Krebs von den Lungen-6 as Disease Severity Marker for COVID-19 Patients: Analytical Verification and Quality Assessment of the Tosoh AIA-360 Compared to Lumipulse G600II
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. KL-6 Assay
2.3. KL-6: Quality Control of Analytical Determinations and Comparison of Results
2.4. Statistical Analysis
3. Results
3.1. Study Population
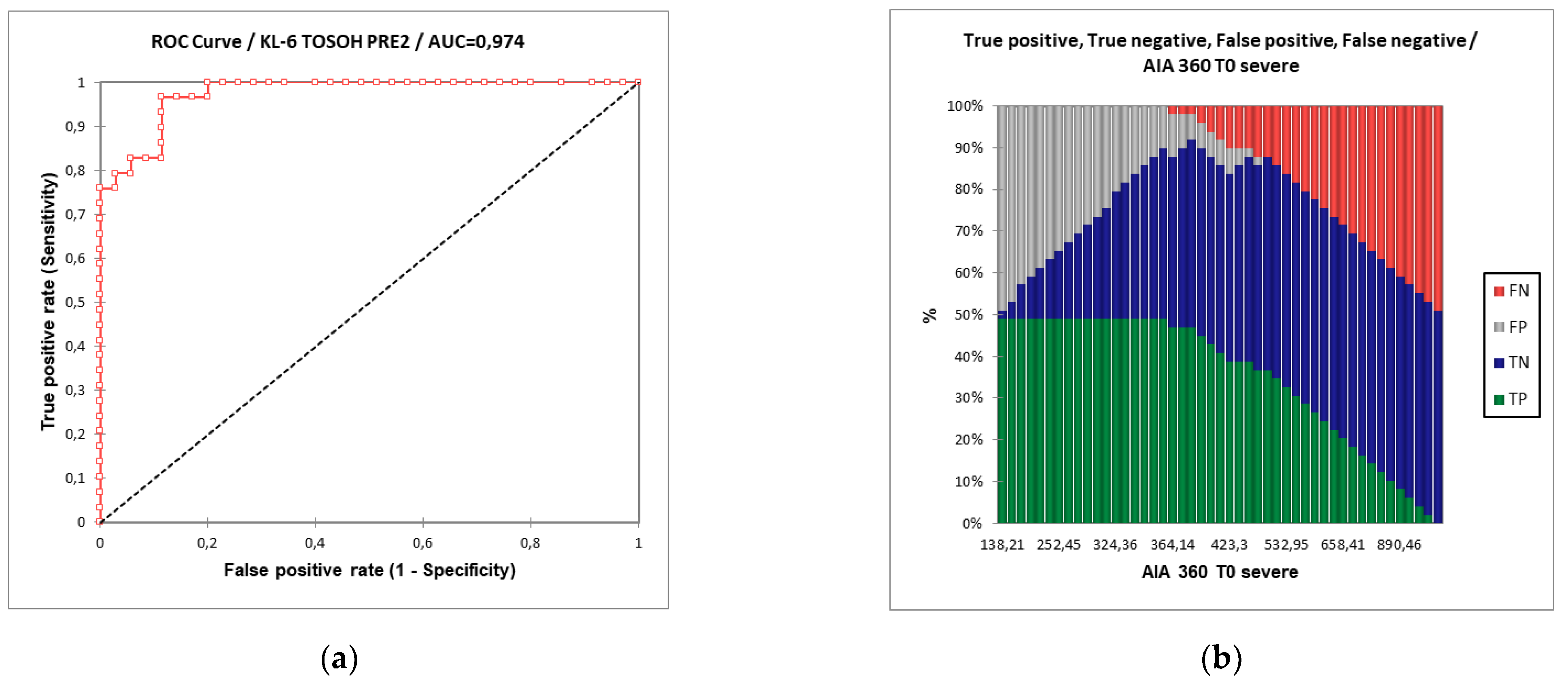
3.2. KL-6 Assay and Analytical Validation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- D’Alessandro, M.; Bergantini, L.; Cameli, P.; Vietri, L.; Lanzarone, N.; Alonzi, V.; Pieroni, M.; M Refini, R.; Sestini, P.; Bonella, F.; et al. Krebs Von Den Lungen-6 as a Biomarker for Disease Severity Assessment in Interstitial Lung Disease: A Comprehensive Review. Biomark. Med. 2020, 14, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, L.-S.; Jin, Y.-P.; Du, S.-S.; Du, Y.-K.; He, X.; Weng, D.; Zhou, Y.; Li, Q.-H.; Shen, L.; et al. Serum Krebs Von Den Lungen-6 Level as a Diagnostic Biomarker for Interstitial Lung Disease in Chinese Patients. Clin. Respir. J. 2017, 11, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; Kohno, N.; Akiyama, M.; Hiwada, K. Monitoring of Serum Kl-6 Antigen in a Patient with Radiation Pneumonia. Chest 1992, 101, 858–860. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Kohno, N.; Kondo, K.; Ueda, S.; Hirasawa, Y.; Watanabe, K.; Takada, Y.; Hiwada, K. Comparative Evaluation of Sialylated Carbohydrate Antigens, Kl-6, Ca19-9 and Slx as Serum Markers for Interstitial Pneumonia. Respirology 1998, 3, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Kondo, K.; Nakajima, M.; Matsushima, T.; Takahashi, T.; Nishimura, M.; Bando, M.; Sugiyama, Y.; Totani, Y.; Ishizaki, T.; et al. Prognostic Value of Circulating Kl-6 in Idiopathic Pulmonary Fibrosis. Respirology 2006, 11, 164–168. [Google Scholar] [CrossRef]
- Ishikawa, N.; Hattori, N.; Yokoyama, A.; Kohno, N. Utility of Kl-6/Muc1 in The Clinical Management of Interstitial Lung Diseases. Respir. Investig. 2012, 50, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Iwata, Y.; Wada, T.; Furuichi, K.; Kitagawa, K.; Kokubo, S.; Kobayashi, M.; Sakai, N.; Yoshimoto, K.; Shimizu, M.; Kobayashi, K.; et al. Serum Levels of Kl-6 Reflect Disease Activity of Interstitial Pneumonia Associated with Anca-Related Vasculitis. Intern. Med. 2001, 40, 1093–1097. [Google Scholar] [CrossRef] [Green Version]
- Bergantini, L.; Bianchi, F.; Cameli, P.; Mazzei, M.A.; Fui, A.; Sestini, P.; Rottoli, P.; Bargagli, E. Prognostic Biomarkers of Sarcoidosis: A Comparative Study of Serum Chitotriosidase, Ace, Lysozyme, and Kl-6. Dis. Markers 2019, 2019, 8565423. [Google Scholar] [CrossRef]
- Bergantini, L.; Bargagli, E.; Cameli, P.; Cekorja, B.; Lanzarone, N.; Pianigiani, L.; Vietri, L.; Bennett, D.; Sestini, P.; Rottoli, P. Serial Kl-6 Analysis in Patients with Idiopathic Pulmonary Fibrosis Treated with Nintedanib. Respir. Investig. 2019, 57, 290–291. [Google Scholar] [CrossRef]
- D’alessandro, M.; Bergantini, L.; Cameli, P.; Lanzarone, N.; Antonietta Mazzei, M.; Alonzi, V.; Sestini, P.; Bargagli, E. Serum Kl-6 Levels in Pulmonary Langerhans’ Cell Histiocytosis. Eur. J. Clin. Investig. 2020, 50, e13242. [Google Scholar] [CrossRef]
- D’alessandro, M.; Bellisai, F.; Bergantini, L.; Cameli, P.; D’alessandro, R.; Mazzei, M.A.; Gentili, F.; Conticini, E.; Selvi, E.; Frediani, B.; et al. Prognostic Role of Kl-6 in Ssc-Ild Patients with Pleuroparenchymal Fibroelastosis. Eur. J. Clin. Investig. 2021, 51, E13543. [Google Scholar] [CrossRef] [PubMed]
- D’alessandro, M.; Bergantini, L.; Cameli, P.; Perillo, F.; Remediani, L.; Refini, R.M.; Pieroni, M.; Mazzei, M.A.; Sestini, P.; Bargagli, E. Prognostic Role of Kl-6 in Lymphangioleiomyomatosis Patients. Minerva Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- D’alessandro, M.; Bergantini, L.; Cameli, P.; Pieroni, M.; Refini, R.M.; Sestini, P.; Bargagli, E. Serum Concentrations of Kl-6 in Patients with Ipf and Lung Cancer and Serial Measurements of Kl-6 in Ipf Patients Treated with Antifibrotic Therapy. Cancers 2021, 13, 689. [Google Scholar] [CrossRef] [PubMed]
- D’alessandro, M.; Bergantini, L.; Torricelli, E.; Cameli, P.; Lavorini, F.; Pieroni, M.; Refini, R.M.; Sestini, P.; Bargagli, E. Systematic Review and Metanalysis of Oncomarkers in Ipf Patients and Serial Changes of Oncomarkers in a Prospective Italian Real-Life Case Series. Cancers 2021, 13, 539. [Google Scholar] [CrossRef]
- Sato, H.; Callister, M.E.J.; Mumby, S.; Quinlan, G.J.; Welsh, K.I.; Dubois, R.M.; Evans, T.W. Kl-6 Levels Are Elevated in Plasma From Patients with Acute Respiratory Distress Syndrome. Eur. Respir. J. 2004, 23, 142–145. [Google Scholar] [CrossRef]
- Nakamura, H.; Tateyama, M.; Tasato, D.; Haranaga, S.; Yara, S.; Higa, F.; Ohtsuki, Y.; Fujita, J. Clinical Utility of Serum Beta-D-Glucan and Kl-6 Levels in Pneumocystis Jirovecii Pneumonia. Intern. Med. 2009, 48, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Urabe, N.; Sakamoto, S.; Sano, G.; Ito, A.; Sekiguchi, R.; Homma, S. Serial Change in Serum Biomarkers During Treatment of Non-Hiv Pneumocystis Pneumonia. J. Infect. Chemother. 2019, 25, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Obinata, K.; Sato, Y.; Hisata, K.; Tadokoro, R.; Tawa, T.; Kinoshita, K. Clinical Significance of the Serum Surfactant Protein D and Kl-6 Levels in Patients with Measles Complicated By Interstitial Pneumonia. Eur. J. Pediatr. 2001, 160, 425–429. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Aoyagi, Y.; Abe, Y.; Go, H.; Imamura, T.; Kaneko, M.; Ito, M.; Katayose, M.; Hashimoto, K.; Hosoya, M. Serum Kl-6 Levels as a Biomarker of Lung Injury in Respiratory Syncytial Virus Bronchiolitis. J. Med. Virol. 2009, 81, 2104–2108. [Google Scholar] [CrossRef]
- Bergantini, L.; Bargagli, E.; D’alessandro, M.; Refini, R.M.; Cameli, P.; Galasso, L.; Scapellato, C.; Montagnani, F.; Scolletta, S.; Franchi, F.; et al. Prognostic Bioindicators in Severe COVID-19 Patients. Cytokine 2021, 141, 155455. [Google Scholar] [CrossRef]
- D’alessandro, M.; Bergantini, L.; Cameli, P.; Curatola, G.; Remediani, L.; Bennett, D.; Bianchi, F.; Perillo, F.; Volterrani, L.; Mazzei, M.A.; et al. Serial Kl-6 Measurements in COVID-19 Patients. Intern. Emerg. Med. 2021, 16, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- D’alessandro, M.; Cameli, P.; Refini, R.M.; Bergantini, L.; Alonzi, V.; Lanzarone, N.; Bennett, D.; Rana, G.D.; Montagnani, F.; Scolletta, S.; et al. Serum Kl-6 Concentrations as a Novel Biomarker of Severe COVID-19. J. Med. Virol. 2020, 92, 2216–2220. [Google Scholar] [CrossRef] [PubMed]
- D’alessandro, M.; Cameli, P.; Bergantini, L.; Franchi, F.; Scolletta, S.; Bargagli, E. Serum Concentrations of Krebs Von Den Lungen-6 in Different COVID-19 Phenotypes. J. Med. Virol. 2020, 93, 657. [Google Scholar] [CrossRef] [PubMed]
- Awano, N.; Inomata, M.; Kuse, N.; Tone, M.; Takada, K.; Muto, Y.; Fujimoto, K.; Akagi, Y.; Mawatari, M.; Ueda, A.; et al. Serum Kl-6 Level Is a Useful Biomarker for Evaluating The Severity of Coronavirus Disease 2019. Respir. Investig. 2020, 58, 440–447. [Google Scholar] [CrossRef]
- Ep05a3: Evaluating Quantitative Measurement Precision. Available online: Https://Clsi.Org/Standards/Products/Method-Evaluation/Documents/Ep05/ (accessed on 26 December 2021).
- Budd, J.R.; Durham, A.P.; Gwise, T.E.; Hawkins, D.M.; Holland, M.; Iriarte, B.; Kallner, A.; Linnet, K.; Magari, R.; Vaks, J.E. Measurement Procedure Comparison and Bias Estimation Using Patient Samples; Clinical Laboratory Standards Institute: Wayne, NY, USA, 2013. [Google Scholar]
- Bland, J.M.; Altman, D.G. Statistical Methods for Assessing Agreement Between Two Methods of Clinical Measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Hilkens, J.; Ligtenberg, M.J.; Vos, H.L.; Litvinov, S.V. Cell Membrane-Associated Mucins and Their Adhesion-Modulating Property. Trends Biochem. Sci. 1992, 17, 359–363. [Google Scholar] [CrossRef]
- Hirasawa, Y.; Kohno, N.; Yokoyama, A.; Inoue, Y.; Abe, M.; Hiwada, K. Kl-6, a Human Muc1 Mucin, Is Chemotactic for Human Fibroblasts. Am. J. Respir. Cell Mol. Biol. 1997, 17, 501–507. [Google Scholar] [CrossRef]
- Bonella, F.; Costabel, U. Biomarkers in Connective Tissue Disease-Associated Interstitial Lung Disease. Semin. Respir. Crit. Care Med. 2014, 35, 181–200. [Google Scholar] [CrossRef]
- Bonella, F.; Volpe, A.; Caramaschi, P.; Nava, C.; Ferrari, P.; Schenk, K.; Ohshimo, S.; Costabel, U.; Ferrari, M. Surfactant Protein D and Kl-6 Serum Levels in Systemic Sclerosis: Correlation with Lung and Systemic Involvement. Sarcoidosis Vasc. Diffus. Lung Dis. 2011, 28, 27–33. [Google Scholar]
- Zhong, D.; Wu, C.; Bai, J.; Hu, C.; Xu, D.; Wang, Q.; Zeng, X. Comparative Diagnostic Efficacy of Serum Krebs Von Den Lungen-6 and Surfactant D for Connective Tissue Disease-Associated Interstitial Lung Diseases: A Meta-Analysis. Medicine 2020, 99, E19695. [Google Scholar] [CrossRef]
- Xue, M.; Guo, Z.; Cai, C.; Sun, B.; Wang, H. Evaluation of The Diagnostic Efficacies of Serological Markers Kl-6, Sp-A, Sp-D, Ccl2, and Cxcl13 in Idiopathic Interstitial Pneumonia. Respiration 2019, 98, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Lanzarone, N.; Gentili, F.; Alonzi, V.; Bergantini, L.; D’alessandro, M.; Rottoli, P.; Refini, R.M.; Pieroni, M.; Vietri, L.; Bianchi, F.; et al. Bronchoalveolar Lavage and Serum Kl-6 Concentrations in Chronic Hypersensitivity Pneumonitis: Correlations With Radiological and Immunological Features. Intern. Emerg. Med. 2020, 15, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Kurishima, K.; Kagohashi, K.; Kawaguchi, M.; Ishikawa, H.; Satoh, H.; Hizawa, N. Serum Kl-6 Levels in Lung Cancer Patients with or Without Interstitial Lung Disease. J. Clin. Lab. Anal. 2010, 24, 295–299. [Google Scholar] [CrossRef] [PubMed]





| Parameters | Severe (n = 29) | Non-Severe (n = 35) | p Value |
|---|---|---|---|
| Age (median IQR) | 71 (62–79) | 63 (57–73) | NS |
| Gender, M/F | 20/9 | 28/7 | NS |
| T0 KL-6 (U/mL), Lumipulse G600II | 827 (599–1103) | 293 (220–345) | <0.0001 |
| T0 KL-6 (U/mL), AIA360 | 658 (508–936) | 282 (209–346) | <0.0001 |
| Blood count | |||
| Lymphocytes % | 13.6 (6.4–15.9) | 18.4 (15.9–22.5) | 0.041 |
| Neutrophils % | 73.5 (67.5–77.1) | 77 (68–81) | NS |
| Eosinophils % | 0.2 (0–1.4) | 0.08 (0.02–0.1) | NS |
| Basophils % | 0.15 (0.10–0.17) | 0.3 (0.2–0.4) | NS |
| Monocytes % | 7.8 (5.38.5) | 7.4 (2.9–7.9) | NS |
| WBC (cells/µL) | 5.2 (3.7–6.9) | 4.9 (3.2–7.1) | NS |
| CRP (mg/L) | 5.84 (2.27–6.9) | 4.64 (0.64–9.7) | 0.037 |
| LDH (U/L) | 351 (295–589) | 252 (232–330) | NS |
| Glycemia (mg/dL) | 104 (105–224) | 96 (76–110) | NS |
| Lipase (U/L) | 24 (14–28) | 20 (17–23) | NS |
| Pancreatic Amylase (U/L) | 30 (18–36) | 28 (16–42) | NS |
| AST (U/L) | 33 (22–42) | 24 (19–39) | NS |
| ALT (U/L) | 32 (13–49) | 21 (13–29) | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
d’Alessandro, M.; Bergantini, L.; Cavallaro, D.; Gangi, S.; Cameli, P.; Conticini, E.; Siena COVID Unit; Frediani, B.; Dotta, F.; Bargagli, E. Krebs von den Lungen-6 as Disease Severity Marker for COVID-19 Patients: Analytical Verification and Quality Assessment of the Tosoh AIA-360 Compared to Lumipulse G600II. Int. J. Environ. Res. Public Health 2022, 19, 2176. https://doi.org/10.3390/ijerph19042176
d’Alessandro M, Bergantini L, Cavallaro D, Gangi S, Cameli P, Conticini E, Siena COVID Unit, Frediani B, Dotta F, Bargagli E. Krebs von den Lungen-6 as Disease Severity Marker for COVID-19 Patients: Analytical Verification and Quality Assessment of the Tosoh AIA-360 Compared to Lumipulse G600II. International Journal of Environmental Research and Public Health. 2022; 19(4):2176. https://doi.org/10.3390/ijerph19042176
Chicago/Turabian Styled’Alessandro, Miriana, Laura Bergantini, Dalila Cavallaro, Sara Gangi, Paolo Cameli, Edoardo Conticini, Siena COVID Unit, Bruno Frediani, Francesco Dotta, and Elena Bargagli. 2022. "Krebs von den Lungen-6 as Disease Severity Marker for COVID-19 Patients: Analytical Verification and Quality Assessment of the Tosoh AIA-360 Compared to Lumipulse G600II" International Journal of Environmental Research and Public Health 19, no. 4: 2176. https://doi.org/10.3390/ijerph19042176
APA Styled’Alessandro, M., Bergantini, L., Cavallaro, D., Gangi, S., Cameli, P., Conticini, E., Siena COVID Unit, Frediani, B., Dotta, F., & Bargagli, E. (2022). Krebs von den Lungen-6 as Disease Severity Marker for COVID-19 Patients: Analytical Verification and Quality Assessment of the Tosoh AIA-360 Compared to Lumipulse G600II. International Journal of Environmental Research and Public Health, 19(4), 2176. https://doi.org/10.3390/ijerph19042176











