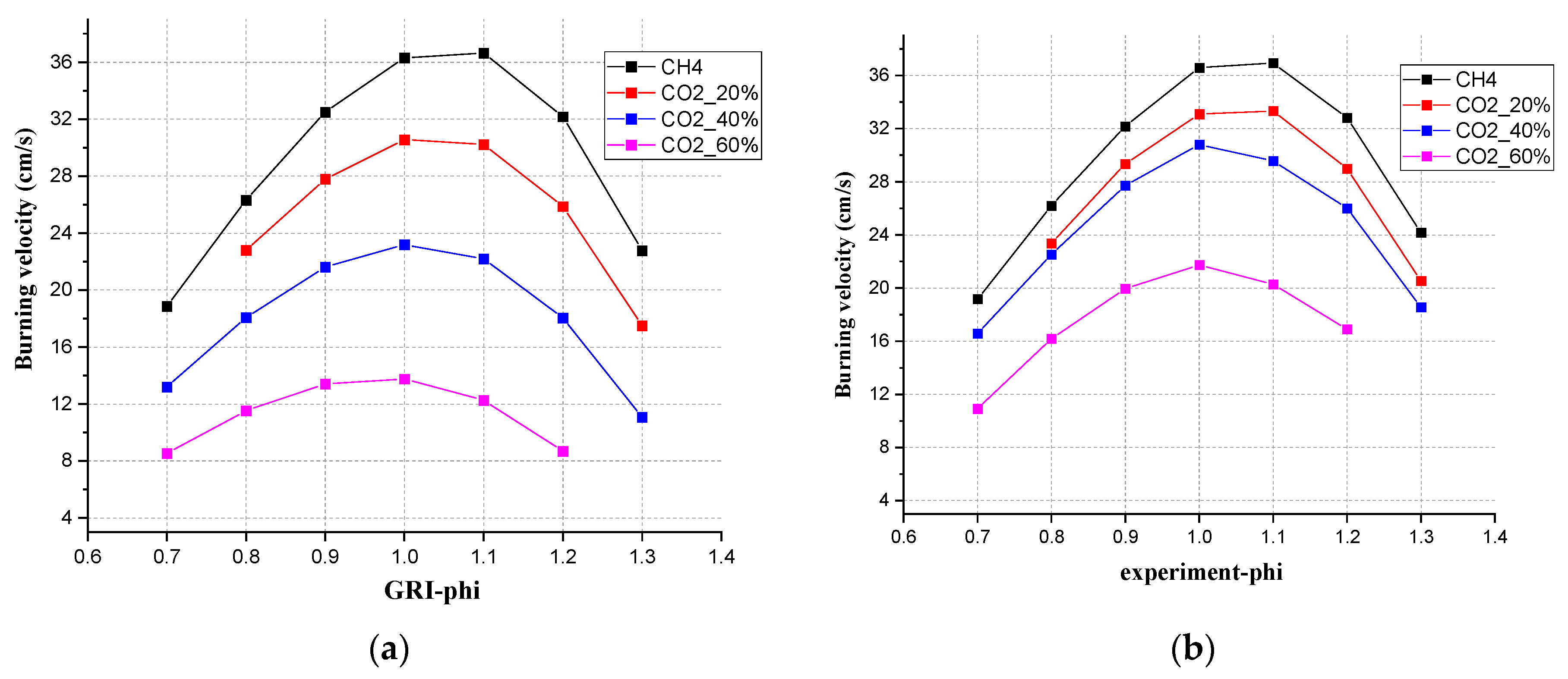
3.1. Effect of CO2 Addition on Laminar Flame Velocity
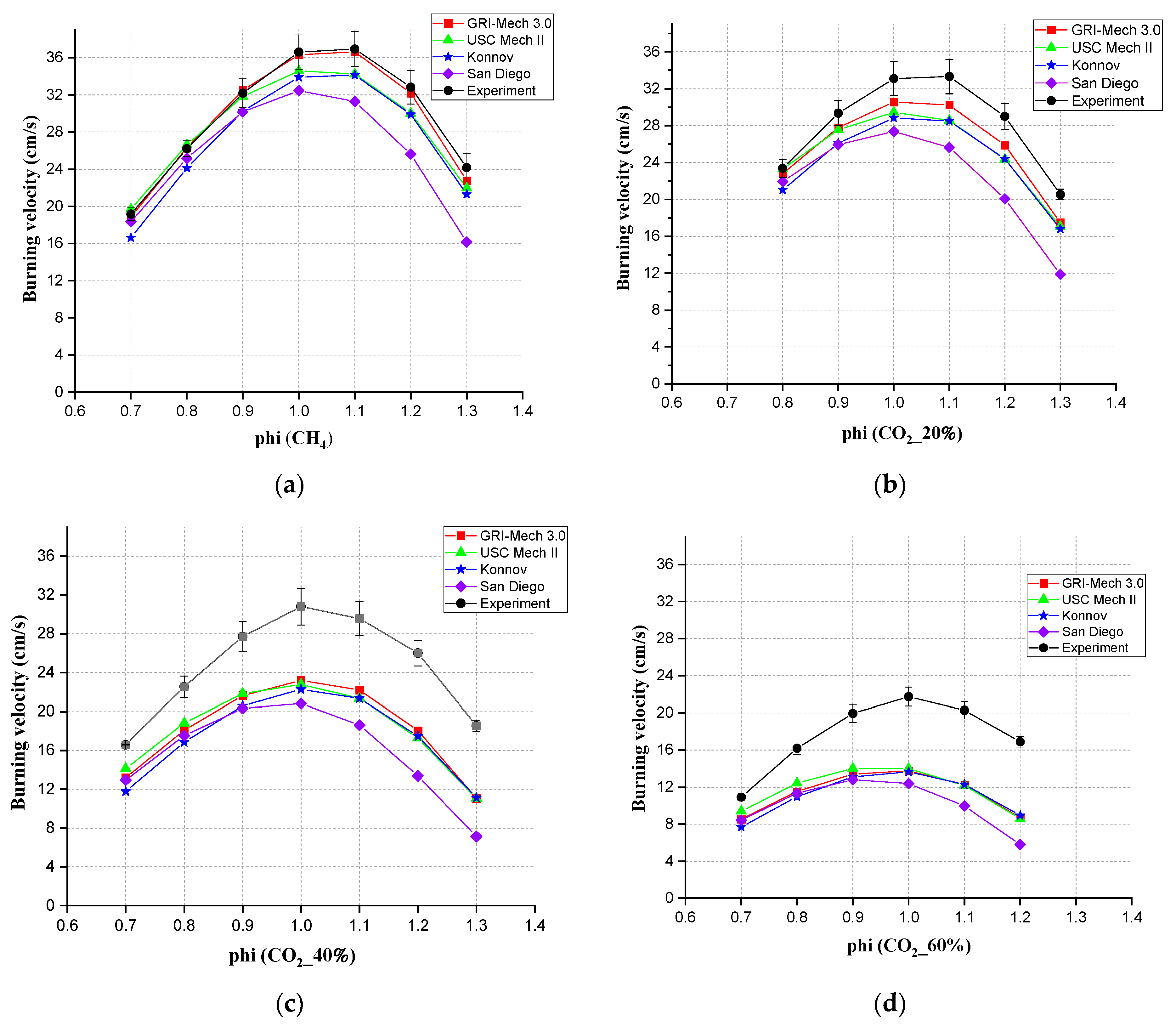
3.2. Comparison between Experimental and Simulation Results Using Different Mechanisms
3.3. Comparison of Experimental and Simulated Values of Different Concentrations of CO2
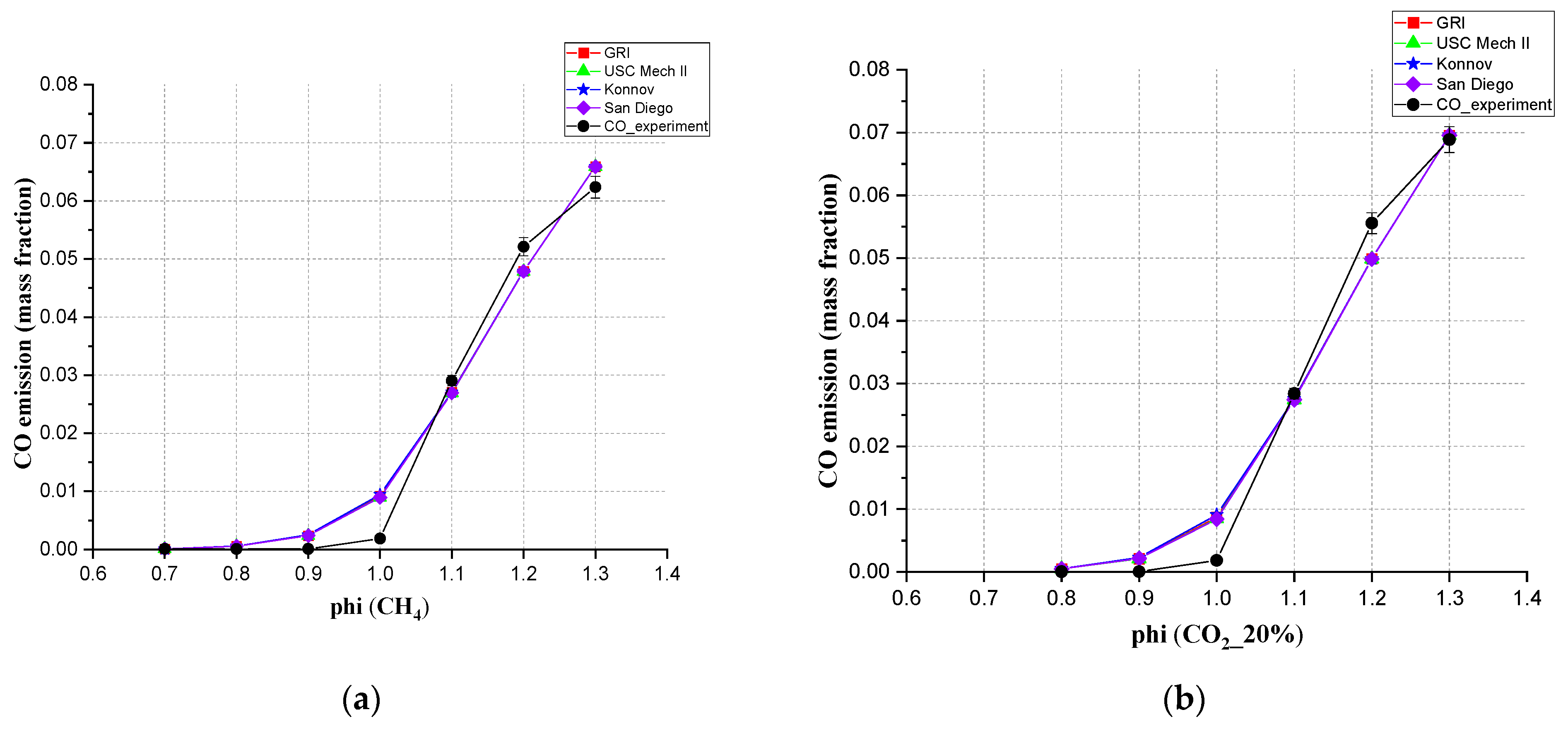
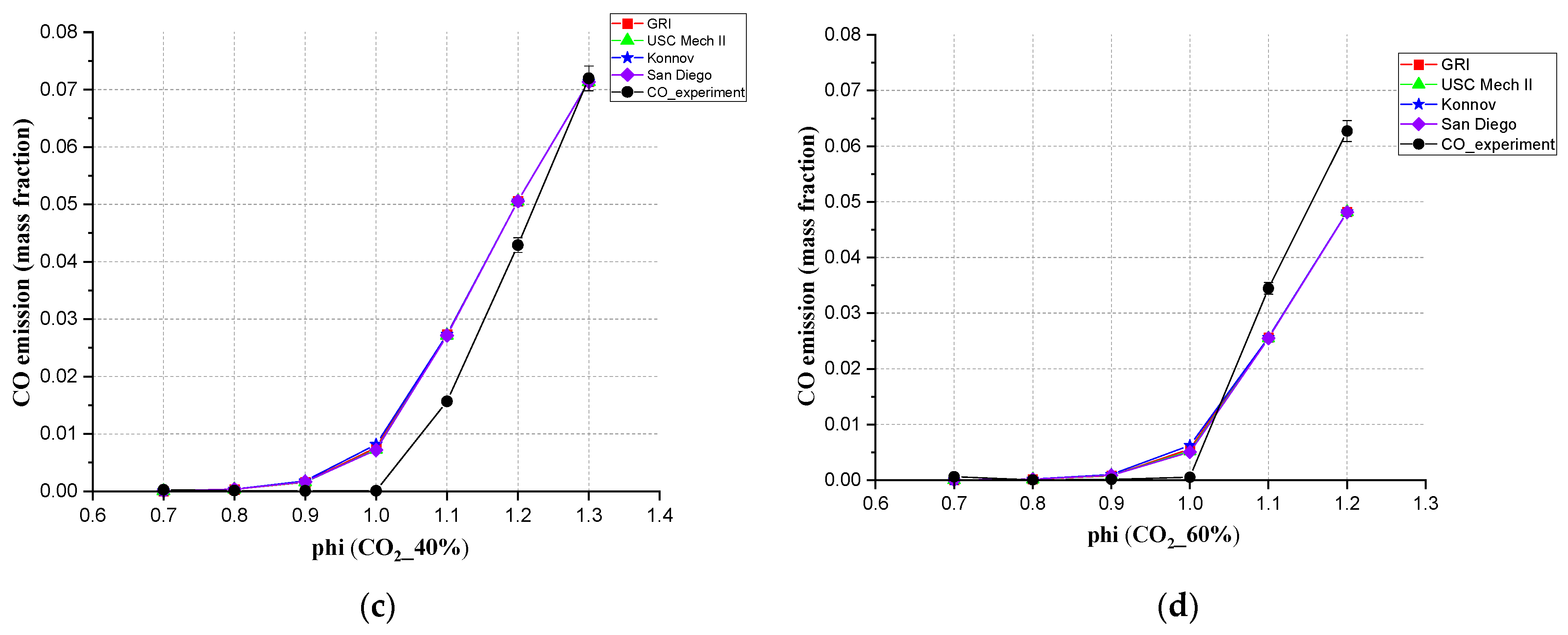
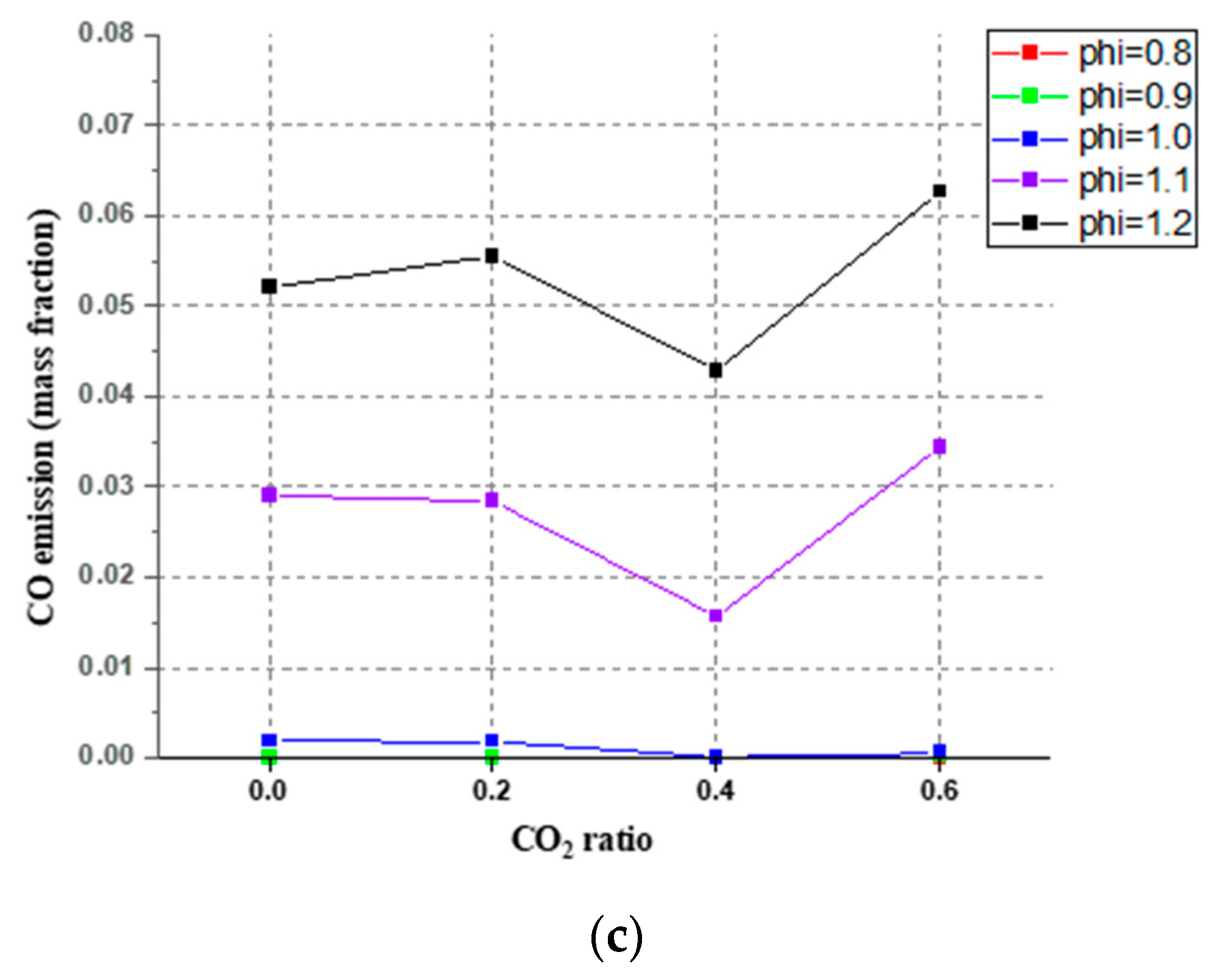
3.4. The Emission of CO, Simulation and Experimental Results at 20 cm Downstream
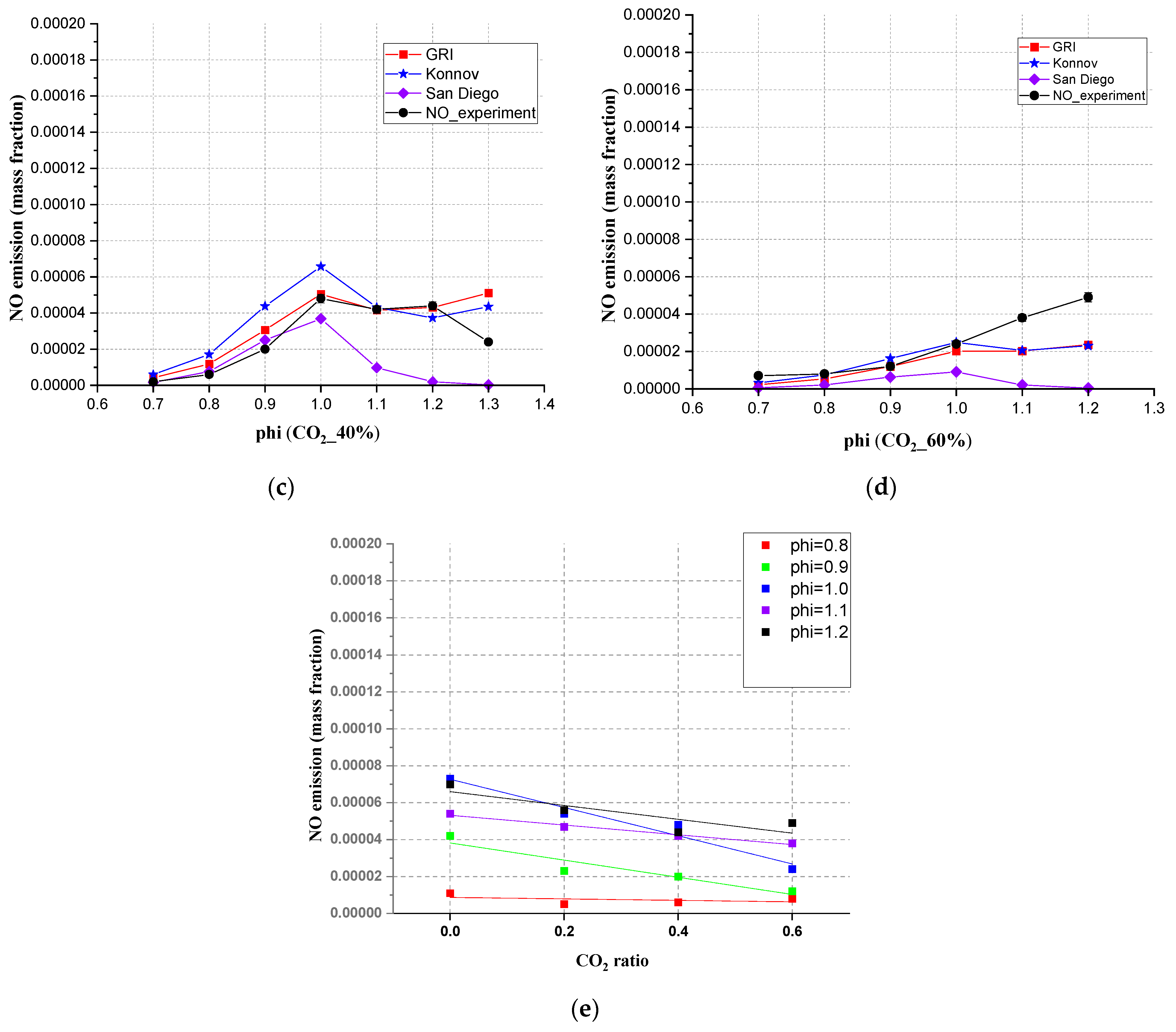
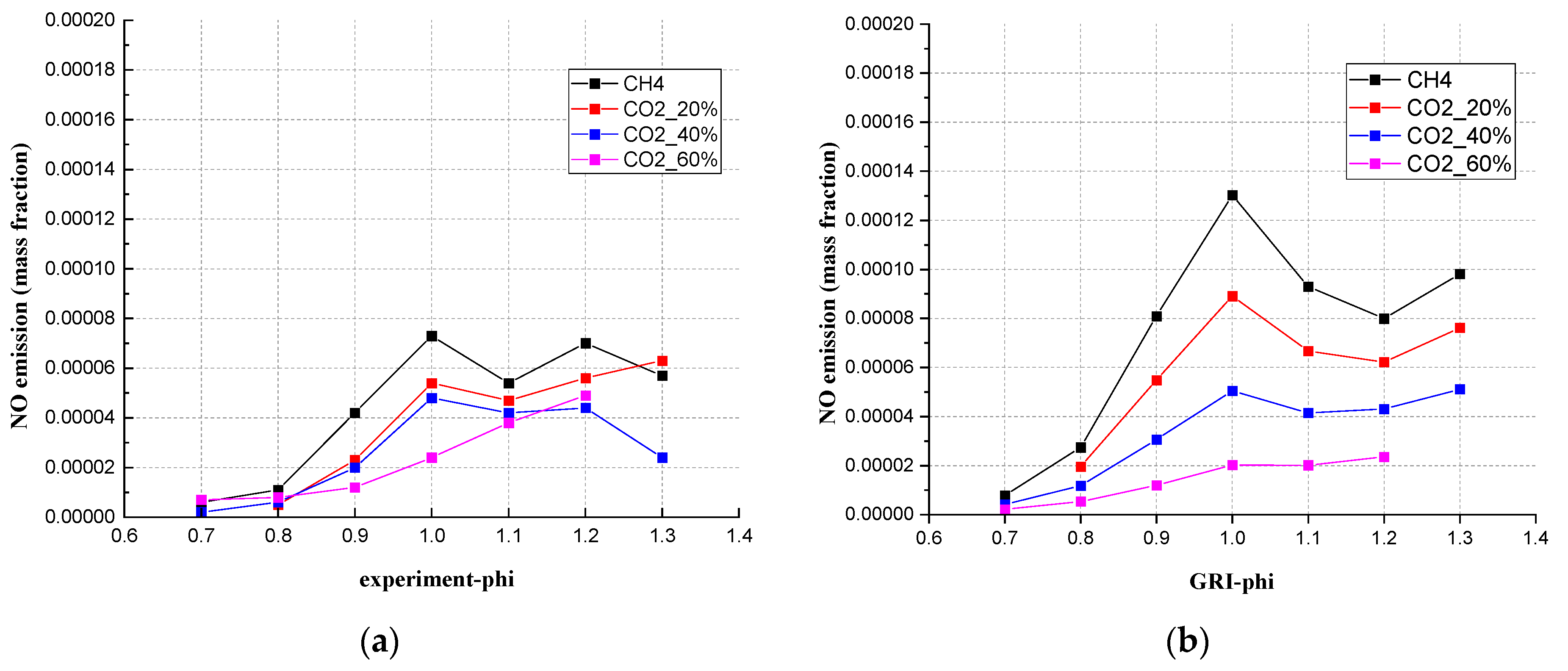
3.5. The Emission of NO between Simulation (20 cm Downstream Position) and Experimental Results
3.6. Burning Velocities with Different Diffusion Methods
4. Conclusions
- For laminar burning velocity simulation, four chemical kinetic mechanisms, GRI-Mech 3.0, San Diego, Konnov, and USC Mech II, all showed the same tendency compared with the experimental results. The simulation results were all lower than the experimental results. Consistent with the conclusion of Nonaka and Pereira [3], GRI Mech 3.0 showed the best agreement when the CO2 content was 20%. USC Mech II showed the best consistency when the CO2 content was between 40% and 60%. Agreement was limited when there were CO2 additions. The burning velocity showed a liner decrease while adding CO2.
- For the CO emission, for the mixed gas, all four mechanisms in Chem1D can be used to predict CO exhaust amount. These four mechanisms all showed a small error compared with the experiments. When CO2 content was higher than 40%, the deviation between simulation and experiment became bigger. In the mixed gas, the proportion of CO2 did affect CO emissions, making the CO emissions decrease first and then increase.
- For the NO emission, when the CO2 content was 40%, compared with the experimental results, the Chem1D can predict the mixed gas NO emission interval range. The NO emissions showed a linear relationship with the addition of CO2. In rich conditions, no matter how much CO2 accounted for, the NO emissions were all below 0.0001 mass fraction.
- All in all, numerical simulation is a good way to predict burning velocities and CO emissions for 1D adiabatic flames. GRI Mech 3.0 is the best kinetic mechanism for this with the current mixtures. Adding CO2 to CH4 decreases the burning velocity but also decreases NO emissions and does not produce more CO emissions.
- Different diffusion approximations were used, complex, mixture averaged, constant Lewis numbers, and unity Lewis numbers. Of course, the complex and mixture averaged approximations showed the best agreement. For CH4, the curves from complex, mixture averaged, and unity Lewis numbers showed a very small deviation compared with the experiment results. Different burning velocity approximations cause different burning rates estimations and, therefore, different CO and NOx emission rates. The exact impact should be investigated in an upcoming survey. For now, we can say that for truth-finding, complex diffusion is advised for use.
- The authors found the mixed ratio for CO2 as 40% was a good balance between SL and pollutant emissions. For SL, the curve from CO2 = 40% did not show a big difference compared with CH4 but had a much lower CO and NO emission specifically at the point of Φ = 1.0. This is due to the inert dilution that lowers the product’s temperature and, therefore, the thermal (Zeldovich) mechanism; thus, adding CO2 into CH4 is a good NOx removal method due to its low cost. Good advice can be provided here for industrial utilization by using 40% CO2 of the natural gas, which can reduce the pollutant emission without drastically reducing the burning velocity. More simulation work to research the ignition delay time, chemical and thermal effects on burning velocity will be undertaken in the future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rasi, S.; Veijanen, A.; Rintala, J. Trace Compounds of Biogas from Different Biogas Production Plants. Energy 2007, 32, 1375–1380. [Google Scholar] [CrossRef]
- Jönsson, O.; Polman, E.; Jensen, J.K.; Eklund, R.; Schyl, H.; Ivarsson, S. Sustainable Gas Enters the European Gas Distribution System; Danish Gas Technology Center: Copenhagen, Denmark, 2003; pp. 1–9. [Google Scholar]
- Nonaka, H.O.B.; Pereira, F.M. Experimental and Numerical Study of CO2 Content Effects on the Laminar Burning Velocity of Biogas. Fuel 2016, 182, 382–390. [Google Scholar] [CrossRef]
- Goswami, M.; Van Griensven, J.G.H.; Bastiaans, R.J.M.; Konnov, A.A.; De Goey, L.P.H. Experimental and Modeling Study of the Effect of Elevated Pressure on Lean High-Hydrogen Syngas Flames. Proc. Combust. Inst. 2015, 35, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Andrews, G.E.; Bradley, D. Determination of Burning Velocities: A Critical Review. Combust. Flame 1972, 18, 133–153. [Google Scholar] [CrossRef]
- Zhao, P.; Yuan, W.; Sun, H.; Li, Y.; Kelley, A.P.; Zheng, X.; Law, C.K. Laminar Flame Speeds, Counterflow Ignition, and Kinetic Modeling of the Butene Isomers. Proc. Combust. Inst. 2015, 35, 309–316. [Google Scholar] [CrossRef]
- de GOEY, L.P.H.; van MAAREN, A.; QUAX, R.M. Stabilization of Adiabatic Premixed Laminar Flames on a Flat Flame Burner. Combust. Sci. Technol. 1993, 92, 201–207. [Google Scholar] [CrossRef]
- Rallis, C.J.; Garforth, A.M. The Determination of Laminar Burning Velocity. Prog. Energy Combust. Sci. 1980, 6, 303–329. [Google Scholar] [CrossRef]
- Cohe, C.; Chauveau, C.; Gökalp, I.; Kurtuluş, D.F. CO2 Addition and Pressure Effects on Laminar and Turbulent Lean Premixed CH4 Air Flames. Proc. Combust. Inst. 2009, 32, 1803–1810. [Google Scholar] [CrossRef]
- Galmiche, B.; Halter, F.; Foucher, F.; Dagaut, P. Effects of Dilution on Laminar Burning Velocity of Premixed Methane/Air Flames. Energy Fuels 2011, 25, 948–954. [Google Scholar] [CrossRef]
- Halter, F.; Foucher, F.; Landry, L.; Mounaïm-Rousselle, C. Effect of Dilution by Nitrogen and/or Carbon Dioxide on Methane and Iso-Octane Air Flames. Combust. Sci. Technol. 2009, 181, 813–827. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Zhang, M.; Gong, J.; Jin, W.; Huang, Z. Experimental and Numerical Study on Laminar Flame Characteristics of Methane Oxy-Fuel Mixtures Highly Diluted with CO2. Energy Fuels 2013, 27, 6231–6237. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, C.; Fu, J.; Jiang, X.; Li, Q.; Wei, L.; Huang, Z. Experimental and Numerical Investigation on Diluted DME Flames: Thermal and Chemical Kinetic Effects on Laminar Flame Speeds. Fuel 2012, 102, 567–573. [Google Scholar] [CrossRef]
- Qiao, L.; Dahm, W.; Faeth, G.; Oran, E. Burning Velocities and Flammability Limits of Premixed Methane/Air/Diluent Flames in Microgravity. In Proceedings of the 46th AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 7–10 January 2008; p. 959. [Google Scholar]
- Van Maaren, A.; Thung, D.S.; DE GOEY, L.R.H. Measurement of Flame Temperature and Adiabatic Burning Velocity of Methane/Air Mixtures. Combust. Sci. Technol. 1994, 96, 327–344. [Google Scholar] [CrossRef] [Green Version]
- Hermanns, R.T.E.; Kortendijk, J.A.; Bastiaans, R.J.M.; De Goey, L.P.H. Laminar Burning Velocities of Methane-Hydrogen-Air Mixtures. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2007. [Google Scholar]
- Dyakov, I.V.; Konnov, A.A.; Ruyck, J.D.; Bosschaart, K.J.; Brock, E.C.M.; De Goey, L.P.H. Measurement of Adiabatic Burning Velocity in Methane-Oxygen-Nitrogen Mixtures. Combust. Sci. Technol. 2001, 172, 81–96. [Google Scholar] [CrossRef]
- Konnov, A.A.; Dyakov, I.V. Measurement of Propagation Speeds in Adiabatic Flat and Cellular Premixed Flames of C2H6+ O2+ CO2. Combust. Flame 2004, 136, 371–376. [Google Scholar] [CrossRef]
- Konnov, A.A.; Dyakov, I.V. Measurement of Propagation Speeds in Adiabatic Cellular Premixed Flames of CH4+ O2+ CO2. Exp. Therm. Fluid Sci. 2005, 29, 901–907. [Google Scholar] [CrossRef]
- Coppens, F.H.V.; De Ruyck, J.; Konnov, A.A. Effects of Hydrogen Enrichment on Adiabatic Burning Velocity and NO Formation in Methane+ Air Flames. Exp. Therm. Fluid Sci. 2007, 31, 437–444. [Google Scholar] [CrossRef]
- Clarke, A.; Stone, R.; Beckwith, P. Measuring the Laminar Burning Velocity of Methane/Diluent/Air Mixtures within a Constant-Volume Combustion Bomb in a Micro-Gravity Environment. Fuel Energy Abstr. 1995, 68, 130–136. [Google Scholar]
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NOx Abatement: A Review. Sci. Total Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef]
- Abbasfard, H.; Hashemi, S.H.; Rahimpour, M.R.; Jokar, S.M.; Ghader, S. Reducing NO x Emissions from a Nitric Acid Plant of Domestic Petrochemical Complex: Enhanced Conversion in Conventional Radial-Flow Reactor of Selective Catalytic Reduction Process. Environ. Technol. 2013, 34, 2867–2879. [Google Scholar] [CrossRef]
- Somers, L.B. The Simulation of Flat Flames with Detailed and Reduced Chemical Models. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 1994. [Google Scholar]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W., Jr.; et al. Gri-Mech 3.0; Gas Research Institute: Chicago, IL, USA, 1999. [Google Scholar]
- William, F.A.; Kalyanasundaram, S.; Cattolica, R.J. The San Diego Mechanism—Chemical-Kinetic Mechanisms for Combustion Applications; Mechanical and Aerospace Engineering (Combustion Research), University of California at San Diego: San Diego, CA, USA, 2012. [Google Scholar]
- Konnov, A. Detailed Reaction Mechanism for Small Hydrocarbons Combustion, Release 0.4. 1998. Available online: http://homepages.vub.ac.be/~akonnov/ (accessed on 10 January 2022).
- Wang, H.; You, X.; Joshi, A.V.; Davis, S.G.; Laskin, A.; Egolfopoulos, F.; Law, C.K. USC Mech Version II. High-Temperature Combustion Reaction Model of H2/CO/C1-C4 Compounds; Combustion Kinetics Laboratory, University of Southern California: Los Angeles, CA, USA, 2007. [Google Scholar]
- Hinton, N.; Stone, R. Laminar Burning Velocity Measurements of Methane and Carbon Dioxide Mixtures (Biogas) over Wide Ranging Temperatures and Pressures. Fuel 2014, 116, 743–750. [Google Scholar] [CrossRef]
- Powling, J. A New Burner Method for the Determination of Low Burning Velocities and Limits of Inflammability. Fuel 1949, 28, 25–28. [Google Scholar]
- Bosschaart, K.J.; De Goey, L.P.H. Detailed Analysis of the Heat Flux Method for Measuring Burning Velocities. Combust. Flame 2003, 132, 170–180. [Google Scholar] [CrossRef]
- Raida, M.B.; Hoetmer, G.J.; Konnov, A.A.; van Oijen, J.A.; de Goey, L.P.H. Laminar Burning Velocity Measurements of Ethanol+air and Methanol+air Flames at Atmospheric and Elevated Pressures Using a New Heat Flux Setup. Combust. Flame 2021, 230, 111435. [Google Scholar] [CrossRef]
- Alekseev, V.A.; Naucler, J.D.; Christensen, M.; Nilsson, E.J.K.; Volkov, E.N.; de Goey, L.P.H.; Konnov, A.A. Experimental Uncertainties of the Heat Flux Method for Measuring Burning Velocities. Combust. Sci. Technol. 2016, 188, 853–894. [Google Scholar] [CrossRef]
- Burcat, A.; Ruscic, B. Third Millenium Ideal Gas and Condensed Phase Thermochemical Database for Combustion (with Update from Active Thermochemical Tables); Argonne National Lab.: Argonne, IL, USA, 2005. [Google Scholar]
- Li, J.; Zhao, Z.; Kazakov, A.; Chaos, M.; Dryer, F.L.; Scire, J.J., Jr. A Comprehensive Kinetic Mechanism for CO, CH2O, and CH3OH Combustion. Int. J. Chem. Kinet. 2007, 39, 109–136. [Google Scholar] [CrossRef]
- Burke, M.P.; Chaos, M.; Ju, Y.; Dryer, F.L.; Klippenstein, S.J. Comprehensive H2/O2 Kinetic Model for High-Pressure Combustion. Int. J. Chem. Kinet. 2012, 44, 444–474. [Google Scholar] [CrossRef]
- Kéromnès, A.; Metcalfe, W.K.; Heufer, K.A.; Donohoe, N.; Das, A.K.; Sung, C.-J.; Herzler, J.; Naumann, C.; Griebel, P.; Mathieu, O.; et al. An Experimental and Detailed Chemical Kinetic Modeling Study of Hydrogen and Syngas Mixture Oxidation at Elevated Pressures. Combust. Flame 2013, 160, 995–1011. [Google Scholar] [CrossRef] [Green Version]
- Bosschaart, K.J.; Versluis, M.; Knikker, R.; Vandermeer, T.H.; Schreel, K.; De Goey, L.P.H.; Van Steenhoven, A.A. The Heat Flux Method for Producing Burner Stabilized Adiabatic Flames: An Evaluation with CARS Thermometry. Combust. Sci. Technol. 2001, 169, 69–87. [Google Scholar] [CrossRef]
- Hermanns, R.T.E.; Konnov, A.A.; Bastiaans, R.J.M.; de Goey, L.P.H.; Lucka, K.; Köhne, H. Effects of Temperature and Composition on the Laminar Burning Velocity of CH4+H2+O2+N2 Flames. Fuel 2010, 89, 114–121. [Google Scholar] [CrossRef]
- Goswami, M.; Derks, S.C.R.; Coumans, K.; Slikker, W.J.; de Andrade Oliveira, M.H.; Bastiaans, R.J.M.; Luijten, C.C.M.; de Goey, L.P.H.; Konnov, A.A. The Effect of Elevated Pressures on the Laminar Burning Velocity of Methane+air Mixtures. Combust. Flame 2013, 160, 1627–1635. [Google Scholar] [CrossRef]
- He, Y.; Wang, Z.; Weng, W.; Zhu, Y.; Zhou, J.; Cen, K. Effects of CO Content on Laminar Burning Velocity of Typical Syngas by Heat Flux Method and Kinetic Modeling. Int. J. Hydrog. Energy 2014, 39, 9534–9544. [Google Scholar] [CrossRef]
- Zahedi, P.; Yousefi, K. Effects of Pressure and Carbon Dioxide, Hydrogen and Nitrogen Concentration on Laminar Burning Velocities and NO Formation of Methane-Air Mixtures. J. Mech. Sci. Technol. 2014, 28, 377–386. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Y.; Zhang, X.; Zhou, G.; Yan, B.; Bastiaans, R.J.M. Experimental and Numerical Study of the Laminar Burning Velocity and Pollutant Emissions of the Mixture Gas of Methane and Carbon Dioxide. Int. J. Environ. Res. Public Health 2022, 19, 2078. https://doi.org/10.3390/ijerph19042078
Wang Y, Wang Y, Zhang X, Zhou G, Yan B, Bastiaans RJM. Experimental and Numerical Study of the Laminar Burning Velocity and Pollutant Emissions of the Mixture Gas of Methane and Carbon Dioxide. International Journal of Environmental Research and Public Health. 2022; 19(4):2078. https://doi.org/10.3390/ijerph19042078
Chicago/Turabian StyleWang, Yalin, Yu Wang, Xueqian Zhang, Guoping Zhou, Beibei Yan, and Rob J. M. Bastiaans. 2022. "Experimental and Numerical Study of the Laminar Burning Velocity and Pollutant Emissions of the Mixture Gas of Methane and Carbon Dioxide" International Journal of Environmental Research and Public Health 19, no. 4: 2078. https://doi.org/10.3390/ijerph19042078





