Abstract
Gold is one of the most valuable materials but is frequently extracted under circumstances that are hazardous to artisanal and small-scale gold miners’ health. A common gold extraction method uses liquid mercury, leading to a high exposure in workers. Therefore, a systematic review according to the PRISMA criteria was conducted in order to examine the health effects of occupational mercury exposure. Researching the databases PubMed®, EMBASE® and Web of ScienceTM yielded in a total of 10,589 results, which were screened by two independent reviewers. We included 19 studies in this review. According to the quantitative assessment, occupational mercury exposure may cause a great variety of signs and symptoms, in particular in the field of neuro-psychological disorders, such as ataxia, tremor or memory problems. However, many reported symptoms were largely unspecific, such as hair loss or pain. Most of the included studies had a low methodological quality with an overall high risk of bias rating. The results demonstrate that occupational mercury exposure seriously affects miners’ health and well-being.
1. Introduction
1.1. Gold Mining
Since ancient times, gold has been one of the most desired and noble elements in the world, with an outstanding variety of application areas. One of the most impressive examples for its use in art is the world-famous death mask of the Egyptian pharaoh Tutankhamun. The jewellery industry processes gold for all conceivable kinds of products. Furthermore, gold is also of considerable relevance as a financial reserve for national banks. Germany, for instance, possessed about 3400 t of gold ingots in 2020 [1]. Hence, the gold price has shown an overall upward trend over the last five decades and amounted to approximately 1800 USD/fine troy ounce in 2020 [2,3], indicating it to remain a promising market.
The total annual gold production is, according to the U.S. Geological Survey, about 3300 t in 2019 [4], of which artisanal and small-scale gold mining (ASGM) is estimated to account for 380–450 t per year [5]. As defined by the United Nations (U.N.), artisanal and small-scale gold miners (ASG miners) are persons who engage in mining as “individual miners or [in] small enterprises with limited capital investment and production” [6]. Nevertheless, official figures on the exact number of labourers are, to our knowledge, not obtainable, but approximate values suggest that about 16 million people work as ASG miners [5]. This form of gold mining is predominantly practised in countries of the global south, for example, in Ghana, Ecuador and Indonesia [5].
Gold mining, and ASGM in particular, is of great importance for low- and middle-income countries where it reveals both positive and negative aspects. On the one hand, a striking positive aspect is its economic weight, for which Ghana is presented as an example: the gold mining industry alone amounted to 7.1% of Ghana’s national gross domestic product (GDP) in 2019 (provisional data) [7] with a general increase in revenues [8]. In addition, the companies that are members of the Ghana Chamber of Mines also support society by, for example, financially sponsoring projects in the fields of health or education [8]. On the other hand, negative effects concern the environment [9,10,11,12], with parts of the tropical rainforest in South America being destroyed in the context of gold mining [9]. Mining waste including chemicals such as cyanide or mercury can pollute the environment, such as water, sediments and soil, finally affecting the human food chain, as well [10,11,12]. In addition, the workers’ health is harmed by their work itself. Nakua et al. observed that ASG miners have a high risk of being injured at work, while the safety precautions are at a low standard [13]. Mercury exposure is known to cause a considerable burden of disease in miners, being responsible for up to more than 2 million DALYs per year, especially in countries of the global south [14].
In general, the mining process can be carried out using various methods, depending on available materials, equipment and knowledge [15,16]. Among other methods, the mercury amalgamation is still used for extracting gold [15,16]. Here, gold-containing rocks are ground and afterwards pulverized into small pieces, and the material thus obtained is mixed with elemental mercury to form an amalgam out of gold and mercury [15,16]. After gathering, this gold amalgam is further processed by smelting, so that the mercury vaporizes and the gold remains behind [15,16].
1.2. Mercury
Mercury (also known as Hg or quicksilver) is a chemical element with a silver–grey colour. It is the only metal that exists in a liquid aggregate state under standard conditions and evaporates on contact with the ambient air. According to an official European Union directive, mercury is categorized as a threat to aquatic ecosystems, as toxic through inhalation and as hazardous to human’s health [17].
Natural processes, such as volcanism, cause atmospheric mercury emissions. However, anthropogenic sources, such as coal combustion or ASGM contribute to at least three-fold higher mercury masses in the atmosphere [18,19,20]. Currently, the ASGM sector is the largest contributor to anthropogenic—man-made, non-natural—mercury emissions [20]. In 2015, its airborne emissions amounted up to 838 t, which represents approximately 38% of the total mercury emissions worldwide [20].
The consequences of mercury exposure are highly dependent on its chemical form (organic or inorganic compounds) as well as its dose and exposure pathway. Mercury can be absorbed via different pathways. ASG miners heat the amalgam to extract gold, leading to an evaporation of metallic (inorganic) mercury [15,16]. Therefore, this paper puts the emphasis on the metallic form. The main absorption route of elemental mercury is via inhalation. In an experimental setting, exposed individuals absorbed 67–87% of the entire inhaled mercury vapour [21]), while absorption of vapour through skin contact and gastrointestinal absorption of ingested metallic mercury played only a subordinate role [22,23]. The exhalative half-life of mercury amounts approximately 2 days [24,25], while the half-life for the urinary excretion is 63 days [25]. However, mercury accumulation occurs in organ tissues, but its exact distribution depends on its chemical form [26,27]. After inhalation of inorganic mercury, the distribution takes place via blood as it crosses most cell membranes, such as the blood–brain barrier or the placenta. The oxidation of elemental mercury in the erythrocytes influences the uptake in the brain with its corresponding typical mercury-related signs and symptoms. In contrast, occupational exposure to organic mercury compounds can be found exemplarily in the production of mercury fulminate. Organic mercury is highly lipophilic and is mainly absorbed via skin and inhalation. The main target organ of organic mercury compounds is the brain.
Internal human mercury burden can be detected in certain biological materials such as blood or urine, but due to the varying specificity for the different forms of Hg, other materials are suitable for the measurements, such as hair [28].
Acute cases of mercury poisoning can occur after contact with its metallic form [29,30,31]. Due to the inhalation of toxic fumes, the resulting symptoms affect, in particular, the pulmonary system with cough and dyspnoea up to acute respiratory distress syndrome (ARDS) [29,30,31]. Additionally, mercury exposure can lead to other unspecific symptoms, such as nausea, diarrhoea, fever or lymphadenopathy [30,31]. The course of mercury poisoning can end lethal, depending on its severity [31]. Likewise, chronic exposure to elemental Hg vapour can trigger a multitude of symptoms, mainly neuro-psychological disorders, such as tremor or erethism [32,33], which may persist in a reduced intensity even after the end of the exposure [32,34]. Further typical symptoms include a dark discolouration of the gum, gingivitis and renal damage [32,33].
In order to curb anthropogenic mercury pollution and to avoid the resulting health and environmental impacts in the future, the U.N. adopted the Minamata Convention on Mercury in 2013 [6]. This convention provided and established regulations to diminish mercury-using practices [6]. Although most of the participating countries have already signed and ratified this convention [35], mercury pollution in the artisanal and small-scale gold mining sector remains a major challenge. The aim of this systematic review was to examine the situation of occupational mercury exposure and mercury-related health effects among ASG miners in middle- and low-income countries in order to give a comprehensive overview on affected workers and their symptoms and diseases.
2. Materials and Methods
2.1. Conceptualization and Literature Research
The foundation of this systematic review was the “The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA)” checklist [36]. A systematic study protocol was submitted to and accepted by the International prospective register of systematic reviews (PROSPERO), in order to ensure the methodological quality of this review. The review protocol is available online (PROSPERO-ID: CRD42021235289).
The main research question was developed according to the PECO (Population, Exposure, Comparison, Outcome) criteria [37]: How does a direct ongoing occupational mercury exposure (E) among gold miners (P) influence their health (such as mercury-related symptoms, diseases and intoxication) (O) in comparison to individuals without an occupational mercury exposure (C) (Table 1)?

Table 1.
Inclusion and Exclusion Criteria.
Exclusively, studies with a peer-reviewed full-text article in English or German were included. These articles had to be published between 1 January 1980 and 31 December 2020. The study design was restricted to the inclusion of prospective studies, observational studies, cross-sectional studies, case-control studies, systematic reviews, non-systematic reviews and meta-analyses. All other kinds of studies were intentionally excluded. Furthermore, only studies that use the amalgam method (or another mercury-related method of gold mining) as well as studies in low- and middle-income countries were included.
To perform a systematic literature research, a search string was created considering three categories: population, exposure and outcome. The selected keywords were combined with the Boolean operators AND (to combine the topics) and OR (to combine the words in a category) to ensure the creation of senseful and complete results. Several words were also ended with *, so that all possible endings were included in the search (for example, symptom* can mean symptom as well as symptoms or symptomatic etc.). For every database (PubMed®, EMBASE® and Web of Science™), a specific search string was individually established according to the given guidelines.
The research was implemented on the 16 February 2021 by one reviewer (Kira Taux). In addition, the complete reference lists of certain key reviews [38,39,40,41,42] were also screened for eligible literature.
2.2. Screening Process
All duplicates were removed from the list of results; then the selection of literature started according to the a priori designed review protocol. The entire screening process was conducted by the same two independent reviewers (Kira Taux and Andrea Kaifie).
A screening of the articles’ titles, then abstracts and finally full texts was performed by these reviewers to determine whether the aforementioned inclusion criteria were met or not. After suitable studies were selected by each reviewer, the results were compared, and disagreement was solved by discussion until consensus was achieved. Each exclusion of a study was individually documented, including the specific reason for exclusion.
2.3. Data Extraction (Quantitative Assessment)
A table containing the following categories was created to outline all relevant data: author, year, study design, setting, time, participants, exposure, measurements, outcome, effect parameters.
Depending on the given statistics, the statistical mean was preferred to report any kind of socio-demographic data (e.g., age or working time), while median values were reported as outcome (for example, laboratory parameters). If no effect parameters were given, odds ratios (OR) were calculated by one author (Andrea Kaifie) if the underlying data were available [43,44,45,46,47,48].
All data were extracted and summarized by one reviewer (Kira Taux), while the second reviewer (Andrea Kaifie) controlled the precision and completeness of the data extraction. Although considered, the creation of a meta-analysis out of the selected studies was not possible because the given data were too heterogeneous.
2.4. Bias Assessment (Qualitative Assessment)
The methodological quality evaluation of each included study was based on the assessment of the following items: selection bias, performance bias, detection bias, attrition bias, reporting bias and other source(s) of bias. Considering this classification, a table for the assessment was established according to the following references.
To evaluate the selection bias, two subsidiary categories (bias and confounder) were considered according to questions 14–26 of the checklist first published by Downs and Black [49]. Answer options were yes, no or unable to determine. If all questions were answered with no, this category was rated as low risk; if at least one question was answered with yes, the category was rated as high risk. If at least one question was answered with unable to determine, the category was rated as unable to determine. The checklist had to be adapted to the design of the included studies, which was mainly cross-sectional. Therefore, questions about participants’ blinding and their compliance concerning the intervention (numbers 14 and 19) were considered as not applicable since the studies did not deal with any intervention. The questions concerning the follow-up (numbers 17 and 26) could not be answered adequately because none of the studies had a follow-up. Ultimately, the questions dealing with random sequence generation and allocation concealment (numbers 23 and 24) were omitted since none of the studies was randomized.
The remaining bias categories (performance, detection, attrition, reporting and other source(s) of bias) were evaluated according to the Cochrane Collaboration [50]. Each topic was rated in analogy to the aforementioned categories: the rating was low risk when all questions of an item were judged as low risk, the rating was high risk when at least one question was assessed as high risk and the rating was unclear risk when at least one question was answered as unable to determine.
A conclusive judgement over all categories was done after all items were evaluated and controlled: studies with an overall low risk of bias had all items assessed as low risk, studies with an overall high risk of bias had at least one category rated as high risk and studies with an unclear risk of bias had at least one category judged as unable to determine.
A blank protocol for this bias assessment is attached as Supplemental Material (Table S1).
3. Results
3.1. Literature Research and Screening Process
The literature research yielded in a total of 10,589 results. After the exclusion of duplicates, 6562 publications were finally taken into consideration. Although the reviewed publications included studies from all over the world, a major portion had to be excluded due to failure to meet the aforementioned inclusion criteria. Title and abstract screening led to the further exclusion of 6464 studies, while the literature review of selected articles [38,39,40,41,42] yielded 5 additional studies to be included in the full-text screening. The subsequent evaluation of the remaining 103 full-texts resulted in the exclusion of 84 articles due to their failure to fulfil the following eligibility criteria: study population (n = 55), outcome (n = 16), exposure (n = 6), study design (n = 4), language (n = 2) or context (n = 1). Eventually, 19 publications were included in this systematic review [43,44,45,46,47,48,51,52,53,54,55,56,57,58,59,60,61,62,63] (Figure 1).
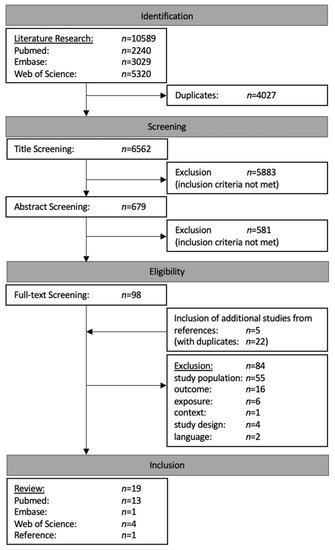
Figure 1.
Flow chart of literature research and screening process.
3.2. Data Extraction (Quantitative Assessment)
The selected articles included 18 cross-sectional studies [43,44,45,46,47,51,52,53,54,55,56,57,58,59,60,61,62,63] and 1 case series [48], whose publication years ranged from 1993 to 2020 [43,44,45,46,47,48,51,52,53,54,55,56,57,58,59,60,61,62,63]. The following geographical regions were covered:
- Africa: Ghana [43,44,51,52], Tanzania [45,53], Zimbabwe [46,54], Sudan [55], Burkina Faso [56], Uganda [57].
- Asia: Indonesia [46,47,58], Pakistan [59,60].
 Table 2. Data extraction.
Table 2. Data extraction.
3.2.1. Africa
Ghana
The first study by Afrifa et al. focused on mercury’s impact on kidney function and discovered that Hg exposure among gold miners was significantly positively associated with elevated levels of urine protein and serum creatinine, while its association with eGFR was significantly negative [43]. None of the surveyed symptoms showed any statistically significant association with mercury exposure [43].
Concerning the thyroid function of gold miners, Afrifa and co-authors found a significantly negative association between mercury blood levels with T3 as well as T4 concentrations; in contrast, its association with TSH was non-significantly positive [51]. However, all measured thyroid values were still in a physiological range [51].
The connection of mercury with blood pressure was illustrated by the study from Rajaee et al. [44]. A significant association between mercury exposure and hypertension could not be demonstrated for miners versus residents [44].
Mensah et al. observed in their study that mercury exposure did not show significant associations with any medical symptoms for all miners, while the association with numbness was statistically significant for the sub-group with previous work experience in another mine [52].
Tanzania
The first study described a variety of symptoms to be more frequent in exposed than in controls, in particular neuro-psychological symptoms, increased salivation or discoloured gums [45]. However, the association between the surveyed symptoms and Hg exposure lacked significance for miners compared to residents [45].
The second study detected a variety of symptoms, such as neuro-psychological disorders (e.g., trembling or numbness), gingivitis or several respiratory symptoms in gold miners [53]. The combination of certain observed symptoms with elevated mercury levels in hair led to the diagnosis of mercury intoxication in approximately 10% of the examined miners [53].
Zimbabwe
Bose-O’Reilly et al. investigated the medical consequences of occupational mercury exposure in children in Zimbabwe and Indonesia [46]. Certain symptoms, such as neuro-psychological disorders (e.g., ataxia or dysdiadochokinesia), increased salivation or discoloured gums, were more frequent in exposed children [46]. In addition, mercury exposure was significantly associated with certain neuro-psychological disorders, for example, ataxia or dysdiadochokinesia [46].
The situation in Zimbabwe was also analysed regarding the disease burden presented in DALYs (Disability-Adjusted Life Years) [54]. The examinations also revealed that a number of symptoms were significantly more common in miners, for example, certain neuro-psychological symptoms [54]. Approximately 3% of the Zimbabwean population was occupationally exposed to Hg, while a total of 2% (which was equivalent to be around 72% of all miners) was considered to be intoxicated, which corresponded to 95,400 DALYs triggered by Hg exposure in the context of ASGM [54].
Sudan
Concerning gold miners in Sudan, one study determined variations in thyroid hormones and demonstrated that both TSH and TT4 were significantly elevated in miners in comparison to a control group, while FT3, FT4 and TT3 were significantly reduced [55]. These results were considered generally compatible to the laboratory parameters of hypothyroidism [55].
Burkina Faso
Highly exposed workers showed certain medical symptoms, such as neuro-psychological disorders (for example, headache or trembling), thoracic pain or cough [56]. A statistically significant positive association was observed between the mercury values in urine with problems to grab as well as with thoracic pain [56].
Uganda
Wanyana et al. described that observed symptoms showed a significant different distribution according to sex: males reported more frequently about headache, while females reported more frequently about psychiatric disorders or memory problems [57]. In addition, mercury exposure was statistically significantly associated with neuro-psychological disorders (such as headache, numbness, dizziness), certain kinds of pain and respiratory symptoms [57].
3.2.2. Asia
Indonesia
Following Bose-O’Reilly et al., the examination revealed that certain symptoms, such as certain neuro-psychological disorders, were significantly more frequent in exposed persons [47]. Moreover, cases of mercury intoxication were significantly more frequent in exposed participants [47].
Ekawanti and Krisnayanti focused on changes in haematological and renal parameters and highlighted that miners’ haemoglobin and haematocrit was significantly reduced in comparison to a control group, which led to a higher frequency of anaemia [58]. In addition, urine protein was significantly elevated as well, leading to a frequent proteinuria among miners [58].
Pakistan
Khan et al. demonstrated that miners complained about various symptoms, such as neuro-psychological disorders, kidney diseases or different kinds of pain [59].
The second study by Riaz et al. also observed that gold miners showed a wide variety of symptoms, for example, gastrointestinal disorders, kidney diseases or respiratory symptoms [60].
3.2.3. South America
Brazil
Lacerda et al. examined the visual performance of gold miners and observed a reduced perimetric area in gold miners. In addition, the colour vision was also significantly worse compared to controls [61]. However, none of the calculated associations or correlations were statistically significant for miners [61].
The study by Branches et al. detected various symptoms in gold miners, for example, neuro-psychological disorders, certain kinds of pain or gingivitis [48]. Nevertheless, the associations between mercury exposure and diagnosed symptoms were not statistically significant [48].
Ecuador
The first study presented a significantly positive association between mercury in blood and urine with both tremor and reaction time, while their association with postural sway showed a significantly inverse association [62].
The second study highlighted that a fluctuating proportion of the clinically examined gold miners manifested particular symptoms, such as neuro-psychological disorders, discolouration of the gums or social problems [63]. A statistically significantly positive correlation between the urinary mercury content and the number of medical symptoms could be detected [63].
3.3. Risk of Bias Assessment (Qualitative Assessment)
The detailed results of the methodological quality assessment can be found in Table 3. The overall risk of bias rating is attached in the Supplemental Material (Table S2). Four studies were evaluated with an overall low risk of bias [43,45,46,47], while fifteen studies were assessed with an overall high risk of bias [44,48,51,52,53,54,55,56,57,58,59,60,61,62,63]. This rating as high-risk was based on a substantial potential for internal validity bias [44,48,52,53,55,56,58,59,60,61,62], internal validity confounder [48,52,53,54,55,56,58,59,60,62], detection bias [44,48,53,54,56,57,58,59,61,63] and/or attrition bias [44,48,51,53,55,56,58,59,60,61]. Other remarks that could not be assigned to the fixed categories but could lead to an increased risk of bias were identified for nine studies [43,48,53,54,57,58,59,60,61].

Table 3.
Bias assessment. (XS: cross sectional study).
4. Discussion
4.1. Data Extraction (Quantitative Assessment)
Although a systematic literature research was conducted, the eligibility criteria only applied to 19 studies, which indicates that there is only limited literature available on the specific topic of mercury-related health effects in ASG miners.
In summary, mercury exposure in the ASGM sector was reported to cause an extraordinarily wide variety of symptoms in diverse organ systems [43,44,45,46,47,48,51,52,53,54,55,56,57,58,59,60,61,62,63]. Interestingly, many symptoms such as pain, hair loss or cough were largely unspecific [43,44,45,46,47,48,51,52,53,54,55,56,57,58,59,60,61,62,63] and not easy to relate to mercury exposure. However, a substantial part of the reported symptoms could be classified to mercury-related neuro-psychological disorders (e.g., tremor, ataxia, memory problems) [43,45,46,47,48,52,53,54,56,57,59,62,63], although the extent varied considerably among the studies. It also has to be considered that working in the ASGM sector in low- or middle-income countries is related to low safety standards and a high probability to suffer from work-related injuries [13]. Challenging working conditions can lead to a high psychological burden, which may cause unspecific somatic symptoms. In a variety of countries with ASGM, medical care availability is often restricted, in particular in terms of occupational health. Therefore, a medical undersupply of work- and non-work-related diseases has to be assumed. Nevertheless, it must be underlined that mercury-related symptoms mean a severe burden of disease in affected persons, in particular for gold miners, who show a high risk of occupational-related mercury exposure.
The organ specific toxicity of mercury exposure has been described in animal studies before [64,65,66]. Akgül and colleagues showed that exposure to mercury vapour caused histological renal damage in rats [64]. Renal damage caused by mercury exposure also has been described in humans, where significantly elevated levels of urine protein or serum creatinine could be observed [43,58]. However, two included studies in this review could not demonstrate significant changes in proteinuria [46,54]. Regarding neuro-psychological abnormalities, Altunkaynak and colleagues observed histological damages in rats’ cerebellum after exposure to mercury vapour [65]. In addition, a further toxicological study in mice detected that post-natal exposure to mercury vapour affects the neuro-behavioral function, such as locomotive activity [66]. These observations support our findings that mercury exposure is in particular connected to neuro-psychological abnormalities [43,45,46,47,48,52,53,54,56,57,59,62,63].
Occupational mercury exposure is not limited to ASGM; it can also occur in other industrial sectors [67,68,69], such as in fluorescent lamp production companies [67]. Mercury-exposed labourers from the fluorescent lamp industry showed statistically significant more frequent psychological symptoms and a worse performance in neuro-psychological tests in comparison to non-exposed controls [67]. These findings confirmed our results, where mercury-exposed gold miners suffered significantly more often from neuro-psychological disorders [45,46,47,54] and showed somewhat significant worse results in neuro-psychological tests [45,46,47,54].
Another sector with an occupational exposure to mercury is the e-waste industry [68]. Decharat et al. examined e-waste workers and highlighted that neuro-psychological disorders, such as headache, were statistically significantly more frequent in exposed workers in comparison to office staff from the same company [68]. These results only partly agree with the findings of this review. For example, we could not observe statistically significant differences for headache between exposed and non-exposed groups [43,45,46]. Only one study could observe a statistically significant association between mercury exposure and headache [57]. However, headache must be considered as an unspecific symptom, which can be caused by a wide range of circumstances and diseases, such as migraine, meningitis or intra-cranial tumours. Consequently, this symptom could have also been caused by other circumstances or pre-existing conditions.
In addition, chlor-alkali workers are occupationally exposed to mercury [69]. These workers showed, according to Neghab et al., a statistically significant association between mercury exposure and neuro-psychological disorders (e.g., memory problems), while other associations with symptoms, such as tremor, showed no statistical significance [69]. However, only two studies in this review found significant differences regarding memory problems [45,47], but in contrast, we detected three studies with non-significant differences [45,46,54]. The reviewed studies used different methods to detect memory problems, from self-reported outcomes to objective neuro-psychological tests [45,46,47,54]. In particular, self-reported memory problems [45,46] are difficult to compare because of a missing generally applicable definition of memory problems, which makes them prone to a recall bias. Similar differing findings have been observed for tremor in miners or mercury-exposed participants [45,46,47,54], where the results differed both in subjective symptoms and clinical examinations [45,46,47,54]. In has to be considered that health consequences of mercury exposure are highly dependent of its chemical form, exposure pathway and dose. In particular the inhalation of inorganic mercury during the amalgamation process leads to a high absorption of and therefore high body burden of toxic metallic mercury. Although ASGM activities are mainly related to occupational inorganic mercury exposure, a burden with organic mercury compounds may be attributed by nutritional habits, such as fish or the ingestion of plant production products. These variables could at least partly explain the differing results.
4.2. General Methodology
Since all included studies were cross-sectional studies or case series, none of them was able to randomize their participants into groups [43,44,45,46,47,48,51,52,53,54,55,56,57,58,59,60,61,62,63]. In addition, a follow-up was not available [43,44,45,46,47,48,51,52,53,54,55,56,57,58,59,60,61,62,63]. This would have been helpful in order to understand the long-term effects of mercury and the clinical course in an exposed population.
Considering the study design, the availability of a control group was handled differently among the studies. The majority of the studies had a control group [44,45,46,47,48,53,54,55,56,58,59,61,62]; only six studies lacked a comparable group [43,51,52,57,60,63]. In addition, the composition of control groups was very heterogeneous among the studies. Some control groups consisted exclusively of indirectly exposed participants (e.g., residents from the same area) [48,56,58,59,62] or non-exposed controls (e.g., participants with no known mercury exposure) [54,55]. In contrast, six studies included more than one control group, respectively [44,45,46,47,53,61].
The included studies also differed in terms of data collection and diagnostic procedures [43,44,45,46,47,48,51,52,53,54,55,56,57,58,59,60,61,62,63]. The data were mainly collected through measurements of defined parameters [43,44,45,46,47,48,51,52,53,54,55,56,57,58,59,60,61,62,63], surveys [43,44,45,46,47,48,51,52,53,56,57,58,59,60,61,62] and/or clinical examinations [44,45,46,47,48,53,54,55,56,57,61,62,63]. Since no generally applicable definition of the signs and symptoms of a mercury intoxication existed, the diagnosis standards varied between studies, as well [45,46,47,53,54,63].
4.3. Specific Methodology
Six studies reported contradictory data in their manuscripts (see Table 3) [43,48,57,59,60,61]. In addition, one of these studies did not provide all the required information, such as the total number of included participants [59], which made it difficult to assess the results.
Another methodological aspect was the inhomogeneous inclusion of different job categories in the miners’ study groups or in the control group. Exemplarily, three studies also included other occupations, such as gold traders, in the miners’ exposure group [56,57,63]. Therefore, a comparability between the individual studies in general, and in particular their specific results, was limited. Moreover, the study conducted by Ekawanti and Krisnayanti also included child gold miners in the control group [58]. Unfortunately, this point was only mentioned in the Section 4, and no further information was made available in this regard [58].
4.4. Strengths and Limitations of This Review
The key strength of this review is the application of a systematic methodology according to the PRISMA scheme [36]. The underlying review protocol was drafted in advance and submitted to PROSPERO (PROSPERO-ID: CRD42021235289) to secure standard methodological claims and transparency. A further strength was the risk of bias assessment, which was created on the basis of relevant literature in order to classify the different findings of the included studies [49,50]. This review provided an overview of the extensive health-related problems caused by ongoing inorganic mercury exposure.
In contrast, the major limitation of this review was the failed attempt to carry out a meta-analysis due to the unsuitable data for this procedure. Another restriction was the limited publication period, ending on 31 December 2020. Therefore, articles that were published later and could have also fulfilled the eligibility criteria could not be considered.
5. Conclusions
This systematic review underlines the substantial adverse health effects in ASG miners in low- and middle-income countries. Research should therefore continue to focus on the situation of workers in the ASGM sector. More high-quality studies are urgently necessary, as most of the included studies only had a low methodological quality, resulting in a high risk of bias. These should include a defined control group, a clear definition of mercury-related diseases and a diagnostic standard to detect mercury intoxication. A comprehensive assessment of confounding factors of reported symptoms and diseases is necessary, as well, since a variety of symptoms observed in the included studies were quite unspecific. Due to the cross-sectional design, a causal relation was difficult to derive. Prospective studies that detect a clear causation between mercury exposure and mercury-related outcome are urgently required in order to increase the pressure for change. Mercury remains a significant health threat; this has been described in several toxicological animal studies before and should be underlined by epidemiological analyses in humans in the ASGM sector [64,65,66].
In the past, a first attempt was made to reduce the mercury burden with the Minamata Convention that initiates National Action Plans in the affected countries [6,35]. As the presented results indicate, these efforts were not as sufficient as intended. Mercury still causes serious health problems in ASG miners, and the topic demands intensified attention. Awareness must be raised in miners and their environment. Mercury-related symptoms can persist for a long time, even after termination of the corresponding exposure [32,34]. Hence, governments should take action to improve the working conditions for miners. Miners need to be convinced of replacing mercury-containing practices in gold extraction with non-hazardous techniques, as have already been attempted in model projects [70,71]. Finally, mercury-intoxicated miners must also be given the opportunity to receive sufficient medical care.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph19042081/s1, Table S1: Methodological Evaluation (Blank Protocol), Table S2: Overall Risk of Bias Rating.
Author Contributions
Elaboration of the research question: K.T. and A.K.; Study protocol—original draft: K.T.; Study protocol—review and editing: K.T. and A.K.; Literature research: K.T.; Screening of the results: K.T. and A.K.; Data extraction and bias assessment—original draft: K.T.; Data extraction and bias assessment—review and editing: K.T. and A.K.; Calculation of missing effect parameters: A.K.; Manuscript—original draft: K.T.; Manuscript—review and editing: K.T., A.K. and T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be requested by the authors of this review.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ASG miner | artisanal and small-scale gold miner |
| ASGM | artisanal and small-scale gold mining |
| B–Hg | mercury concentration in blood |
| H–Hg | mercury concentration in hair |
| HBM | human biological monitoring |
| Hg | mercury |
| I–Hg | inorganic mercury concentration |
| MeHg | methylmercury |
| N–Hg | mercury concentration in nails |
| O–Hg | organic mercury concentration |
| P–Hg | mercury concentration in plasma |
| T–Hg | total mercury concentration |
| U–Hg | mercury concentration in urine |
| UBA | German Umweltbundesamt, German Environment Agency |
| µg/gCR | microgram per gram creatinine |
References
- Deutsche Bundesbank. Goldbestand der Deutschen Bundesbank. Available online: https://www.bundesbank.de/resource/blob/743058/c70ab6299bc0a4edae91b321e784e081/mL/goldbarrenliste-data.pdf (accessed on 22 February 2021).
- World Bank Group. Commodity Markets Outlook: Causes and Consequences of Metal Price Shocks, April 2021; World Bank: Washington, DC, USA, 2021; License: Creative Commons Attribution CC BY 3.0 IGO. [Google Scholar]
- LBMA. LBMA Precious Metal Prices. Available online: https://www.lbma.org.uk/prices-and-data/precious-metal-prices (accessed on 30 September 2021).
- U.S. Geological Survey. Mineral Commodity Summaries 2021: U.S. Geological Survey; US Geological Survey: Reston, VA, USA, 2021; p. 200. [CrossRef]
- Seccatore, J.; Veiga, M.; Origliasso, C.; Marin, T.; De Tomi, G. An Estimation of the Artisanal Small-Scale Production of Gold in the World. Sci. Total Environ. 2014, 496, 662–667. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Minamata Convention on Mercury. Available online: https://treaties.un.org/doc/Treaties/2013/10/20131010%2011-16%20AM/CTC-XXVII-17.pdf (accessed on 23 September 2021).
- Ghana Statistical Service (GSS). Rebased 2013-2019 Annual Gross Domestic Product. Available online: https://statsghana.gov.gh/gssmain/storage/img/marqueeupdater/Annual_2013_2019_GDP.pdf (accessed on 22 September 2021).
- The Ghana Chamber of Mines. 2020 Mining Industry Statistics and Data. Available online: http://ghanachamberofmines.org/wp-content/uploads/2021/09/2020-Mining-Industry-Statistics-and-Data.pdf (accessed on 22 September 2021).
- Alvarez-Berríos, N.L.; Aide, T.M. Global Demand for Gold Is Another Threat for Tropical Forests. Environ. Res. Lett. 2015, 10, 014006. [Google Scholar] [CrossRef] [Green Version]
- Marshall, B.G.; Veiga, M.M.; da Silva, H.A.M.; Guimarães, J.R.D. Cyanide Contamination of the Puyango-Tumbes River Caused by Artisanal Gold Mining in Portovelo-Zaruma, Ecuador. Curr. Envir. Health Rpt. 2020, 7, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J.D.; Weeks, J.M.; Calvez, J.P.S.; Beinhoff, C. Impacts of Mercury Contaminated Mining Waste on Soil Quality, Crops, Bivalves, and Fish in the Naboc River Area, Mindanao, Philippines. Sci. Total Environ. 2006, 354, 198–211. [Google Scholar] [CrossRef] [Green Version]
- Marshall, B.G.; Veiga, M.M.; Kaplan, R.J.; Adler Miserendino, R.; Schudel, G.; Bergquist, B.A.; Guimarães, J.R.D.; Sobral, L.G.S.; Gonzalez-Mueller, C. Evidence of Transboundary Mercury and Other Pollutants in the Puyango-Tumbes River Basin, Ecuador–Peru. Environ. Sci. Processes Impacts 2018, 20, 632–641. [Google Scholar] [CrossRef]
- Nakua, E.K.; Owusu-Dabo, E.; Newton, S.; Koranteng, A.; Otupiri, E.; Donkor, P.; Mock, C. Injury Rate and Risk Factors among Small-Scale Gold Miners in Ghana. BMC Public Health 2019, 19, 1368. [Google Scholar] [CrossRef] [Green Version]
- Steckling, N.; Tobollik, M.; Plass, D.; Hornberg, C.; Ericson, B.; Fuller, R.; Bose-O’Reilly, S. Global Burden of Disease of Mercury Used in Artisanal Small-Scale Gold Mining. Ann. Glob. Health 2017, 83, 234–247. [Google Scholar] [CrossRef] [Green Version]
- Stoffersen, B.; Køster-Rasmussen, R.; Cardeño, J.I.C.; Appel, P.W.U.; Smidth, M.; Na-Oy, L.D.; Lardizabal, D.L.; Onos, R.W. Comparison of Gold Yield with Traditional Amalgamation and Direct Smelting in Artisanal Small-Scale Gold Mining in Uganda. J. Health Pollut. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Hylander, L.D.; Plath, D.; Miranda, C.R.; Lücke, S.; Öhlander, J.; Rivera, A.T.F. Comparison of Different Gold Recovery Methods with Regard to Pollution Control and Efficiency. Clean Soil Air Water 2007, 35, 52–61. [Google Scholar] [CrossRef]
- European Union. Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008R1272&from=en (accessed on 23 September 2021).
- Bagnato, E.; Allard, P.; Parello, F.; Aiuppa, A.; Calabrese, S.; Hammouya, G. Mercury Gas Emissions from La Soufrière Volcano, Guadeloupe Island (Lesser Antilles). Chem. Geol. 2009, 266, 267–273. [Google Scholar] [CrossRef]
- Cui, Z.; Li, Z.; Zhang, Y.; Wang, X.; Li, Q.; Zhang, L.; Feng, X.; Li, X.; Shang, L.; Yao, Z. Atmospheric Mercury Emissions from Residential Coal Combustion in Guizhou Province, Southwest China. Energy Fuels 2019, 33, 1937–1943. [Google Scholar] [CrossRef]
- AMAP/UN Environment. Technical Background Report for the Global Mercury Assessment 2018. In Arctic Monitoring and Assessment Programme, Oslo, Norway/UN Environment Programme; Viii + 426 Pp Including E-Annexes; Chemicals and Health Branch: Geneva, Switzerland, 2019. [Google Scholar]
- Kudsk, F.N. Absorption of Mercury Vapour from the Respiratory Tract in Man. Acta Pharmacol. Et Toxicol. 1965, 23, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Hursh, J.B.; Clarkson, T.W.; Miles, E.F.; Goldsmith, L.A. Percutaneous Absorption of Mercury Vapor by Man. Arch. Environ. Health Int. J. 1989, 44, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Geijersstam, E.; Sandborgh-Englund, G.; Jonsson, F.; Ekstrand, J. Mercury Uptake and Kinetics after Ingestion of Dental Amalgam. J. Dent. Res. 2001, 80, 1793–1796. [Google Scholar] [CrossRef]
- Sandborgh-Englund, G.; Elinder, C.-G.; Johanson, G.; Lind, B.; Skare, I.; Ekstrand, J. The Absorption, Blood Levels, and Excretion of Mercury after a Single Dose of Mercury Vapor in Humans. Toxicol. Appl. Pharmacol. 1998, 150, 146–153. [Google Scholar] [CrossRef]
- Jonsson, F.; Sandborgh-Englund, G.; Johanson, G. A Compartmental Model for the Kinetics of Mercury Vapor in Humans. Toxicol. Appl. Pharmacol. 1999, 155, 161–168. [Google Scholar] [CrossRef]
- Falnoga, I.; Tušek-Žnidarič, M.; Horvat, M.; Stegnar, P. Mercury, Selenium, and Cadmium in Human Autopsy Samples from Idrija Residents and Mercury Mine Workers. Environ. Res. 2000, 84, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, N.; Suzuki, T.; Akagi, H. Mercury Concentration in Organs of Contemporary Japanese. Arch. Environ. Health Int. J. 1989, 44, 298–303. [Google Scholar] [CrossRef]
- Institut für Wasser-, Boden- und Lufthygiene des Umweltbundesamtes. Stoffmonographie Quecksilber – Referenz- Und Human-Biomonitoring-(HBM)-Werte. Bundesgesundheitsblatt Gesundh. Gesundh. 1999, 42, 522–532. [Google Scholar] [CrossRef]
- Cortes, J.; Peralta, J.; Díaz-Navarro, R. Acute Respiratory Syndrome Following Accidental Inhalation of Mercury Vapor. Clin. Case Rep. 2018, 6, 1535–1537. [Google Scholar] [CrossRef]
- Cicek-Senturk, G.; Altay, F.A.; Ulu-Kilic, A.; Gurbuz, Y.; Tutuncu, E.; Sencan, I. Acute Mercury Poisoning Presenting as Fever of Unknown Origin in an Adult Woman: A Case Report. J. Med. Case Rep. 2014, 8, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowens, B.; Guerrero-Betancourt, D.; Gottlieb, C.A.; Boyes, R.J.; Eichenhorn, M.S. Respiratory Failure and Death Following Acute Inhalation of Mercury Vapor. Chest 1991, 99, 185–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishi, R.; Doi, R.; Fukuchi, Y.; Satoh, H.; Satoh, T.; Ono, A.; Moriwaka, F.; Tashiro, K.; Takahata, N. Subjective Symptoms and Neurobehavioral Performances of Ex-Mercury Miners at an Average of 18 Years after the Cessation of Chronic Exposure to Mercury Vapor. Environ. Res. 1993, 62, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Feng, X.; Qiu, G.; Li, Z.; Fu, X.; Sakamoto, M.; Liu, X.; Wang, D. Mercury Exposures and Symptoms in Smelting Workers of Artisanal Mercury Mines in Wuchuan, Guizhou, China. Environ. Res. 2008, 107, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Zachi, E.C.; Taub, A.; Faria, M.D.A.M.; Ventura, D.F. Neuropsychological Alterations in Mercury Intoxication Persist Several Years after Exposure. Dement. Neuropsychol. 2008, 2, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Minamata Convention on Mercury. Parties and Signatories. Available online: https://www.mercuryconvention.org/en/parties (accessed on 23 September 2021).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 88, 105906. [Google Scholar] [CrossRef]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A Framework for Formulating Good Questions to Explore the Association of Environmental and Other Exposures with Health Outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef]
- Afrifa, J.; Opoku, Y.K.; Gyamerah, E.O.; Ashiagbor, G.; Sorkpor, R.D. The Clinical Importance of the Mercury Problem in Artisanal Small-Scale Gold Mining. Front. Public Health 2019, 7, 131. [Google Scholar] [CrossRef]
- Gibb, H.; O’Leary, K.G. Mercury Exposure and Health Impacts among Individuals in the Artisanal and Small-Scale Gold Mining Community: A Comprehensive Review. Environ. Health Perspect. 2014, 122, 667–672. [Google Scholar] [CrossRef]
- Berzas Nevado, J.J.; Rodríguez Martín-Doimeadios, R.C.; Guzmán Bernardo, F.J.; Jiménez Moreno, M.; Herculano, A.M.; do Nascimento, J.L.M.; Crespo-López, M.E. Mercury in the Tapajós River Basin, Brazilian Amazon: A Review. Environ. Int. 2010, 36, 593–608. [Google Scholar] [CrossRef]
- Vianna, A.D.S.; Matos, E.P.D.; Jesus, I.M.D.; Asmus, C.I.R.F.; Câmara, V.D.M. Human Exposure to Mercury and Its Hematological Effects: A Systematic Review. Cad. Saude Publica 2019, 35, e00091618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chirico, F.; Scoditti, E.; Viora, C.; Magnavita, N. How Occupational Mercury Neurotoxicity Is Affected by Genetic Factors. A Systematic Review. Appl. Sci. 2020, 10, 7706. [Google Scholar] [CrossRef]
- Afrifa, J.; Essien-Baidoo, S.; Ephraim, R.K.D.; Nkrumah, D.; Dankyira, D.O. Reduced Egfr, Elevated Urine Protein and Low Level of Personal Protective Equipment Compliance among Artisanal Small Scale Gold Miners at Bibiani-Ghana: A Cross-Sectional Study. BMC Public Health 2017, 17, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajaee, M.; Sánchez, B.; Renne, E.; Basu, N. An Investigation of Organic and Inorganic Mercury Exposure and Blood Pressure in a Small-Scale Gold Mining Community in Ghana. Int. J. Environ. Res. Public Health 2015, 12, 10020–10038. [Google Scholar] [CrossRef] [Green Version]
- Bose-O’Reilly, S.; Drasch, G.; Beinhoff, C.; Tesha, A.; Drasch, K.; Roider, G.; Taylor, H.; Appleton, D.; Siebert, U. Health Assessment of Artisanal Gold Miners in Tanzania. Sci. Total Environ. 2010, 408, 796–805. [Google Scholar] [CrossRef]
- Bose-O’Reilly, S.; Lettmeier, B.; Gothe, R.M.; Beinhoff, C.; Siebert, U.; Drasch, G. Mercury as a Serious Health Hazard for Children in Gold Mining Areas. Environ. Res. 2008, 107, 89–97. [Google Scholar] [CrossRef]
- Bose-O’Reilly, S.; Drasch, G.; Beinhoff, C.; Rodrigues-Filho, S.; Roider, G.; Lettmeier, B.; Maydl, A.; Maydl, S.; Siebert, U. Health Assessment of Artisanal Gold Miners in Indonesia. Sci. Total Environ. 2010, 408, 713–725. [Google Scholar] [CrossRef]
- Branches, F.J.; Erickson, T.B.; Aks, S.E.; Hryhorczuk, D.O. The Price of Gold: Mercury Exposure in the Amazonian Rain Forest. J. Toxicol. Clin. Toxicol. 1993, 31, 295–306. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The Feasibility of Creating a Checklist for the Assessment of the Methodological Quality Both of Randomised and Non-Randomised Studies of Health Care Interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.; Altman, D.; Sterne, J. Chapter 8: Assessing Risk of Bias in Included Studies. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011); Higgins, J.P.T., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2011; Available online: www.handbook.cochrane.org (accessed on 23 September 2021).
- Afrifa, J.; Ogbordjor, W.D.; Duku-Takyi, R. Variation in Thyroid Hormone Levels Is Associated with Elevated Blood Mercury Levels among Artisanal Small-Scale Miners in Ghana. PLoS ONE 2018, 13, e0203335. [Google Scholar] [CrossRef]
- Mensah, E.K.; Afari, E.; Wurapa, F.; Sackey, S.; Quainoo, A.; Kenu, E.; Nyarko, K.M. Exposure of Small-Scale Gold Miners in Prestea to Mercury, Ghana, 2012. Pan Afr. Med. J. 2016, 25, 6. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Nakachi, S.; Cheu, T.; Hamada, H.; Ono, Y.; Tsuda, T.; Yanagida, K.; Kizaki, T.; Ohno, H. Monitoring of Mercury Pollution in Tanzania: Relation between Head Hair Mercury and Health. Sci. Total Environ. 1999, 227, 249–256. [Google Scholar] [CrossRef]
- Steckling, N.; Bose-O’Reilly, S.; Pinheiro, P.; Plass, D.; Shoko, D.; Drasch, G.; Bernaudat, L.; Siebert, U.; Hornberg, C. The Burden of Chronic Mercury Intoxication in Artisanal Small-Scale Gold Mining in Zimbabwe: Data Availability and Preliminary Estimates. Environ. Health 2014, 13, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tayrab, E. Thyroid Function in Sudanese Gold Miners with Chronic Mercury Exposure. Eur. J. Pharm. Med. Res. 2017, 4, 177–180. [Google Scholar]
- Tomicic, C.; Vernez, D.; Belem, T.; Berode, M. Human Mercury Exposure Associated with Small-Scale Gold Mining in Burkina Faso. Int. Arch. Occup. Environ. Health 2011, 84, 539–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanyana, M.W.; Agaba, F.E.; Sekimpi, D.K.; Mukasa, V.N.; Kamese, G.N.; Douglas, N.; Ssempebwa, J.C. Mercury Exposure Among Artisanal and Small-Scale Gold Miners in Four Regions in Uganda. J. Health Pollut. 2020, 10, 200613. [Google Scholar] [CrossRef]
- Ekawanti, A.; Krisnayanti, B.D. Effect of Mercury Exposure on Renal Function and Hematological Parameters among Artisanal and Small-Scale Gold Miners at Sekotong, West Lombok, Indonesia. J. Health Pollut. 2015, 5, 25–32. [Google Scholar] [CrossRef]
- Khan, S.; Shah, M.T.; Din, I.U.; Rehman, S. Mercury Exposure of Workers and Health Problems Related with Small-Scale Gold Panning and Extraction. J. Chem. Soc. Pak. 2012, 34, 870–876. [Google Scholar]
- Riaz, A.; Khan, S.; Shah, M.T.; Li, G.; Gul, N.; Shamshad, I. Mercury Contamination in the Blood, Urine, Hair and Nails of the Gold Washers and Its Human Health Risk during Extraction of Placer Gold along Gilgit, Hunza and Indus Rivers in Gilgit-Baltistan, Pakistan. Environ. Technol. Innov. 2016, 5, 22–29. [Google Scholar] [CrossRef]
- Lacerda, E.M.D.C.B.; Souza, G.D.S.; Cortes, M.I.T.; Rodrigues, A.R.; Pinheiro, M.C.N.; Silveira, L.C.D.L.; Ventura, D.F. Comparison of Visual Functions of Two Amazonian Populations: Possible Consequences of Different Mercury Exposure. Front. Neurosci. 2020, 13, 1428. [Google Scholar] [CrossRef] [Green Version]
- Harari, R.; Harari, F.; Gerhardsson, L.; Lundh, T.; Skerfving, S.; Strömberg, U.; Broberg, K. Exposure and Toxic Effects of Elemental Mercury in Gold-Mining Activities in Ecuador. Toxicol. Lett. 2012, 213, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Schutzmeier, P.; Berger, U.; Bose-O’Reilly, S. Gold Mining in Ecuador: A Cross-Sectional Assessment of Mercury in Urine and Medical Symptoms in Miners from Portovelo/Zaruma. Int. J. Environ. Res. Public Health 2016, 14, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akgül, N.; Altunkaynak, B.Z.; Altunkaynak, M.E.; Deniz, Ö.G.; Ünal, D.; Akgül, H.M. Inhalation of Mercury Vapor Can Cause the Toxic Effects on Rat Kidney. Ren. Fail. 2016, 38, 465–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altunkaynak, B.Z.; Akgül, N.; Yahyazedeh, A.; Makaracı, E.; Akgül, H.M. A Stereological Study of the Effects of Mercury Inhalation on the Cerebellum. Biotech. Histochem. 2019, 94, 42–47. [Google Scholar] [CrossRef]
- Yoshida, M.; Lee, J.-Y.; Satoh, M.; Watanabe, C. Neurobehavioral Effects of Postnatal Exposure to Low-Level Mercury Vapor and/or Methylmercury in Mice. J. Toxicol. Sci. 2018, 43, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Al-Batanony, M.A.; Abdel-Rasul, G.M.; Abu-Salem, M.A.; Al-Dalatony, M.M.; Allam, H.K. Occupational Exposure to Mercury among Workers in a Fluorescent Lamp Factory, Quisna Industrial Zone, Egypt. Int. J. Occup. Environ. Med. 2013, 4, 149–156. [Google Scholar]
- Decharat, S. Urinary Mercury Levels Among Workers in E-Waste Shops in Nakhon Si Thammarat Province, Thailand. J. Prev. Med. Public Health 2018, 51, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Neghab, M.; Amin Norouzi, M.; Choobineh, A.; Reza Kardaniyan, M.; Hassan Zadeh, J. Health Effects Associated With Long-Term Occupational Exposure of Employees of a Chlor-Alkali Plant to Mercury. Int. J. Occup. Saf. Ergon. 2012, 18, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Appel, P.W.U.; Andersen, A.; Na-Oy, L.D.; Onos, R. Introduction of Mercury-Free Gold Extraction Methods to Medium-Scale Miners and Education of Health Care Providers to Reduce the Use of Mercury in Sorata, Bolivia. J. Health Pollut. 2015, 5, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Køster-Rasmussen, R.; Westergaard, M.L.; Brasholt, M.; Gutierrez, R.; Jørs, E.; Thomsen, J.F. Mercury Pollution from Small-Scale Gold Mining Can Be Stopped by Implementing the Gravity-Borax Method--A Two-Year Follow-Up Study from Two Mining Communities in the Philippines. New Solut. 2016, 25, 567–587. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).