Phthalate Esters in Tap Water, Southern Thailand: Daily Exposure and Cumulative Health Risk in Infants, Lactating Mothers, Pregnant and Nonpregnant Women
Abstract
:1. Introduction
2. Materials and Methods
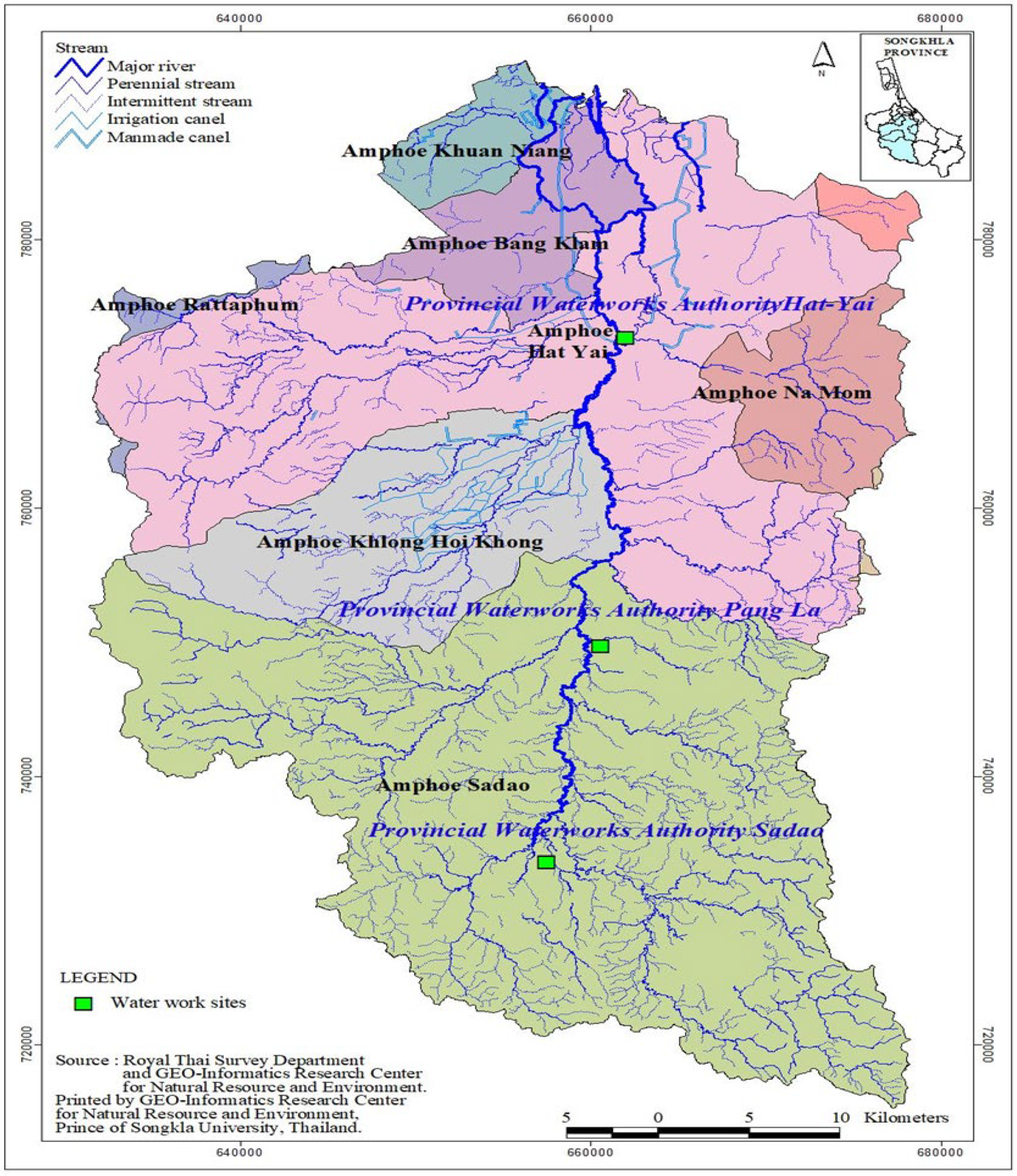
2.1. Sample Collection
2.2. Chemicals and Reagents
2.3. Sample Pretreatment
2.4. Instrumental Analysis
2.5. Quality Control/Assurance
2.6. PAE Removal Efficiency by Conventional Treatment Process
2.7. Health Risk Assessment
2.8. Statistical Analysis
3. Results
3.1. Occurrence and Fate of PAEs in Treatment Plants
3.2. Comparison with Studies in Other Locations
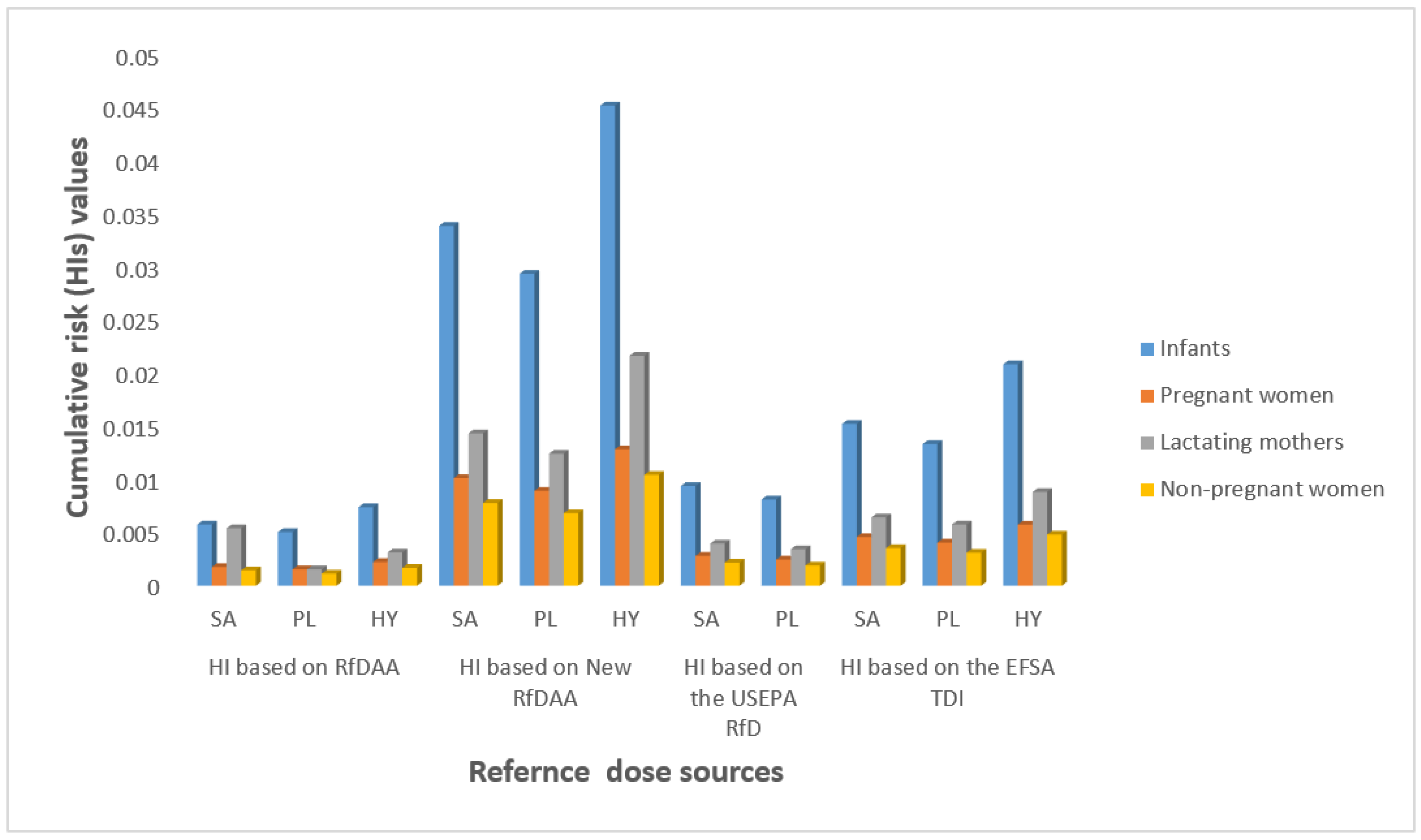
3.3. Estimated Daily Intake and Risk
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staples, C.A.; Peterson, D.R.; Parkerton, T.F.; Adams, W.J. The environmental fate of phthalate esters: A literature review. Chemosphere 1997, 35, 667–749. [Google Scholar] [CrossRef]
- Yan, X.; Calafat, A.; Lashley, S.; Smulian, J.; Ananth, C.; Barr, D.; Robson, M.G. Phthalates Biomarker Identification and Exposure Estimates in a Population of Pregnant Women. Hum. Ecol. Risk Assess. 2009, 15, 565–578. [Google Scholar] [CrossRef] [Green Version]
- Net, S.; Sempéré, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, fate, behavior and ecotoxicological state of phthalates in different environmental matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Hirata-Koizumi, M.; Ema, M. Potential adverse effects of phthalic acid esters on human health: A review of recent studies on reproduction. Regul. Toxicol. Pharmacol. 2008, 50, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S. Pharmaceuticals and Endocrine Disrupting Compounds in U.S. Drinking Water. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gou, Y.-Y.; Lin, S.; Que, D.; Tayo, L.L.; Lin, D.-Y.; Chen, K.-C.; Chen, F.-A.; Chiang, P.-C.; Wang, G.-S.; Hsu, Y.-C.; et al. Estrogenic effects in the influents and effluents of the drinking water treatment plants. Environ. Sci. Pollut. Res. 2016, 23, 8518–8528. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.M.; Koo, H.J. Hershberger Assay for Antiandrogenic Effects of Phthalates. J. Toxicol. Environ. Health Part A 2007, 70, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Lyche, J.L.; Gutleb, A.C.; Bergman, Å.; Eriksen, G.S.; Murk, A.J.; Ropstad, E.; Saunders, M.; Skaare, J.U. Reproductive and Developmental Toxicity of Phthalates. J. Toxicol. Environ. Health Part B 2009, 12, 225–249. [Google Scholar] [CrossRef]
- Shi, W.; Hu, X.X.; Zhang, F.X.; Hu, G.J.; Hao, Y.Q.; Zhang, X.W.; Liu, H.L.; Wei, S.; Wang, X.R.; Giesy, J.P.; et al. Occurrence of thyroid hormone activities in drinking water from Eastern China: Contributions of phthalate esters. Environ. Sci. Technol. 2012, 46, 1811–1818. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z. Endocrine disrupting compounds in drinking water supply system and human health risk implication. Environ. Int. 2017, 106, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; LI, A.Q.; Guan, Y.B.; Yan, L.; Cheng, X.M.; Ping, L.; Qunl, S.; Xinluo, Y.; Qihuang, H.; Chen, Y.; et al. Influence of polluted SY River on child growth and sex hormones. Biomed. Environ. Sci. 2012, 25, 291–296. [Google Scholar] [PubMed]
- Liu, X.; Shi, J.; Bo, T.; Zhang, H.; Wu, W.; Chen, Q. Occurrence of phthalic acid esters in source waters: A nation-wide survey in China during the period of 2009 to 2012. Environ. Pollut. 2014, 184, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Z.; Shen, J. Occurrence and Removal Characteristics of Phthalate Esters from Typical Water Sources in Northeast China. J. Anal. Methods Chem. 2013, 2013, 419349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Shi, J.; Bo, T.; Li, H.; Crittenden, J.C. Occurrence and risk assessment of selected phthalates in drinking water from waterworks in China. Environ. Sci. Pollut. Res. 2015, 22, 10690–10698. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Shen, J.; Chen, Z.; Kang, J.; Li, T.; Wu, X.; Kong, X.; Fan, L. Profiles and risk assessment of phthalate acid esters (PAEs) in drinking water sources and treatment plants, East China. Environ. Sci. Pollut. Res. 2017, 24, 23646–23657. [Google Scholar] [CrossRef] [PubMed]
- Sulentic, R.O.; Dumitrascu, I.; Deziel, N.C.; Gurzau, A.E. Phthalate Exposure from Drinking Water in Romanian Adolescents. Int. J. Environ. Res. Public Health 2018, 15, 2109. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Wang, F.; Zhang, L.; Shan, C.; Bai, Z.; Sun, Z.; Liu, L.; Shen, B. A comprehensive assessment of human exposure to phthalates from environmental media and food in Tianjin, China. J. Hazard. Mater. 2014, 279, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Martine, B.; Marie-Jeanne, T.; Cendrine, D.; Fabrice, A.; Marc, C. Assessment of Adult Human Exposure to Phthalate Esters in the Urban Centre of Paris (France). Bull. Environ. Contam. Toxicol. 2012, 90, 91–96. [Google Scholar] [CrossRef]
- Abtahi, M.; Dobaradaran, S.; Torabbeigi, M.; Jorfi, S.; Gholamnia, R.; Koolivand, A.; Darabi, H.; Kavousi, A.; Saeedi, R. Health risk of phthalates in water environment: Occurrence in water resources, bottled water, and tap water, and burden of disease from exposure through drinking water in tehran, Iran. Environ. Res. 2019, 173, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Tabtong, W.; Boontanon, S.K.; Boontanon, N. Fate and Risk Assessment of Perfluoroalkyl Substances (PFASs) in Water Treatment Plants and Tap Water in Bangkok, Thailand. Procedia Environ. Sci. 2015, 28, 750–757. [Google Scholar] [CrossRef] [Green Version]
- Adeniyi, A.A.; Okedeyi, O.O.; Yusuf, K.A. Flame ionization gas chromatographic determination of phthalate esters in water, surface sediments and fish species in the Ogun river catchments, Ketu, Lagos, Nigeria. Environ. Monit. Assess. 2010, 172, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Fatoki, O.; Bornman, M.; Ravandhalala, L.; Chimuka, L.; Genthe, B.; Adeniyi, A. Phthalate ester plasticizers in freshwater systems of Venda, South Africa and potential health effects. Water SA 2010, 36, 1. [Google Scholar] [CrossRef] [Green Version]
- Kingsley, O.; Witthayawirasak, B. Occurrence, Ecological and Health Risk Assessment of Phthalate Esters in Surface Water of U-Tapao Canal, Southern, Thailand. Toxics 2020, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Sirivithayapakorn, S.; Thuyviang, K. Dispersion and Ecological Risk Assessment of Di (2-Ethyl hexyl) Phthalate (DEHP) in the Surface Waters of Thailand. Bull. Environ. Contam. Toxicol. 2010, 84, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, O.; Witthayawirasak, B. Deterministic Assessment of the Risk of Phthalate Esters in Sediments of U-Tapao Canal, Southern Thailand. Toxics 2020, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.C. Prenatal Exposures to Environmental Chemicals and Children’s Neurodevelopment: An Update. Saf. Health Work 2013, 4, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, C.; Mahalingam, S.; Flaws, J.A. Environmental Contaminants Affecting Fertility and Somatic Health. Semin. Reprod. Med. 2017, 35, 241–249. [Google Scholar] [CrossRef]
- Zeman, F.A.; Boudet, C.; Tack, K.; Barneaud, A.F.; Brochot, C.; Péry, A.R.R.; Oleko, A.; Vandentorren, S. Exposure assessment of phthalates in French pregnant women: Results of the ELFE pilot study. Int. J. Hyg. Environ. Health 2013, 216, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.C.; Kuo, P.L.; Guo, Y.L.; Liao, P.C.; Lee, C.C. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum. Reprod. 2007, 22, 2715–2722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risnes, K.R.; Vatten, L.J.; Baker, J.L.; Jameson, K.; Sovio, U.; Kajantie, E.; Osler, M.; Morley, R.; Jokela, M.; Painter, R.C.; et al. Effects of diisononyl phthalate on Daniorerio reproduction. Environ. Pollut. 2017, 231, 1051–1062. [Google Scholar]
- Meeker, J.D. Exposure to environmental endocrine disruptors and child development. Arch. Pediatr. Adolesc. Med. 2012, 166, 952–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertelsen, R.J.; Carlsen, K.C.L.; Calafat, A.M.; Hoppin, J.; Håland, G.; Mowinckel, P.; Carlsen, K.-H.; Løvik, M. Urinary Biomarkers for Phthalates Associated with Asthma in Norwegian Children. Environ. Health Perspect. 2013, 121, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franken, C.; Lambrechts, N.; Govarts, E.; Koppen, G.; Den Hond, E.; Ooms, D.; Voorspoels, S.; Bruckers, L.; Loots, I.; Nelen, V.; et al. Phthalate-induced oxidative stress and association with asthma-related air way inflammation in adolescents. Int. J. Hyg. Environ. Health 2017, 220, 468–477. [Google Scholar] [CrossRef]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 127, 204–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathyanarayana, S. Phthalates and children’s health. Curr. Probl. Pediatr. Adolesc. Health Care 2008, 38, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.S.; Engel, S.M.; Berkowitz, G.S.; Ye, X.; Silva, M.J.; Zhu, C.; Wetmur, J.; Calafat, A.M. Prenatal phenol and phthalate exposures and birth outcomes. Environ. Health Perspect. 2008, 116, 1092. [Google Scholar] [CrossRef] [PubMed]
- Adibi, J.J.; Whyatt, R.M.; Hauser, R.; Bhat, H.K.; Davis, B.J.; Calafat, A.M.; Hoepner, L.A.; Perera, F.P.; Tang, D.; Williams, P.L. Transcriptional Biomarkers of Steroidogenesis and Trophoblast Differentiation in the Placenta in Relation to Prenatal Phthalate Exposure. Environ. Health Perspect. 2010, 118, 291–296. [Google Scholar] [CrossRef]
- Neier, K.; Marchlewicz, E.H.; Dolinoy, D.C.; Padmanabhan, V. Assessing human health risk to endocrine disrupting chemicals: A focus on prenatal exposures and oxidative stress. Endocr. Disruptors 2015, 3, e1069916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasper-Sonnenberg, M.; Koch, H.M.; Wittsiepe, J.; Wilhelm, M. Levels of phthalate metabolites in urine among mother-child-pairs—Results from the Duisburg birth cohort study, Germany. Int. J. Hyg. Environ. Health 2012, 215, 373–382. [Google Scholar] [CrossRef]
- Russo, M.V.; Notardonato, I.; Cinelli, G.; Avino, P. Evaluation of an analytical method for determining phthalate esters in wine samples by solid-phase extraction and gas chromatography coupled with ion-trap mass spectrometer detector. Anal. Bioanal. Chem. 2012, 402, 1373–1381. [Google Scholar] [CrossRef]
- Kortenkamp, A.; Faust, M. Combined exposures to anti-androgenic chemicals steps towards cumulative risk assessment. Int. J. Androl. 2010, 33, 463–472. [Google Scholar] [CrossRef]
- Kortenkamp, A.; Koch, H.M. Refined reference doses and new procedures for phthalate mixture risk assessment focused on male developmental toxicity. Int. J. Hyg. Environ. Health 2020, 224, 113428. [Google Scholar] [CrossRef] [PubMed]
- DRI. Dietary reference intakes for water, po-tassium, sodium, chloride, and sulfate. In Dietary Reference Intakes: The Essential Guide to Nutrient Requirements DRI; Institute of Medicine of National Academy Press: Washington, DC, USA, 2006. [Google Scholar]
- U.S. EPA. Exposure Factors Handbook; (Final); EPA/600/R-09/052F; U.S. Environmental Protection Agency: Washington, DC, USA, 2011.
- U.S. EPA. Drinking Water Standards and Health Advisories; EPA 822-S-12-001; Office of Water U.S. Environmental Protection Agency: Washington, DC, USA, 2012.
- Jeddi, M.Z.; Gorji, M.E.; Rietjens, I.M.C.M.; Louisse, J.; De Bruin, Y.B.; Liska, R. Biomonitoring and Subsequent Risk Assessment of Combined Exposure to Phthalates in Iranian Children and Adolescents. Int. J. Environ. Res. Public Health 2018, 15, 2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. EPA. Integrated Risk Information System (IRIS), U.S. Environmental Protection Agency. Di (2-Ethylhexyl) Phthalate (DEHP) (CASRN 117–81-7). 2012. Available online: http://www.epa.gov/iris/subst/0014.htm (accessed on 15 July 2020).
- Li, N.; Wang, D.; Zhou, Y.; Ma, M.; Li, J.; Wang, Z. Dibutyl Phthalate Contributes to the Thyroid Receptor Antagonistic Activity in Drinking Water Processes. Environ. Sci. Technol. 2010, 44, 6863–6868. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Morueco, N.; González-Alonso, S.; Valcárcel, Y. Phthalate occurrence in rivers and tap water from central Spain. Sci. Total Environ. 2014, 500–501, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Loraine, G.A.; Pettigrove, M.E. Seasonal Variations in Concentrations of Pharmaceuticals and Personal Care Products in Drinking Water and Reclaimed Wastewater in Southern California. Environ. Sci. Technol. 2006, 40, 687–695. [Google Scholar] [CrossRef]
- Health Canada. Report on Human Biomonitoring of Environmental Chemicals in Canada: Results of the Canadian Health Measures Survey Cycle 1 (2007–2009). 2010. Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/ewh-semt/alt_formats/hecs-sesc/pdf/pubs/contaminants/chms-ecms/report-rapport-eng.pdf (accessed on 11 January 2021).
- Zare Jeddi, M.; Rastkari, N.; Ahmadkhaniha, R.; Yunesian, M. Endocrine disruptor phthalates in bottled water: Daily exposure and health risk assessment in pregnant and lactating women. Environ. Monit. Assess. 2016, 188, 534. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, Z.; Ala, A.; Babanezhad, E.; Mohammadpour, A.R. Evaluation of exposure to phthalate esters through the use of various brands of drinking water bottled in polyethylene terephthalate (PET) containers under different storage conditions. Environ. Health Eng. Manag. J. 2019, 6, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Kim, T.S.; Shin, J.-H.; Moon, H.J.; Kang, I.H.; Kim, I.Y.; Oh, J.Y.; Han, S.Y. Neonatal Exposure to DI (n-Butyl) Phthalate (DBP) Alters Male Reproductive-Tract Development. J. Toxicol. Environ. Health Part A 2004, 67, 2045–2060. [Google Scholar] [CrossRef]
- Hu, X.; Shi, W.; Wei, S.; Zhang, X.; Feng, J.; Hu, G.; Chen, S.; Giesy, J.P.; Yu, H. Occurrence and Potential Causes of Androgenic Activities in Source and Drinking Water in China. Environ. Sci. Technol. 2013, 47, 10591–10600. [Google Scholar] [CrossRef]
- Kim Hyun, K. Transformation and Bioactivities of Phthalates Leached from PVC Exposed to Disinfected Waters. Master’s Thesis, The Hong Kong University of Science and Technology, Clear Water Bay, Hong Kong, 2016. [Google Scholar]
- Gavala, H.N.; Yenal, U.; Ahring, B.K. Thermal and enzymatic pretreatment of sludge containing phthalate esters prior to mesophilic anaerobic digestion. Biotechnol. Bioeng. 2004, 85, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Li, X.; Graham, N. Aqueous oxidation of dimethyl phthalate in a Fe(VI)-TiO2-UV reaction system. Water Res. 2008, 42, 1413–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera-Utrilla, J.; Ocampo-Pérez, R.; Méndez-Díaz, J.D.; Sánchez-Polo, M. Environmental impact of phthalic acid esters and their removal from water and sediments by different technologies—A review. J. Environ. Manag. 2012, 109, 164–178. [Google Scholar] [CrossRef]
- Forner, I.; Constantinos, P.; Ioannis, C.M.; Maria, F.; Fabiana, P.; Di Marzo, V.; Calduch-Giner, J.; Pérez-Sánchez, J.; Carnevali, O. Effects of diisononyl phthalate (DiNP) on the endocannabinoid andreproductive systems of male gilt-head sea bream (Sparus aurata) during the spawning season. Arch. Toxicol. 2019, 93, 727–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Zijl, M.C.; Aneck-Hahn, N.H.; Swart, P.; Hayward, S.; Genthe, B.; De Jager, C. Estrogenic activity, chemical levels and health risk assessment of municipal distribution point water from Pretoria and Cape Town, South Africa. Chemosphere 2017, 186, 305–313. [Google Scholar] [CrossRef] [Green Version]
- EFSA European Food Safety Authority. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Butylbenzylphthalate (BBP) for use in food contact materials. EFSA J. 2005, 3, 241. [Google Scholar] [CrossRef]
- EFSA European Food Safety Authority. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to di-Butylphthalate (DBP) for use in food contact materials. EFSA J. 2005, 3, 242. [Google Scholar] [CrossRef]
- EFSA European Food Safety Authority. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Bis(2-ethylhexyl)phthalate (DEHP) for use in food contact materials. EFSA J. 2005, 3, 243. [Google Scholar] [CrossRef]
- TEACH Phthalates. TEACH Chemical Summary. U.S. EPA, Toxicity and Exposure Assessment for Children’s Health (TEACH). 2007. Available online: https://archive.epa.gov/region5/teach/web/html/teachsummaries.html (accessed on 28 October 2020).
- Zare Jeddi, M.; Ahmadkhaniha, R.; Yunesian, M.; Rastkari, N. Magnetic solid-phase extraction based on modified magnetic nanoparticles for the determination of phthalate. J. Chromatogr. Sci. 2015, 53, 385–391. [Google Scholar] [CrossRef] [Green Version]
- Clark, K.; Cousins, I.T.; Mackay, D. Assessment of Critical Exposure Pathways. In The Handbook of Environmental Chemistry; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2002; pp. 227–262. [Google Scholar]
- Jurewicz, J.; Hanke, W. Exposure to phthalates: Reproductive outcome and children health. A review of epidemiological studies. Int. J. Occup. Med. Environ. Health 2011, 24, 115–141. [Google Scholar] [CrossRef]
- Polanska, K.; Ligocka, D.; Sobala, W.; Hanke, W. Phthalate exposure and child development: The Polish Mother and Child Cohort Study. Early Hum. Dev. 2014, 90, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Ejaredar, M.; Nyanza, E.C.; Ten Eycke, K.; Dewey, D. Phthalate exposure and childrens neurodevelopment: A systematic review. Environ. Res. 2015, 142, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, N.; Distel, E.; de Oliveira, E.; Gabriel, C.; Frydas, I.S.; Anesti, O.; Attignon, E.A.; Odena, A.; Diaz, R.; Aggerbeck, M.; et al. Multi-omics analysis reveals that co-exposure to phthalates and metals disturbs urea cycle and choline metabolism. Environ. Res. 2020, 192, 110041. [Google Scholar] [CrossRef] [PubMed]
- Howdeshell, K.L.; Hotchkiss, A.K.; Gray, L.E. Cumulative effects of antiandrogenic chemical mixtures and their relevance to human health risk assessment. Int. J. Hyg. Environ. Health 2017, 220, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Braun, J.M.; Sathyanarayana, S.; Hauser, R. Phthalate exposure and children’s health. Curr. Opin. Pediatr. 2013, 25, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, C.; Uhl, M.; Weiss, S.; Koch, H.M.; Scharf, S.; König, J. Human biomonitoring of phthalate exposure in Austrian children and adults and cumulative risk assessment. Int. J. Hyg. Environ. Health 2015, 218, 489–499. [Google Scholar] [CrossRef]
- Jane, C. Caldwell. DEHP: Genotoxicity and potential carcinogenic mechanisms—A review. Mutat. Res. Rev. Mutat. Res. 2012, 751, 82–157. [Google Scholar] [CrossRef]

| PAEs | Linearity R2 | Target Ions (m/z) | Retention Time (min) | Recovery (%) n = 3 | RSD (%) | LOQ n = 7 μg/L | LOD n = 7 µg/L |
|---|---|---|---|---|---|---|---|
| DBP | 0.999 | 223, 205, 167 | 7.57 | 84 | 5.9 | 0.11 | 0.07 |
| BBP | 0.999 | 205, 149, 91 | 8.77 | 69 | 6.2 | 0.07 | 0.03 |
| DEHP | 0.999 | 279, 167, 149 | 9.29 | 99 | 7.2 | 0.16 | 0.10 |
| DnOP | 0.999 | 279, 261, 149 | 9.84 | 93 | 6.8 | 0.18 | 0.13 |
| DiNP | 0.999 | 293, 127 | 9.93 | 110 | 7.6 | 0.29 | 0.18 |
| DIDP | 0.999 | 307, 141 | 10.44 | 119 | 8.4 | 0.82 | 0.12 |
| PAEs | SADAO PW (µg/L) | PHANGALA PW (µg/L) | HAT YAI PW (µg/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean ± SD | Min | Max | Mean ± SD | Min | Max | Mean ± SD | |
| DBP | ND | 2.04 | 1.89 ± 0.15 | ND | 1.82 | 1.68 ± 0.18 | ND | 3.36 | 2.21 ± 0.58 |
| DEHP | 1.84 | 2.68 | 2.18 ± 0.36 | 1.69 | 2.14 | 1.93 ± 0.20 | 2.88 | 4.84 | 3.71 ± 0.82 |
| DiNP | ND | 1.74 | 1.47 ± 0.23 | ND | 1.68 | 1.63 ± 0.06 | ND | 2.47 | 2.04 ± 0.29 |
| BBP | ND | ND | ND | ND | ND | ND | <LOD | <LOD | <LOD |
| DnOP | ND | ND | ND | ND | ND | ND | <LOD | <LOD | <LOD |
| DIDP | ND | ND | ND | ND | ND | ND | <LOD | <LOD | <LOD |
| ∑PAEs | 1.84 | 6.46 | 5.54 ± 0.74 | 1.69 | 5.64 | 5.27 ± 0.48 | 2.88 | 10.67 | 8.16 ± 1.69 |
| Location | Method of Treatment | Raw Water | Tap Water | References | ||
|---|---|---|---|---|---|---|
| DBP | DEHP | DBP | DEHP | |||
| Taiwan | Conventional treatment process | <MDL–0.76 | <MDL–2.50 | <MDL–0.84 | <MDL–2.88 | Guo et al., 2016 |
| China | Conventional treatment process | 0.05–4.49 | 0.13–6.57 | 0.02–1.71 | 0.05–2.36 | Liu et al., 2013 |
| China | Conventional treatment process | 0.02–0.08 | 0.18–0.75 | 0.01–0.03 | 0.07–0.31 | Kong et al., 2017 |
| USA | Conventional treatment process | 1.44–8.34 | 2.67–5.94 | (mean, 2.73) | 2.43–2.68 | Loraine and Pettigrove, 2006 |
| USA | Advanced process | 0.05–0.06 | 0.12–0.17 | ND | ND | Benotti et al., 2009 |
| China | Advanced process | 14.00–100 | 0.46–7.00 | 0.07–0.19 | 0.01–0.05 | Hu et al., 2013 |
| Taiwan | Advanced process | 0.08–0.09 | 0.13–0.16 | 0.01–0.07 | 0.02–0.12 | Yang et al., 2014 |
| Thailand | Conventional treatment process | ND-3.36 | 1.69–4.84 | ND-1.07 | 0.59–1.24 | Present study |
| Sadao PW | Phangla PW | Hat Yai PW | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DBP | DEHP | DiNP | DBP | DEHP | DiNP | DBP | DEHP | DiNP | |
| MC in Tap Water (μg/L) | 0.78 | 0.98 | 0.64 | 0.68 | 0.85 | 0.52 | 1.07 | 1.24 | 0.99 |
| Infants | |||||||||
| EDI | 1.17 × 10−1 | 1.47 × 10−1 | 9.6 × 10−2 | 1.02 × 10−1 | 1.28 × 10−1 | 7.8 × 10−2 | 1.61 × 10−1 | 1.86 × 10−1 | 1.49 × 10−1 |
| 1.2 × 10−3 | 7.35 × 10−3 | 8.35 × 10−4 | 1.02 × 10−3 | 6.4 × 10−3 | 6.78 × 10−4 | 1.61 × 10−3 | 9.3 × 10−3 | 1.30 × 10−3 | |
| 7.8 × 10−4 | 4.9 × 10−3 | 6.4 × 10−5 | 6.8 × 10−4 | 4.3 × 10−3 | 5.2 × 10−5 | 1.07 × 10−3 | 6.2 × 10−3 | 9.93 × 10−5 | |
| 1.75 × 10−2 | 1.47 × 10−2 | 1.63 × 10−3 | 1.52 × 10−2 | 1.28 × 10−2 | 1.32 × 10−3 | 2.40 × 10−2 | 1.86 × 10−2 | 2.53 × 10−3 | |
| 1.17 × 10−2 | 2.94 × 10−3 | 6.4 × 10−4 | 1.02 × 10−2 | 2.56 × 10−3 | 5.2 × 10−4 | 1.61 × 10−2 | 3.72 × 10−3 | 9.93 × 10−4 | |
| CVD | 1.17 × 101 | 2.94 × 10−1 | 6.4 × 10−2 | 1.02 × 101 | 2.56 × 10−1 | 5.2 × 10−2 | 1.61 × 101 | 3.72 × 10−1 | 9.93 × 10−2 |
| Pregnant women | |||||||||
| EDI | 3.5 × 10−2 | 4.4 × 10−2 | 2.9 × 10−2 | 3.1 × 10−2 | 3.9 × 10−2 | 2.4 × 10−2 | 4.8 × 10−2 | 5.6 × 10−2 | 4.5 × 10=2 |
| 3.5 × 10−4 | 2.2 × 10−3 | 2.52 × 10−4 | 3.1 × 10−4 | 2.0 × 10−3 | 2.09 × 10−4 | 4.8 × 10−4 | 2.8 × 10−3 | 3.91 × 10−4 | |
| 2.3 × 10−4 | 1.5 × 10−3 | 1.93 × 10−5 | 2.1 × 10−4 | 1.3 × 10−3 | 1.6 × 10−5 | 2.9 × 10−4 | 1.9 × 10−3 | 3.0 × 10−5 | |
| 5.2 × 10−3 | 4.4 × 10−3 | 4.92 × 10−4 | 4.6 × 10−3 | 3.9 × 10−3 | 4.07 × 10−4 | 6.4 × 10−3 | 5.6 × 10−3 | 7.63 × 10−4 | |
| 3.5 × 10−3 | 8.8 × 10−4 | 1.93 × 10−4 | 3.1 × 10−3 | 7.8 × 10−4 | 1.6 × 10−4 | 4.8 × 10−3 | 1.12 × 10−3 | 3.0 × 10−4 | |
| CVD | 3.5 × 10−1 | 8.8 × 10−2 | 1.93 × 10−2 | 3.1 × 10−1 | 7.8 × 10−2 | 1.6 × 10−2 | 4.3 × 10−1 | 1.12 × 10−1 | 3.0 × 10−2 |
| Lactating mothers | |||||||||
| EDI | 4.9 × 10−2 | 6.2 × 10−2 | 4.1 × 10−2 | 4.3 × 10−2 | 5.4 × 10−2 | 3.3 × 10−2 | 6.8 × 10−2 | 7.9 × 10−2 | 6.3 × 10−2 |
| 4.9 × 10−4 | 3.1 × 10−3 | 3.57 × 10−4 | 4.3 × 10−4 | 2.7 × 10−3 | 2.87 × 10−4 | 6.8 × 10−4 | 3.95 × 10−3 | 5.48 × 10−4 | |
| 3.3 × 10−4 | 2.1 × 10−4 | 2.73 × 10−5 | 2.9 × 10−4 | 1.8 × 10−3 | 2.2 × 10−5 | 4.5 × 10−4 | 2.6 × 10−3 | 4.2 × 10−5 | |
| 7.3 × 10−3 | 6.2 × 10−3 | 6.95 × 10−4 | 6.4 × 10−3 | 5.4 × 10−3 | 5.6 × 10−4 | 1.02 × 10−2 | 7.9 × 10−3 | 1.07 × 10−4 | |
| 4.9 × 10−3 | 1.24 × 10−3 | 2.73 × 10−4 | 4.3 × 10−3 | 1.08 × 10−3 | 2.2 × 10−4 | 6.8 × 10−3 | 1.58 × 10−3 | 4.2 × 10−4 | |
| CVD | 4.9 × 10−1 | 6.2 × 10−1 | 2.7 × 10−2 | 4.3 × 10−1 | 5.4 × 10−1 | 2.2 × 10−2 | 6.8 × 10−1 | 7.9 × 10−1 | 4.2 × 10−2 |
| Non-pregnant women | |||||||||
| EDI | 2.7 × 10−2 | 3.4 × 10−2 | 2.2 × 10−2 | 2.4 × 10−2 | 2.9 × 10−2 | 1.8 × 10−2 | 3.7 × 10−2 | 4.3 × 10−2 | 3.4 × 10−2 |
| 2.7 × 10−4 | 1.7 × 10−3 | 1.91 × 10−4 | 2.4 × 10−4 | 1.5 × 10−3 | 1.57 × 10−4 | 3.7 × 10−4 | 2.2 × 10−3 | 2.96 × 10−4 | |
| 18 × 10−4 | 1.1 × 10−3 | 1.47 × 10−5 | 1.6 × 10−4 | 9.6 × 10−4 | 1.2 × 10−5 | 2.5 × 10−4 | 1.4 × 10−3 | 2.27 × 10−5 | |
| 4.0 × 10−3 | 3.4 × 103 | 3.73 × 10−4 | 3.6 × 10−3 | 2.9 × 10−3 | 3.05 × 10−4 | 5.5 × 10−3 | 4.3 × 10−3 | 5.76 × 10−4 | |
| 2.7 × 10−3 | 6.8 × 10−4 | 1.47 × 10−4 | 2.4 × 10−3 | 5.8 × 10−4 | 1.2 × 10−4 | 3.7 × 10−3 | 8.6 × 10−4 | 2.27 × 10−4 | |
| CVD | 2.7 × 10−1 | 6.8 × 10−2 | 1.47 × 10−2 | 2.4 × 10−1 | 5.8 × 10−2 | 1.2 × 10−2 | 3.7 × 10−1 | 8.6 × 10−2 | 2.27 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okpara, K.E.; Phoungthong, K.; Agbozu, I.; Edwin-Isotu, E.; Techato, K. Phthalate Esters in Tap Water, Southern Thailand: Daily Exposure and Cumulative Health Risk in Infants, Lactating Mothers, Pregnant and Nonpregnant Women. Int. J. Environ. Res. Public Health 2022, 19, 2187. https://doi.org/10.3390/ijerph19042187
Okpara KE, Phoungthong K, Agbozu I, Edwin-Isotu E, Techato K. Phthalate Esters in Tap Water, Southern Thailand: Daily Exposure and Cumulative Health Risk in Infants, Lactating Mothers, Pregnant and Nonpregnant Women. International Journal of Environmental Research and Public Health. 2022; 19(4):2187. https://doi.org/10.3390/ijerph19042187
Chicago/Turabian StyleOkpara, Kingsley Ezechukwu, Khamphe Phoungthong, Iwekumo Agbozu, Edeh Edwin-Isotu, and Kuaanan Techato. 2022. "Phthalate Esters in Tap Water, Southern Thailand: Daily Exposure and Cumulative Health Risk in Infants, Lactating Mothers, Pregnant and Nonpregnant Women" International Journal of Environmental Research and Public Health 19, no. 4: 2187. https://doi.org/10.3390/ijerph19042187
APA StyleOkpara, K. E., Phoungthong, K., Agbozu, I., Edwin-Isotu, E., & Techato, K. (2022). Phthalate Esters in Tap Water, Southern Thailand: Daily Exposure and Cumulative Health Risk in Infants, Lactating Mothers, Pregnant and Nonpregnant Women. International Journal of Environmental Research and Public Health, 19(4), 2187. https://doi.org/10.3390/ijerph19042187








