Association between Maternal Exposure to Ambient Air Pollution and the Risk of Preterm Birth: A Birth Cohort Study in Chongqing, China, 2015–2020
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exposure Assessment
2.3. Statistical Analysis
3. Results
3.1. Descriptive Statistics of Objects
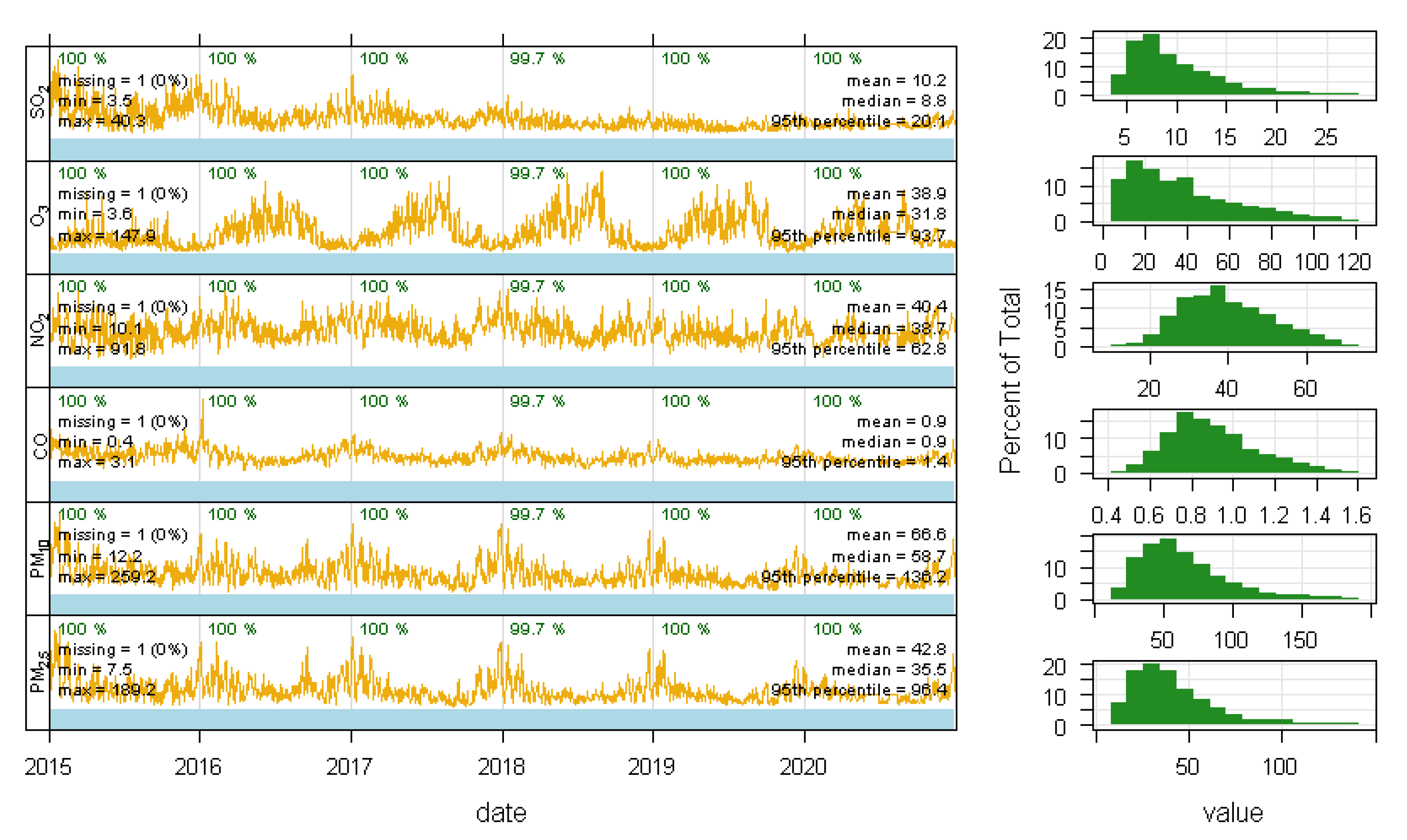
3.2. Descriptive Statistics of Air Pollutants
3.3. Associations between Air Pollutants and PTB
4. Discussion
4.1. Summary of Results
4.2. Comparisons with Other Studies Worldwide
4.3. Analysis of Sensitive Exposure Windows
4.4. Adjusted for Covariates in Single and Multi-Pollutant Model
4.5. Assets and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, G.; Stingone, J.A.; Desrosiers, T.A.; Olshan, A.F.; Nembhard, W.N.; Shaw, G.M.; Pruitt, S.; Romitti, P.A.; Yazdy, M.M.; Browne, M.L.; et al. Maternal exposure to outdoor air pollution and congenital limb deficiencies in the National Birth Defects Prevention Study. Environ. Res. 2019, 179, 108716. [Google Scholar] [CrossRef] [PubMed]
- Frutos, V.; González-Comadran, M.; Solà, I.; Jacquemin, B.; Carreras, R.; Vizcaíno, M.A.C. Impact of air pollution on fertility: A systematic review. Gynecol. Endocrinol. 2014, 31, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, C.; Meng, X.; Niu, Y.; Lin, Z.; Liu, Y.; Liu, J.; Qi, J.; You, J.; Tse, L.A.; et al. Associations between short-term exposure to ambient sulfur dioxide and increased cause-specific mortality in 272 Chinese cities. Environ. Int. 2018, 117, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.-Q.; Chen, Y.; Mi, B.; Dang, S.-N.; Zhao, D.-D.; Liu, R.; Wang, H.-L.; Yan, H. Ambient air pollution and adverse birth outcomes: A systematic review and meta-analysis. J. Zhejiang Univ. Sci. B 2019, 20, 238–252. [Google Scholar] [CrossRef]
- Nyadanu, S.D.; Tessema, G.A.; Mullins, B.; Kumi-Boateng, B.; Bell, M.L.; Pereira, G. Ambient Air Pollution, Extreme Temperatures and Birth Outcomes: A Protocol for an Umbrella Review, Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8658. [Google Scholar] [CrossRef]
- Simoncic, V.; Enaux, C.; Deguen, S.; Kihal-Talantikite, W. Adverse Birth Outcomes Related to NO2 and PM Exposure: European Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8116. [Google Scholar] [CrossRef]
- Chen, J.; Fang, J.; Zhang, Y.; Xu, Z.; Byun, H.-M.; Li, P.-H.; Deng, F.; Guo, X.; Guo, L.; Wu, S. Associations of adverse pregnancy outcomes with high ambient air pollution exposure: Results from the Project ELEFANT. Sci. Total Environ. 2020, 761, 143218. [Google Scholar] [CrossRef]
- Kwag, Y.; Kim, M.-H.; Ye, S.; Oh, J.; Yim, G.; Kim, Y.; Kim, E.; Lee, S.; Koh, T.; Ha, E. The Combined Effects of Fine Particulate Matter and Temperature on Preterm Birth in Seoul, 2010–2016. Int. J. Environ. Res. Public Health 2021, 18, 1463. [Google Scholar] [CrossRef]
- Conforti, A.; Mascia, M.; Cioffi, G.; De Angelis, C.; Coppola, G.; De Rosa, P.; Pivonello, R.; Alviggi, C.; De Placido, G. Air pollution and female fertility: A systematic review of literature. Reprod. Biol. Endocrinol. 2018, 16, 117. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.-Y.; Guo, Y.; Zhou, H.; Wang, X.; Wang, Q.; Shen, H.; Zhang, Y.; Yan, D.; Zhang, Y.; et al. Effect of airborne particulate matter of 2.5 μm or less on preterm birth: A national birth cohort study in China. Environ. Int. 2018, 121, 1128–1136. [Google Scholar] [CrossRef]
- Chen, Q.; Ren, Z.; Liu, Y.; Qiu, Y.; Yang, H.; Zhou, Y.; Wang, X.; Jiao, K.; Liao, J.; Ma, L. The Association between Preterm Birth and Ambient Air Pollution Exposure in Shiyan, China, 2015–2017. Int. J. Environ. Res. Public Health 2021, 18, 4326. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Chen, Y.; Wu, H.; Zeng, J.; Zeng, Z.; Li, W.; Zhang, Q.; Huo, X.; Feng, W.; Lin, J.; et al. Ambient air pollution and markers of fetal growth: A retrospective population-based cohort study of 2.57 million term singleton births in China. Environ. Int. 2019, 135, 105410. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Peng, L.; Wu, X.; Xu, G.; Cheng, P.; Hao, J.; Huang, Z.; Xu, M.; Chen, S.; Zhang, C.; et al. Long-term impact of ambient air pollution on preterm birth in Xuzhou, China: A time series study. Environ. Sci. Pollut. Res. 2021, 28, 41039–41050. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Miao, H.; Chen, Y.; Fu, Y.; Wu, Y.; Zhao, Q.; Li, B. Maternal exposure to gaseous ambient air pollutants increases the risk of preterm birth in the Pearl River Delta, China 2014–2017. Sci. Total Environ. 2019, 671, 959–970. [Google Scholar] [CrossRef]
- Liang, Z.; Yang, Y.; Qian, Z.; Ruan, Z.; Chang, J.; Vaughn, M.G.; Zhao, Q.; Lin, H. Ambient PM2.5 and birth outcomes: Estimating the association and attributable risk using a birth cohort study in nine Chinese cities. Environ. Int. 2019, 126, 329–335. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, Y.; Wang, W.; Chen, R.; Liu, Y.; Liu, C.; Kan, H.; Gao, Y.; Tian, Y. Critical windows for maternal fine particulate matter exposure and adverse birth outcomes: The Shanghai birth cohort study. Chemosphere 2019, 240, 124904. [Google Scholar] [CrossRef]
- Xiao, Q.; Chen, H.; Strickland, M.J.; Kan, H.; Chang, H.H.; Klein, M.; Yang, C.; Meng, X.; Liu, Y. Associations between birth outcomes and maternal PM2.5 exposure in Shanghai: A comparison of three exposure assessment approaches. Environ. Int. 2018, 117, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiang, X.; Mi, B.; Song, H.; Dong, M.; Zhang, S.; Bi, Y.; Zhao, Y.; Li, Q.; Zhang, Q.; et al. Association between early prenatal exposure to ambient air pollution and birth defects: Evidence from newborns in Xi’an, China. J. Public Health 2018, 41, 494–501. [Google Scholar] [CrossRef]
- Leiser, C.L.; Hanson, H.A.; Sawyer, K.; Steenblik, J.; Al-Dulaimi, R.; Madsen, T.; Gibbins, K.; Hotaling, J.M.; Ibrahim, Y.O.; VanDerslice, J.A.; et al. Acute effects of air pollutants on spontaneous pregnancy loss: A case-crossover study. Fertil. Steril. 2019, 111, 341–347. [Google Scholar] [CrossRef]
- Ananth, C.V.; Kioumourtzoglou, M.-A.; Huang, Y.; Ross, Z.; Friedman, A.M.; Williams, M.A.; Wang, S.; Mittleman, M.; Schwartz, J. Exposures to Air Pollution and Risk of Acute-onset Placental Abruption. Epidemiology 2018, 29, 631–638. [Google Scholar] [CrossRef]
- Percy, Z.; DeFranco, E.; Xu, F.; Hall, E.S.; Haynes, E.N.; Jones, D.; Muglia, L.J.; Chen, A. Trimester specific PM2.5 exposure and fetal growth in Ohio, 2007–2010. Environ. Res. 2019, 171, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Dastoorpoor, M.; Khanjani, N.; Moradgholi, A.; Sarizadeh, R.; Cheraghi, M.; Estebsari, F. Prenatal exposure to ambient air pollution and adverse pregnancy outcomes in Ahvaz, Iran: A generalized additive model. Int. Arch. Occup. Environ. Health 2021, 94, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Kang, C.-M.; Osorio-Yáñez, C.; Barrow, T.M.; Zhang, R.; Zhang, Y.; Li, C.; Liu, H.; Li, P.-H.; Guo, L.; et al. Prenatal PM2.5 exposure and the risk of adverse births outcomes: Results from Project ELEFANT. Environ. Res. 2020, 191, 110232. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, J.; Chen, D.; Sun, P.; Ma, X. The association between air pollution and preterm birth and low birth weight in Guangdong, China. BMC Public Health 2019, 19, 3. [Google Scholar] [CrossRef]
- Mekonnen, Z.K.; Oehlert, J.W.; Eskenazi, B.; Shaw, G.M.; Balmes, J.R.; Padula, A.M. The relationship between air pollutants and maternal socioeconomic factors on preterm birth in California urban counties. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 503–513. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, L.; Bai, X.; Du, W.; Shen, G.; Fei, J.; Wang, Y.; Chen, A.; Chen, Y.; Zhao, M. Maternal ambient air pollution exposure with spatial-temporal variations and preterm birth risk assessment during 2013–2017 in Zhejiang Province, China. Environ. Int. 2019, 133, 105242. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, Y.; Gao, Y.; Tian, Y. Maternal fine particulate matter (PM2.5) exposure and adverse birth outcomes: An updated systematic review based on cohort studies. Environ. Sci. Pollut. Res. 2019, 26, 13963–13983. [Google Scholar] [CrossRef]
- Klepac, P.; Locatelli, I.; Korošec, S.; Künzli, N.; Kukec, A. Ambient air pollution and pregnancy outcomes: A comprehensive review and identification of environmental public health challenges. Environ. Res. 2018, 167, 144–159. [Google Scholar] [CrossRef]
- Fleischer, N.L.; Merialdi, M.; Van Donkelaar, A.; Vadillo-Ortega, F.; Martin, R.; Betran, A.P.; Souza, J.P.; O´neill, M.S. Outdoor Air Pollution, Preterm Birth, and Low Birth Weight: Analysis of the World Health Organization Global Survey on Maternal and Perinatal Health. Environ. Health Perspect. 2014, 122, 425–430. [Google Scholar] [CrossRef]
- Qian, Z.; Liang, S.; Yang, S.; Trevathan, E.; Huang, Z.; Yang, R.; Wang, J.; Hu, K.; Zhang, Y.; Vaughn, M.; et al. Ambient air pollution and preterm birth: A prospective birth cohort study in Wuhan, China. Int. J. Hyg. Environ. Health 2015, 219, 195–203. [Google Scholar] [CrossRef]
- Capobussi, M.; Tettamanti, R.; Marcolin, L.; Piovesan, L.; Bronzin, S.; Gattoni, M.E.; Polloni, I.; Sabatino, G.; Tersalvi, C.A.; Auxilia, F.; et al. Air Pollution Impact on Pregnancy Outcomes in Como, Italy. J. Occup. Environ. Med. 2016, 58, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Cassidy-Bushrow, A.E.; Burmeister, C.; Lamerato, L.; Lemke, L.D.; Mathieu, M.; O’Leary, B.F.; Sperone, F.G.; Straughen, J.K.; Reiners, J.J. Prenatal airshed pollutants and preterm birth in an observational birth cohort study in Detroit, Michigan, USA. Environ. Res. 2020, 189, 109845. [Google Scholar] [CrossRef]
- Xiong, L.; Xu, Z.; Tan, J.; Wang, H.; Liu, Z.; Wang, A.; Xie, D.; Kong, F. Acute effects of air pollutants on adverse birth outcomes in Changsha, China. Medicine 2019, 98, e14127. [Google Scholar] [CrossRef]
- Zeng, X.; Jin, S.; Chen, X.; Qiu, Y. Association between Ambient Air Pollution and Pregnancy Outcomes in Patients Undergoing In Vitro Fertilization in Chengdu, China: A retrospective study. Environ. Res. 2020, 184, 109304. [Google Scholar] [CrossRef]
- Chen, G.; Guo, Y.; Abramson, M.J.; Williams, G.; Li, S. Exposure to low concentrations of air pollutants and adverse birth outcomes in Brisbane, Australia, 2003–2013. Sci. Total Environ. 2018, 622, 721–726. [Google Scholar] [CrossRef]
- Lavigne, E.; Burnett, R.T.; Stieb, D.M.; Evans, G.J.; Pollitt, K.J.G.; Chen, H.; Van Rijswijk, D.; Weichenthal, S. Fine Particulate Air Pollution and Adverse Birth Outcomes: Effect Modification by Regional Nonvolatile Oxidative Potential. Environ. Health Perspect. 2018, 126, 077012. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, P.; Ilango, S.; Bruckner, T.A.; Wang, Q.; Basu, R.; Benmarhnia, T. Ambient Fine Particulate Matter and Preterm Birth in California: Identification of Critical Exposure Windows. Am. J. Epidemiol. 2019, 188, 1608–1615. [Google Scholar] [CrossRef]
- Rappazzo, K.M.; Daniels, J.L.; Messer, L.C.; Poole, C.; Lobdell, D.T. Exposure to Elemental Carbon, Organic Carbon, Nitrate, and Sulfate Fractions of Fine Particulate Matter and Risk of Preterm Birth in New Jersey, Ohio, and Pennsylvania (2000–2005). Environ. Health Perspect. 2015, 123, 1059–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Luo, X.; Zhao, C.; Ng, R.W.C.; Lim, C.E.D.; Zhang, B.; Liu, T. The association between fine particulate matter exposure during pregnancy and preterm birth: A meta-analysis. BMC Pregnancy Childbirth 2015, 15, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronlund, C.J.; Yang, A.J.; Conlon, K.C.; Bergmans, R.S.; Le, H.Q.; Batterman, S.A.; Wahl, R.L.; Cameron, L.; O’Neill, M.S. Time series analysis of total and direct associations between high temperatures and preterm births in Detroit, Michigan. BMJ Open 2020, 10, e032476. [Google Scholar] [CrossRef]
- Mandakh, Y.; Rittner, R.; Flanagan, E.; Oudin, A.; Isaxon, C.; Familari, M.; Hansson, S.R.; Malmqvist, E. Maternal Exposure to Ambient Air Pollution and Risk of Preeclampsia: A Population-Based Cohort Study in Scania, Sweden. Int. J. Environ. Res. Public Health 2020, 17, 1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Tang, Y.; Song, X.; Lazar, L.; Li, Z.; Zhao, J. Impact of ambient PM2.5 on adverse birth outcome and potential molecular mechanism. Ecotoxicol. Environ. Saf. 2018, 169, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Q.; He, S.; Wu, K.; Ren, M.; Dong, H.; Di, J.; Yu, Z.; Huang, C. Ambient air pollution and gestational diabetes mellitus: A review of evidence from biological mechanisms to population epidemiology. Sci. Total Environ. 2020, 719, 137349. [Google Scholar] [CrossRef] [PubMed]
- Janssen, B.G.; Saenen, N.D.; Roels, H.A.; Madhloum, N.; Gyselaers, W.; Lefebvre, W.; Penders, J.; Vanpoucke, C.; Vrijens, K.; Nawrot, T.S. Fetal Thyroid Function, Birth Weight, andin UteroExposure to Fine Particle Air Pollution: A Birth Cohort Study. Environ. Health Perspect. 2017, 125, 699–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudra, C.B.; Williams, M.A.; Sheppard, L.; Koenig, J.Q.; Schiff, M.A. Ambient Carbon Monoxide and Fine Particulate Matter in Relation to Preeclampsia and Preterm Delivery in Western Washington State. Environ. Health Perspect. 2011, 119, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Q.; Yan, L.; Wang, H.; Yu, J.; Tang, J.; Yao, H.; Li, S.; Zhang, Y.; Guo, Y. The association between maternal exposure to ambient particulate matter of 2.5 μm or less during pregnancy and fetal congenital anomalies in Yinchuan, China: A population-based cohort study. Environ. Int. 2018, 122, 316–321. [Google Scholar] [CrossRef]
- Wang, Q.; Benmarhnia, T.; Zhang, H.; Knibbs, L.D.; Sheridan, P.; Li, C.; Bao, J.; Ren, M.; Wang, S.; He, Y.; et al. Identifying windows of susceptibility for maternal exposure to ambient air pollution and preterm birth. Environ. Int. 2018, 121, 317–324. [Google Scholar] [CrossRef]
- Song, Y.; Huang, B.; He, Q.; Chen, B.; Wei, J.; Mahmood, R. Dynamic assessment of PM2.5 exposure and health risk using remote sensing and geo-spatial big data. Environ. Pollut. 2019, 253, 288–296. [Google Scholar] [CrossRef]
- Wang, Q.; Benmarhnia, T.; Li, C.; Knibbs, L.; Bao, J.; Ren, M.; Zhang, H.; Wang, S.; Zhang, Y.; Zhao, Q.; et al. Seasonal analyses of the association between prenatal ambient air pollution exposure and birth weight for gestational age in Guangzhou, China. Sci. Total Environ. 2018, 649, 526–534. [Google Scholar] [CrossRef] [Green Version]


| Variables | PTB | Non-PTB | Total | p Value |
|---|---|---|---|---|
| (n = 33,669, 5.88%) | (n = 538,447, 94.12%) | (n = 572,116) | ||
| Gestational age | 34.75 ± 1.81 | 38.97 ± 1.05 | 38.73 ± 1.49 | <0.001 |
| Birth weight | 2459.07 ± 530.76 | 3328.69 ± 403.31 | 3277.52 ± 459.94 | <0.001 |
| Maternal age | 30.00 ± 5.07 | 28.77 ± 4.93 | 28.84 ± 4.95 | <0.001 |
| <20 years | 453 (1.35%) | 7753 (1.44%) | 8206 (1.43%) | <0.001 |
| 20–24 years | 4218 (12.53%) | 93,062 (17.28%) | 97,280 (17%) | |
| 25–29 years | 11,249 (33.41%) | 218,675 (40.61%) | 229,924 (40.19%) | |
| 30–34 years | 11,531 (34.25%) | 152,067 (28.24%) | 163,598 (28.6%) | |
| ≥35 years | 6185 (18.29%) | 66,426 (12.34%) | 72,611 (12.69%) | |
| Missing | 33 (0.9%) | 464 (0.09%) | 497 (0.09%) | |
| Father age | 32.34 ± 5.39 | 31.06 ± 5.59 | 31.14 ± 5.62 | <0.001 |
| <20 years | 96 (0.29%) | 1644 (0.31%) | 1740 (0.31%) | <0.001 |
| 20–24 years | 2062 (6.12%) | 46,922 (8.71%) | 48,984 (8.74%) | |
| 25–29 years | 9068 (26.93%) | 182,964 (33.98%) | 192,032 (34.27%) | |
| 30–34 years | 11,258 (33.44%) | 173,157 (32.16%) | 184,415 (32.91%) | |
| ≥35 years | 10,241 (30.42%) | 122,923 (22.83%) | 133,164 (23.77%) | |
| Missing | 944 (2.04%) | 10,837 (2.0%) | 11,781 (1.27%) | |
| Season of conception | ||||
| Spring | 7993 (23.74%) | 127,356 (23.65%) | 135,349 (23.66%) | <0.001 |
| Summer | 8186 (24.31%) | 128,145 (23.80%) | 136,331 (23.82%) | |
| Autumn | 8458 (25.12%) | 146,884 (27.28%) | 155,342 (27.15%) | |
| Winter | 9032 (26.83%) | 136,062 (25.27%) | 145,094 (25.36%) |
| Pollutants (μg/m3) | Mean | SD | Min | Max | Percentiles | ||
|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | |||||
| Trimester1 | |||||||
| PM2.5 | 42.86 | 14.75 | 16.03 | 112.32 | 32.47 | 41.29 | 50.56 |
| PM10 | 67.88 | 17.74 | 28.79 | 149.27 | 56.26 | 65.93 | 76.88 |
| NO2 | 39.03 | 7.96 | 7.76 | 75.41 | 34.60 | 38.28 | 42.93 |
| CO (mg/m3) | 1.01 | 0.22 | 0.48 | 1.68 | 0.82 | 0.98 | 1.13 |
| SO2 | 10.02 | 3.94 | 3.15 | 27.45 | 6.96 | 9.18 | 12.26 |
| O3 | 37.78 | 19.69 | 3.70 | 110.68 | 19.74 | 36.41 | 52.51 |
| Temperature (°C) | 20.07 | 5.38 | 8.98 | 30.52 | 15.64 | 20.11 | 24.16 |
| Relative humidity (%) | 75.30 | 4.12 | 64.52 | 84.45 | 73.06 | 75.24 | 78.25 |
| Trimester2 | |||||||
| PM2.5 | 41.76 | 14.16 | 16.59 | 107.25 | 31.08 | 40.08 | 49.93 |
| PM10 | 66.49 | 17.27 | 28.41 | 147.33 | 54.94 | 64.22 | 76.54 |
| NO2 | 38.86 | 7.47 | 9.75 | 73.61 | 34.81 | 37.95 | 42.89 |
| CO (mg/m3) | 1.02 | 0.23 | 0.48 | 1.68 | 0.83 | 1.00 | 1.14 |
| SO2 | 9.61 | 3.60 | 3.21 | 25.46 | 6.84 | 8.76 | 11.68 |
| O3 | 39.98 | 19.12 | 5.43 | 112.74 | 20.58 | 39.33 | 53.22 |
| Temperature (°C) | 20.31 | 5.04 | 9.85 | 29.09 | 16.13 | 20.32 | 24.37 |
| Relative humidity (%) | 75.27 | 3.81 | 66.78 | 82.87 | 72.81 | 75.24 | 78.00 |
| Trimester3 | |||||||
| PM2.5 | 41.42 | 14.58 | 2.83 | 130.62 | 28.72 | 40.59 | 51.90 |
| PM10 | 66.18 | 18.07 | 5.90 | 177.25 | 52.68 | 64.66 | 78.41 |
| NO2 | 38.96 | 7.63 | 4.95 | 76.31 | 34.49 | 38.16 | 43.22 |
| CO (mg/m3) | 1.04 | 0.23 | 0.14 | 1.82 | 0.88 | 1.04 | 1.17 |
| SO2 | 9.68 | 3.48 | 0.77 | 30.14 | 7.06 | 8.99 | 11.48 |
| O3 | 37.34 | 19.16 | 2.38 | 34.61 | 18.84 | 37.16 | 51.28 |
| Temperature (°C) | 19.86 | 5.00 | 5.61 | 34.61 | 16.43 | 20.04 | 23.02 |
| Relative humidity (%) | 75.50 | 4.03 | 48.38 | 88.13 | 73.34 | 75.82 | 78.27 |
| Entire pregnancy | |||||||
| PM2.5 | 41.62 | 10.01 | 17.82 | 83.65 | 34.39 | 42.69 | 47.50 |
| PM10 | 66.39 | 12.61 | 28.79 | 121.46 | 59.12 | 67.30 | 73.66 |
| NO2 | 38.95 | 6.46 | 10.78 | 68.19 | 35.50 | 38.35 | 41.15 |
| CO (mg/m3) | 1.02 | 0.18 | 0.54 | 1.52 | 0.89 | 1.01 | 1.14 |
| SO2 | 9.73 | 3.14 | 3.21 | 22.11 | 7.32 | 9.12 | 11.27 |
| O3 | 38.45 | 13.34 | 8.27 | 105.65 | 30.24 | 39.07 | 47.38 |
| Temperature (°C) | 20.25 | 2.73 | 13.13 | 29.09 | 18.43 | 20.42 | 21.86 |
| Relative humidity (%) | 75.25 | 2.17 | 66.89 | 80.37 | 73.85 | 75.21 | 76.81 |
| Pollutants (μg/m3) | PM2.5 | PM10 | CO | NO2 | O3 | SO2 | Temperature | Relative Humidity |
|---|---|---|---|---|---|---|---|---|
| PM2.5 | 1.000 | |||||||
| PM10 | 0.910 *** | 1.000 | ||||||
| CO (mg/m3) | 0.546 *** | 0.527 *** | 1.000 | |||||
| NO2 | 0.476 *** | 0.540 *** | 0.400 *** | 1.000 | ||||
| O3 | −3.390 *** | −0.272 *** | −0.449 *** | −0.281 *** | 1.000 | |||
| SO2 | 0.519 *** | 0.558 *** | 0.381 *** | 0.305 *** | −0.196 *** | 1.000 | ||
| Temperature (°C) | −0.244 *** | −0.170 *** | −0.207 *** | −0.133 *** | 0.378 *** | −0.121 *** | 1.000 | |
| Relative humidity (%) | −0.027 *** | −0.010 *** | 0.104 *** | −0.047 *** | −0.326 *** | −0.136 *** | −0.441 *** | 1.000 |
| Pollutant | Model | Trimester1 | Trimester2 | Trimester3 | Entire Pregnancy | ||||
|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | ||
| PM2.5 | Model 1 | 0.958 | (0.943, 0.973) | 1.002 | (0.984, 1.020) | 1.119 | (1.101, 1.138) | 1.185 | (1.157, 1.215) |
| Model 2 | 0.988 | (0.972, 1.004) | 0.998 | (0.980, 1.017) | 1.036 | (1.021, 1.051) | 1.101 | (1.075, 1.128) | |
| Model 3 | 0.987 | (0.968, 1.006) | 1.001 | (0.979, 1.024) | 1.008 | (0.991, 1.024) | 1.064 | (1.031, 1.099) | |
| PM10 | Model 1 | 0.976 | (0.963, 0.988) | 0.940 | (0.980, 1.008) | 1.107 | (1.094, 1.121) | 1.078 | (1.059, 1.098) |
| Model 2 | 0.999 | (0.986, 1.012) | 0.976 | (0.962, 0.989) | 1.027 | (1.016, 1.038) | 1.038 | (1.020, 1.057) | |
| Model 3 | 1.002 | (0.985, 1.018) | 0.998 | (0.996, 0.999) | 1.005 | (0.992, 1.018) | 1.004 | (0.977, 1.030) | |
| SO2 | Model 1 | 0.733 | (0.697, 0.771) | 0.806 | (0.764, 0.849) | 0.897 | (0.852, 0.945) | 0.893 | (0.840, 0.950) |
| Model 2 | 0.960 | (0.912, 1.010) | 0.957 | (0.908, 1.008) | 1.014 | (0.964, 1.067) | 1.021 | (0.960, 1.085) | |
| Model 3 | 0.988 | (0.933, 1.047) | 0.973 | (0.915, 1.034) | 1.011 | (0.957, 1.068) | 0.973 | (0.901, 1.051) | |
| NO2 | Model 1 | 0.935 | (0.920, 0.951) | 0.919 | (0.903, 0.936) | 1.014 | (0.997, 1.031) | 0.886 | (0.870, 0.903) |
| Model 2 | 0.998 | (0.981, 1.016) | 0.999 | (0.981, 1.017) | 1.016 | (0.999, 1.033) | 0.982 | (0.963, 1.002) | |
| Model 3 | 1.004 | (0.986, 1.023) | 1.002 | (0.982, 1.022) | 0.976 | (0.959, 0.993) | 0.991 | (0.970, 1.013) | |
| O3 | Model 1 | 0.926 | (0.914, 0.937) | 0.922 | (0.910, 0.934) | 0.948 | (0.937, 0.960) | 0.921 | (0.907, 0.934) |
| Model 2 | 1.000 | (0.988, 1.013) | 0.996 | (0.934, 1.009) | 0.976 | (0.966, 0.987) | 0.987 | (0.974, 1.001) | |
| Model 3 | 1.004 | (0.990, 1.018) | 1.004 | (0.990, 1.019) | 0.999 | (0.988, 1.010) | 1.021 | (1.002, 1.039) | |
| CO | Model 1 | 1.857 | (1.733, 1.991) | 2.194 | (2.035, 2.366) | 1.758 | (1.635, 1.890) | 2.207 | (2.031, 2.398) |
| Model 2 | 1.081 | (1.007, 1.162) | 1.116 | (1.035, 1.204) | 1.167 | (1.090, 1.250) | 1.098 | (1.011, 1.192) | |
| Model 3 | 1.094 | (1.008, 1.188) | 1.117 | (1.024, 1.218) | 1.050 | (0.978, 1.127) | 0.878 | (0.790, 0.976) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Ming, X.; Yang, Y.; Hu, Y.; He, Z.; Chen, H.; Li, Y.; Zhou, X.; Yin, P. Association between Maternal Exposure to Ambient Air Pollution and the Risk of Preterm Birth: A Birth Cohort Study in Chongqing, China, 2015–2020. Int. J. Environ. Res. Public Health 2022, 19, 2211. https://doi.org/10.3390/ijerph19042211
Zhou W, Ming X, Yang Y, Hu Y, He Z, Chen H, Li Y, Zhou X, Yin P. Association between Maternal Exposure to Ambient Air Pollution and the Risk of Preterm Birth: A Birth Cohort Study in Chongqing, China, 2015–2020. International Journal of Environmental Research and Public Health. 2022; 19(4):2211. https://doi.org/10.3390/ijerph19042211
Chicago/Turabian StyleZhou, Wenzheng, Xin Ming, Yunping Yang, Yaqiong Hu, Ziyi He, Hongyan Chen, Yannan Li, Xiaojun Zhou, and Ping Yin. 2022. "Association between Maternal Exposure to Ambient Air Pollution and the Risk of Preterm Birth: A Birth Cohort Study in Chongqing, China, 2015–2020" International Journal of Environmental Research and Public Health 19, no. 4: 2211. https://doi.org/10.3390/ijerph19042211






