Effects of Environmental Exposure to Cadmium and Lead on the Risks of Diabetes and Kidney Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Simultaneous Blood and Urine Sampling and Biochemical Analysis
2.3. Analysis of Blood Concentrations of Cd and Pb
2.4. Toxic Metal Exposure Profiling
2.5. Estimated Glomerular Filtration Rates (eGFR)
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. Logistic Regression Analysis
3.3. Correlation Analysis
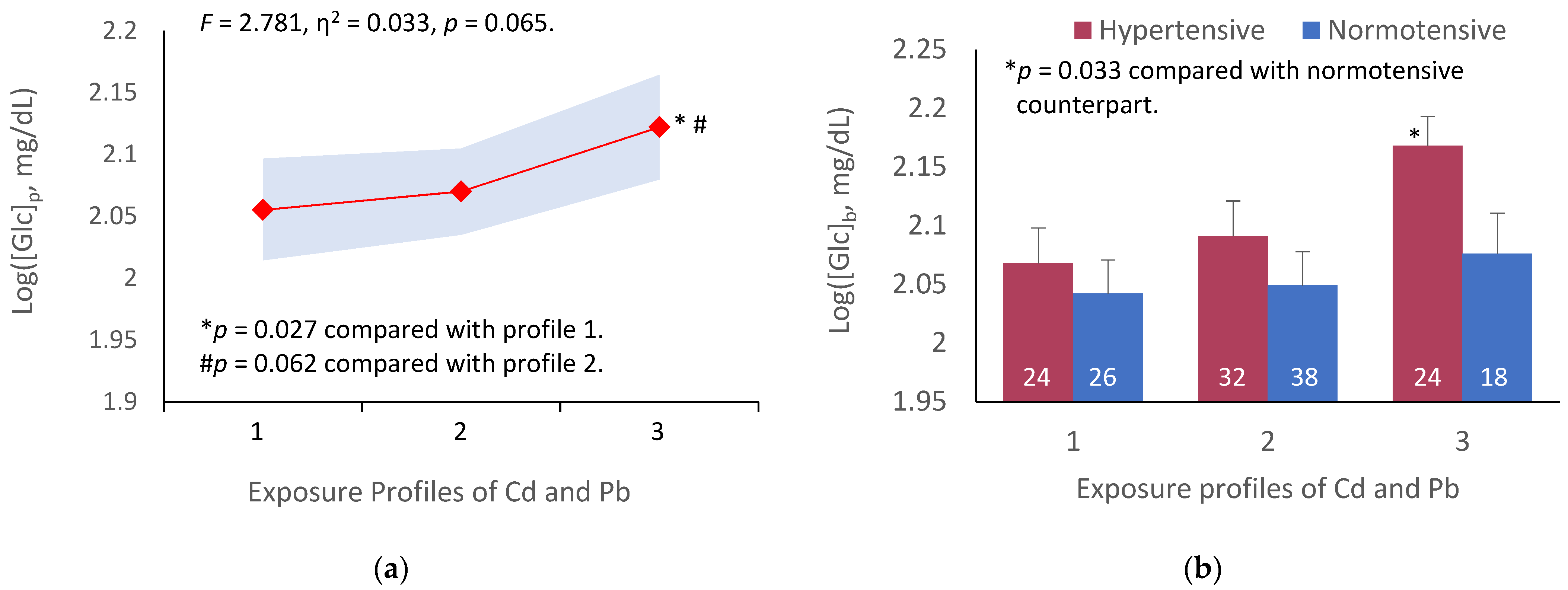
3.4. Covariance Analysis of Fasting Plasma Glucose Variation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, G.G.; Il’yasova, D.; Ivanova, A. Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care 2003, 26, 468–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallia, A.; Allen, N.B.; Badon, S.; El Muayed, M. Association between urinary cadmium levels and prediabetes in the NHANES 2005–2010 population. Int. J. Hyg. Environ. Health 2014, 217, 854–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, B.B.; Reilly, R.; Walsh, B.; Vu, G.T. Cadmium is associated with type 2 diabetes in a Superfund Site Lead Smelter community in Dallas, Texas. Int. J. Environ. Res. Public Health 2020, 17, 4558. [Google Scholar] [CrossRef]
- Shi, P.; Yan, H.; Fan, X.; Xi, S. A benchmark dose analysis for urinary cadmium and type 2 diabetes mellitus. Environ. Pollut. 2021, 273, 116519. [Google Scholar] [CrossRef]
- Son, H.S.; Kim, S.G.; Suh, B.S.; Park, D.U.; Kim, D.S.; Yu, S.D.; Hong, Y.S.; Park, J.D.; Lee, B.K.; Moon, J.D.; et al. Association of cadmium with diabetes in middle-aged residents of abandoned metal mines: The first health effect surveillance for residents in abandoned metal mines. Ann. Occup. Environ. Med. 2015, 27, 20. [Google Scholar] [CrossRef] [Green Version]
- Nie, X.; Wang, N.; Chen, Y.; Chen, C.; Han, B.; Zhu, C.; Chen, Y.; Xia, F.; Cang, Z.; Lu, M.; et al. Blood cadmium in Chinese adults and its relationships with diabetes and obesity. Environ. Sci. Pollut. Res. Int. 2016, 23, 18714–18723. [Google Scholar] [CrossRef] [PubMed]
- Simić, A.; Hansen, A.F.; Åsvold, B.O.; Romundstad, P.R.; Midthjell, K.; Syversen, T.; Flaten, T.P. Trace element status in patients with type 2 diabetes in Norway: The HUNT3 Survey. J. Trace Elem. Med. Biol. 2017, 41, 91–98. [Google Scholar] [CrossRef]
- Hansen, A.F.; Simić, A.; Åsvold, B.O.; Romundstad, P.R.; Midthjell, K.; Syversen, T.; Flaten, T.P. Trace elements in early phase type 2 diabetes mellitus-A population-based study. The HUNT study in Norway. J. Trace Elem. Med. Biol. 2017, 40, 46–53. [Google Scholar] [CrossRef]
- Grau-Perez, M.; Pichler, G.; Galan-Chilet, I.; Briongos-Figuero, L.S.; Rentero-Garrido, P.; Lopez-Izquierdo, R.; Navas-Acien, A.; Weaver, V.; García-Barrera, T.; Gomez-Ariza, J.L.; et al. Urine cadmium levels and albuminuria in a general population from Spain: A gene-environment interaction analysis. Environ. Int. 2017, 106, 27–36. [Google Scholar] [CrossRef]
- Myong, J.P.; Kim, H.R.; Baker, D.; Choi, B. Blood cadmium and moderate-to-severe glomerular dysfunction in Korean adults: Analysis of KNHANES 2005−2008 data. Int. Arch. Occup. Environ. Health 2012, 85, 885–893. [Google Scholar] [CrossRef]
- Navas-Acien, A.; Tellez-Plaza, M.; Guallar, E.; Muntner, P.; Silbergeld, E.; Jaar, B.; Weaver, V. Blood cadmium and lead and chronic kidney disease in US adults: A joint analysis. Am. J. Epidemiol. 2009, 170, 1156–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraro, P.M.; Costanzi, S.; Naticchia, A.; Sturniolo, A.; Gambaro, G. Low level exposure to cadmium increases the risk of chronic kidney disease: Analysis of the NHANES 1999–2006. BMC Public Health 2010, 10, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madrigal, J.M.; Ricardo, A.C.; Persky, V.; Turyk, M. Associations between blood cadmium concentration and kidney function in the U.S. population: Impact of sex, diabetes and hypertension. Environ. Res. 2018, 169, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Ho, W.C.; Caffrey, J.L.; Sonawane, B. Low serum zinc is associated with elevated risk of cadmium nephrotoxicity. Environ. Res. 2014, 134, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Oh, S.; Kang, H.; Kim, S.; Lee, G.; Li, L.; Kim, C.T.; An, J.N.; Oh, Y.K.; Lim, C.S.; et al. Environment-wide association study of CKD. Clin. J. Am. Soc. Nephrol. 2020, 15, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Soveri, I.; Berg, U.B.; Bjork, J.; Elinder, C.-G.; Grubb, A.; Mejare, I.; Sterner, G.; Sten-Erik Bäck, S.-E.; SBU GFR Review Group. Measuring GFR: A systematic review. Am. J. Kidney Dis. 2014, 64, 411–424. [Google Scholar] [CrossRef]
- Levey, A.S.; Becker, C.; Inker, L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA 2015, 313, 837–846. [Google Scholar] [CrossRef] [Green Version]
- White, C.A.; Allen, C.M.; Akbari, A.; Collier, C.P.; Holland, D.C.; Day, A.G.; Knoll, G.A. Comparison of the new and traditional CKD-EPI GFR estimation equations with urinary inulin clearance: A study of equation performance. Clin. Chim. Acta 2019, 488, 189–195. [Google Scholar] [CrossRef]
- Murton, M.; Goff-Leggett, D.; Bobrowska, A.; Garcia Sanchez, J.J.; James, G.; Wittbrodt, E.; Stephen Nolan, S.; Sörstadius, E.; Pecoits-Filho, R.; Tuttle, K. Burden of chronic kidney disease by KDIGO categories of glomerular filtration rate and albuminuria: A systematic review. Adv. Ther. 2021, 38, 180–200. [Google Scholar] [CrossRef]
- Reilly, R.; Spalding, S.; Walsh, B.; Wainer, J.; Pickens, S.; Royster, M.; Villanacci, J.; Little, B.B. Chronic environmental and occupational lead exposure and kidney function among African Americans: Dallas Lead Project II. Int. J. Environ. Res. Public Health 2018, 15, 2875. [Google Scholar] [CrossRef] [Green Version]
- Sommar, J.N.; Svensson, M.K.; Björ, B.M.; Elmståhl, S.I.; Hallmans, G.; Lundh, T.; Schön, S.M.; Skerfving, S.; Bergdahl, I.A. End-stage renal disease and low-level exposure to lead, cadmium and mercury; a population-based, prospective nested case-referent study in Sweden. Environ. Health 2013, 12, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harari, F.; Sallsten, G.; Christensson, A.; Petkovic, M.; Hedblad, B.; Forsgard, N.; Melander, O.; Nilsson, P.M.; Borné, Y.; Engström, G.; et al. Blood lead levels and decreased kidney function in a population-based cohort. Am. J. Kidney Dis. 2018, 72, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satarug, S. Dietary cadmium intake and its effects on kidneys. Toxics 2018, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Satarug, S.; Gobe, G.C.; Vesey, D.A.; Phelps, K.R. Cadmium and lead exposure, nephrotoxicity, and mortality. Toxics 2020, 8, 86. [Google Scholar] [CrossRef]
- Buser, M.C.; Ingber, S.Z.; Raines, N.; Fowler, D.A.; Scinicariello, F. Urinary and blood cadmium and lead and kidney function: NHANES 2007–2012. Int. J. Hyg. Environ. Health 2016, 219, 261–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Schaumberg, D.A.; Park, S.K. Cadmium and lead exposure and risk of cataract surgery in U.S. adults. Int. J. Hyg. Environ. Health 2016, 219, 850–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, Y.K.; Lewin, M.D.; Ruiz, P.; Eichner, J.E.; Mumtaz, M.M. Prevalence and associated demographic characteristics of exposure to multiple metals and their species in human populations: The United States NHANES, 2007–2012. J. Toxicol. Environ. Health A 2017, 80, 502–512. [Google Scholar] [CrossRef]
- Jin, R.; Zhu, X.; Shrubsole, M.J.; Yu, C.; Xia, Z.; Dai, Q. Associations of renal function with urinary excretion of metals: Evidence from NHANES 2003–2012. Environ. Int. 2018, 121, 1355–1362. [Google Scholar] [CrossRef]
- Saravanabhavan, G.; Werry, K.; Walker, M.; Haines, D.; Malowany, M.; Khoury, C. Human biomonitoring reference values for metals and trace elements in blood and urine derived from the Canadian Health Measures Survey 2007–2013. Int. J. Hyg. Environ. Health 2017, 220, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Liao, K.W.; Pan, W.H.; Liou, S.H.; Sun, C.W.; Huang, P.C.; Wang, S.L. Levels and temporal variations of urinary lead, cadmium, cobalt, and copper exposure in the general population of Taiwan. Environ. Sci. Pollut. Res. Int. 2019, 26, 6048–6064. [Google Scholar] [CrossRef]
- Kim, N.S.; Ahn, J.; Lee, B.K.; Park, J.; Kim, Y. Environmental exposures to lead, mercury, and cadmium among South Korean teenagers (KNHANES 2010–2013): Body burden and risk factors. Environ. Res. 2017, 156, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Buchet, J.P.; Lauwerys, R.; Roels, H.; Bernard, A.; Bruaux, P.; Claeys, F.; Ducoffre, G.; de Plaen, P.; Staessen, J.; Amery, A.; et al. Renal effects of cadmium body burden of the general population. Lancet 1990, 336, 699–702. [Google Scholar] [CrossRef]
- Akesson, A.; Lundh, T.; Vahter, M.; Bjellerup, P.; Lidfeldt, J.; Nerbrand, C.; Samsioe, G.; Strömberg, U.; Skerfving, S. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ. Health Perspect. 2005, 113, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Barregard, L.; Bergstrom, G.; Fagerberg, B. Cadmium, type 2 diabetes, and kidney damage in a cohort of middle-aged women. Environ. Res. 2014, 135, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Haswell-Elkins, M.; Satarug, S.; O’Rourke, P.; Moore, M.; Ng, J.; McGrath, V.; Walmby, M. Striking association between urinary cadmium level and albuminuria among Torres Strait Islander people with diabetes. Environ. Res. 2008, 106, 379–383. [Google Scholar] [CrossRef]
- Hwangbo, Y.; Weaver, V.M.; Tellez-Plaza, M.; Guallar, E.; Lee, B.K.; Navas-Acien, A. Blood cadmium and estimated glomerular filtration rate in Korean adults. Environ. Health Perspect. 2011, 119, 1800–1805. [Google Scholar] [CrossRef] [Green Version]
- Bökenkamp, A. Proteinuria-take a closer look! Pediatr. Nephrol. 2020, 35, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Hornung, R.W.; Reed, L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- Flannery, B.M.; Dolan, L.C.; Hoffman-Pennesi, D.; Gavelek, A.; Jones, O.E.; Kanwal, R.; Wolpert, B.; Gensheimer, K.; Dennis, S.; Fitzpatrick, S.U.S. Food and Drug Administration’s interim reference levels for dietary lead exposure in children and women of childbearing age. Regul. Toxicol. Pharmacol. 2020, 110, 104516. [Google Scholar] [CrossRef]
- Padilla, M.A.; Elobeid, M.; Ruden, D.M.; Allison, D.B. An examination of the association of selected toxic metals with total and central obesity indices: NHANES 99-02. Int. J. Environ. Res. Public Health 2010, 7, 3332–3347. [Google Scholar] [CrossRef]
- Jain, R.B. Effect of pregnancy on the levels of blood cadmium, lead, and mercury for females aged 17-39 years old: Data from National Health and Nutrition Examination Survey 2003–2010. J. Toxicol. Environ. Health A 2013, 76, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Garner, R.; Levallois, P. Cadmium levels and sources of exposure among Canadian adults. Health Rep. 2016, 27, 10–18. [Google Scholar] [PubMed]
- Akbar, L.; Zuk, A.M.; Martin, I.D.; Liberda, E.N.; Tsuji, L.J.S. Potential obesogenic effect of a complex contaminant mixture on Cree First Nations adults of Northern Québec, Canada. Environ. Res. 2021, 192, 110478. [Google Scholar] [CrossRef] [PubMed]
- Sonthon, P.; Promthet, S.; Changsirikulchai, S.; Rangsin, R.; Thinkhamrop, B.; Rattanamongkolgul, S.; Hurst, C.P. The impact of the quality of care and other factors on progression of chronic kidney disease in Thai patients with type 2 diabetes mellitus: A nationwide cohort study. PLoS ONE 2017, 12, e0180977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhooge, W.; Hond, E.D.; Koppen, G.; Bruckers, L.; Nelen, V.; Van De Mieroop, E.; Bilau, M.; Croes, K.; Baeyens, W.; Schoeters, G.; et al. Internal exposure to pollutants and body size in Flemish adolescents and adults: Associations and dose-response relationships. Environ. Int. 2010, 36, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Liu, Q.; He, X.; Liu, H.; Gu, A.; Jiang, Z. Association between level of urinary trace heavy metals and obesity among children aged 6-19 years: NHANES 1999–2011. Environ. Sci. Pollut. Res. Int. 2017, 24, 11573–11581. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Boonprasert, K.; Gobe, G.C.; Ruenweerayut, R.; Johnson, D.W.; Na-Bangchang, K.; Vesey, D.A. Chronic exposure to cadmium is associated with a marked reduction in glomerular filtration rate. Clin. Kidney. J. 2018, 12, 468–475. [Google Scholar] [CrossRef]
- Satarug, S.; Gobe, G.C.; Ujjin, P.; Vesey, D.A. A comparison of the nephrotoxicity of low doses of cadmium and lead. Toxics 2020, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Satarug, S.; Vesey, D.A.; Ruangyuttikarn, W.; Nishijo, M.; Gobe, G.C.; Phelps, K.R. The source and pathophysiologic significance of excreted cadmium. Toxics 2019, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Satarug, S.; Vesey, D.A.; Nishijo, M.; Ruangyuttikarn, W.; Gobe, G.C.; Phelps, K.R. The effect of cadmium on GFR is clarified by normalization of excretion rates to creatinine clearance. Int. J. Mol. Sci. 2021, 22, 1762. [Google Scholar] [CrossRef]
- Filippini, T.; Wise, L.A.; Vinceti, M. Cadmium exposure and risk of diabetes and prediabetes: A systematic review and dose-response meta-analysis. Environ. Int. 2021, 158, 106920. [Google Scholar] [CrossRef] [PubMed]
- Gerich, J.E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: Therapeutic implications. Diabet. Med. 2010, 27, 136–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFronzo, R.A.; Davidson, J.A.; Prato, S.D. The role of the kidneys in glucose homeostasis: A new path towards normalizing glycaemia. Diabetes Obes. Metab. 2012, 14, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Fay, M.J.; Alt, L.A.C.; Ryba, D.; Salamah, R.; Peach, R.; Papaeliou, A.; Zawadzka, S.; Weiss, A.; Patel, N.; Rahman, A.; et al. Cadmium nephrotoxicity is associated with altered microRNA expression in the rat renal cortex. Toxics 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, R.; Olsen, A.; Nguyen, J.; Wong, W.; El Muayed, M.; Edwards, J. Pancreatic islets accumulate cadmium in a rodent model of cadmium-induced hyperglycemia. Int. J. Mol. Sci. 2020, 22, 360. [Google Scholar] [CrossRef] [PubMed]
| Variables | All Participants n = 176 | Cadmium and Lead Exposure a | p | ||
|---|---|---|---|---|---|
| Profile 1, n 53 | Profile 2, n 71 | Profile 3, n 52 | |||
| Women, % | 80.7 | 90.6 | 76.1 | 76.9 | 0.092 |
| Diabetes diagnosis b, % | 50.0 | 35.8 | 46.5 | 69.2 | 0.002 |
| Diabetes duration, years | 9.3 ± 7.6 | 8.8 ± 7.8 | 9.2 ± 7.9 | 9.5 ± 7.4 | 0.910 |
| Smoking, % | 9.7 | 1.9 | 9.9 | 17.9 | 0.028 |
| Hypertension c % | 52.0 | 48.0 | 45.1 | 65.4 | 0.067 |
| SBP, mmHg | 138 ± 17 | 137 ± 17 | 136 ± 15 | 142 ± 19 | 0.122 |
| DBP, mmHg | 84 ± 9.5 | 83 ± 9.4 | 83 ± 8.7 | 86 ±10.4 | 0.226 |
| Age, years | 59.9 ± 9.7 | 59.5 ± 10.6 | 60.8 ± 9.2 | 58.9 ± 9.4 | 0.639 |
| BMI, kg/m2 | 25.4 ± 4.7 | 25.8 ± 4.3 | 25.0 ± 4.0 | 25.6 ± 5.9 | 0.426 |
| BMI classification d | |||||
| Thin, % | 6.8 | 5.7 | 4.2 | 11.5 | 0.472 |
| Normal, % | 28.4 | 26.4 | 32.4 | 25.0 | 0.162 |
| Overweight, % | 54.0 | 56.6 | 54.9 | 50.0 | 0.247 |
| Obesity, % | 10.8 | 11.3 | 8.5 | 13.5 | 0.949 |
| [cr]p, mg/dL | 0.87 ± 0.24 | 0.82 ± 0.15 | 0.89 ± 0.19 | 0.90 ± 0.36 | 0.133 |
| [cr]u, mg/dL | 89.2 ± 54.1 | 92.2 ± 51.7 | 90.1 ± 53.7 | 84.8 ± 57.9 | 0.499 |
| [Alb]u, mg/dL | 30.9 ± 79.9 | 23.8 ± 64.1 | 34.4 ± 90.2 | 33.8 ± 81.1 | 0.564 |
| [Pb]b, µg/dL | 4.67 ± 4.88 | 2.12 ± 0.00 | 4.58 ± 5.30 | 7.38 ±5.38 | <0.001 |
| [Cd]b, µg/L | 0.59 ± 0.74 | 0.05 ± 0.05 | 0.65 ± 0.78 | 1.05 ± 0.72 | <0.001 |
| eGFR e, mL/min/1.73 m2 | 79 ± 18 | 81 ± 16 | 77 ± 17 | 81 ± 21 | 0.269 |
| ≤60, % | 16.5 | 11.3 | 22.5 | 13.5 | 0.196 |
| ACR, mg/g creatinine | 41.4 ± 103.8 | 28.4 ±75.8 | 38.5 ± 92.3 | 59.3 ± 139.2 | 0.387 |
| <30, % | 78.4 | 82.7 | 79.1 | 72.9 | 0.155 |
| 30−299, % | 17.4 | 15.4 | 16.4 | 20.8 | 0.786 |
| ≥300, % | 4.2 | 1.9 | 4.5 | 6.3 | 0.565 |
| [Glc]p f mg/dL | 132 ± 61 | 118 ± 41 | 128 ± 66 | 150 ± 68 | 0.031 |
| <110, % | 49.4 | 58.5 | 52.1 | 36.5 | 0.055 |
| 110−126, % | 11.9 | 15.1 | 12.7 | 7.7 | 0.368 |
| 127−179, % | 25.6 | 17.0 | 26.8 | 32.7 | 0.155 |
| ≥180, % | 13.1 | 9.4 | 8.5 | 23.1 | 0.154 |
| Independent Variables/Factors | Number of Subjects | Diabetes Diagnosis a | ||||
|---|---|---|---|---|---|---|
| β Coefficients (SE) | POR | 95% CI | p | |||
| Lower | Upper | |||||
| Age, years | 173 | 0.014 (0.019) | 1.015 | 0.977 | 1.054 | 0.457 |
| BMI, kg/m2 | 173 | −0.062 (0.040) | 0.939 | 0.869 | 1.015 | 0.115 |
| Smoking status | 173 | −0.951 (0.723) | 0.386 | 0.094 | 1.593 | 0.188 |
| Hypertension | 173 | −0.321 (0.334) | 0.725 | 0.377 | 1.394 | 0.335 |
| Gender | 173 | −0.450 (0.542) | 0.638 | 0.220 | 1.846 | 0.407 |
| eGFR, mL/min/1.73 m2 | ||||||
| >60 | 144 | Referent | ||||
| ≤60 | 29 | 1.074 (0.492) | 2.926 | 1.115 | 7.679 | 0.029 |
| Cd-Pb exposure b | ||||||
| Profile 1 c | 50 | Referent | ||||
| Profile 2 | 71 | 1.096 (0.419) | 2.991 | 1.317 | 6.794 | 0.009 |
| Profile 3 | 52 | 1.431 (0.452) | 4.182 | 1.725 | 10.14 | 0.002 |
| Independent Variables/Factors | Model 1 a, n 173 | Model 2 b, n 173 | Model 3 c, n 173 | |||
|---|---|---|---|---|---|---|
| POR (95% CI) | p | POR (95% CI) | p | POR (95% CI) | p | |
| Age, years | 1.008 (0.975, 1.042) | 0.653 | 1.033 (0.997, 1.069) | 0.073 | 1.066 (1.011,1.124) | 0.018 |
| BMI, kg/m2 | 0.940 (0.875, 1.011) | 0.095 | 0.958 (0.891, 1.029) | 0.238 | 1.062 (0.959, 1.176) | 0.250 |
| Smoking status | 0.567 (0.194, 1.662) | 0.301 | 0.638 (0.209, 1.941) | 0.428 | 0.246 (0.029, 2.063) | 0.196 |
| Cd-Pb exposure d | ||||||
| Profile 1 | Referent | Referent | Referent | |||
| Profile 2 | 1.940 (0.911, 4.132) | 0.086 | 2.290 (1.073, 4.885) | 0.032 | 3.383 (1.136, 10.08) | 0.029 |
| Profile 3 | 2.794 (1.228, 6.375) | 0.014 | 2.964 (1.288, 6.822) | 0.011 | 3.407 (1.061, 10.94) | 0.039 |
| Variables | Pearson’s Correlation Coefficients | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| [Glc]b | Age | BMI | Gender | SMK | [Pb]b | [Cd]b | ACR | eGFR | |
| [Glc]b | 1 | ||||||||
| Age | −0.193 * | 1 | |||||||
| BMI | 0.078 | −0.285 *** | 1 | ||||||
| Gender | −0.057 | 0.186 * | 0.140 # | 1 | |||||
| SMK | −0.040 | −0.018 | −0.152 * | −0.620 *** | 1 | ||||
| [Pb]b | 0.194 * | −0.071 | 0.060 | −0.108 | 0.153 * | 1 | |||
| [Cd]b | 0.049 | 0.053 | −0.047 | −0.034 | 0.184 * | 0.148 * | 1 | ||
| ACR | 0.288 *** | 0.114 | 0.003 | 0.009 | −0.031 | 0.047 | 0.098 | 1 | |
| eGFR | 0.092 | −0.454 *** | 0.172 * | −0.139 | 0.040 | 0.090 | −0.123 | −0.176 * | 1 |
| Cd/Pb profiles | 0.216 ** | −0.022 | −0.017 | −0.134 | 0.202 ** | 0.604 ** | 0.724 *** | 0.102 | −0.008 |
| Independent Variables/Factors | Number of Subjects b | Albuminuria a | ||||
|---|---|---|---|---|---|---|
| β Coefficients (SE) | POR | 95% CI | p | |||
| Lower | Upper | |||||
| Age, years | 164 | −0.040 (0.028) | 0.961 | 0.910 | 1.014 | 0.147 |
| BMI, kg/m2 | 164 | −0.027 (0.047) | 0.974 | 0.888 | 1.068 | 0.570 |
| Smoking status | 164 | −1.287 (0.914) | 0.276 | 0.046 | 1.656 | 0.159 |
| Hypertension | 164 | −1.225 (0.482) | 0.294 | 0.114 | 0.755 | 0.011 |
| Gender | 164 | −1.825 (0.644) | 0.161 | 0.046 | 0.570 | 0.005 |
| eGFR, mL/min/1.73 m2 | ||||||
| >60 | 138 | Referent | ||||
| ≤60 | 26 | 1.129 (0.575) | 3.093 | 1.002 | 9.552 | 0.050 |
| [Glc]p, mg/dL | ||||||
| <110 | 81 | Referent | ||||
| 110−126 | 20 | 0.453 (0.634) | 1.574 | 0.454 | 5.457 | 0.475 |
| 127−179 | 43 | 1.210 (0.816) | 3.353 | 0.678 | 16.58 | 0.138 |
| ≥180 | 20 | 1.597 (0.653) | 4.937 | 1.373 | 17.76 | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yimthiang, S.; Pouyfung, P.; Khamphaya, T.; Kuraeiad, S.; Wongrith, P.; Vesey, D.A.; Gobe, G.C.; Satarug, S. Effects of Environmental Exposure to Cadmium and Lead on the Risks of Diabetes and Kidney Dysfunction. Int. J. Environ. Res. Public Health 2022, 19, 2259. https://doi.org/10.3390/ijerph19042259
Yimthiang S, Pouyfung P, Khamphaya T, Kuraeiad S, Wongrith P, Vesey DA, Gobe GC, Satarug S. Effects of Environmental Exposure to Cadmium and Lead on the Risks of Diabetes and Kidney Dysfunction. International Journal of Environmental Research and Public Health. 2022; 19(4):2259. https://doi.org/10.3390/ijerph19042259
Chicago/Turabian StyleYimthiang, Supabhorn, Phisit Pouyfung, Tanaporn Khamphaya, Saruda Kuraeiad, Paleeratana Wongrith, David A. Vesey, Glenda C. Gobe, and Soisungwan Satarug. 2022. "Effects of Environmental Exposure to Cadmium and Lead on the Risks of Diabetes and Kidney Dysfunction" International Journal of Environmental Research and Public Health 19, no. 4: 2259. https://doi.org/10.3390/ijerph19042259








