Antimicrobial Photodynamic Coatings Reduce the Microbial Burden on Environmental Surfaces in Public Transportation—A Field Study in Buses
Abstract
:1. Introduction
2. Materials and Methods
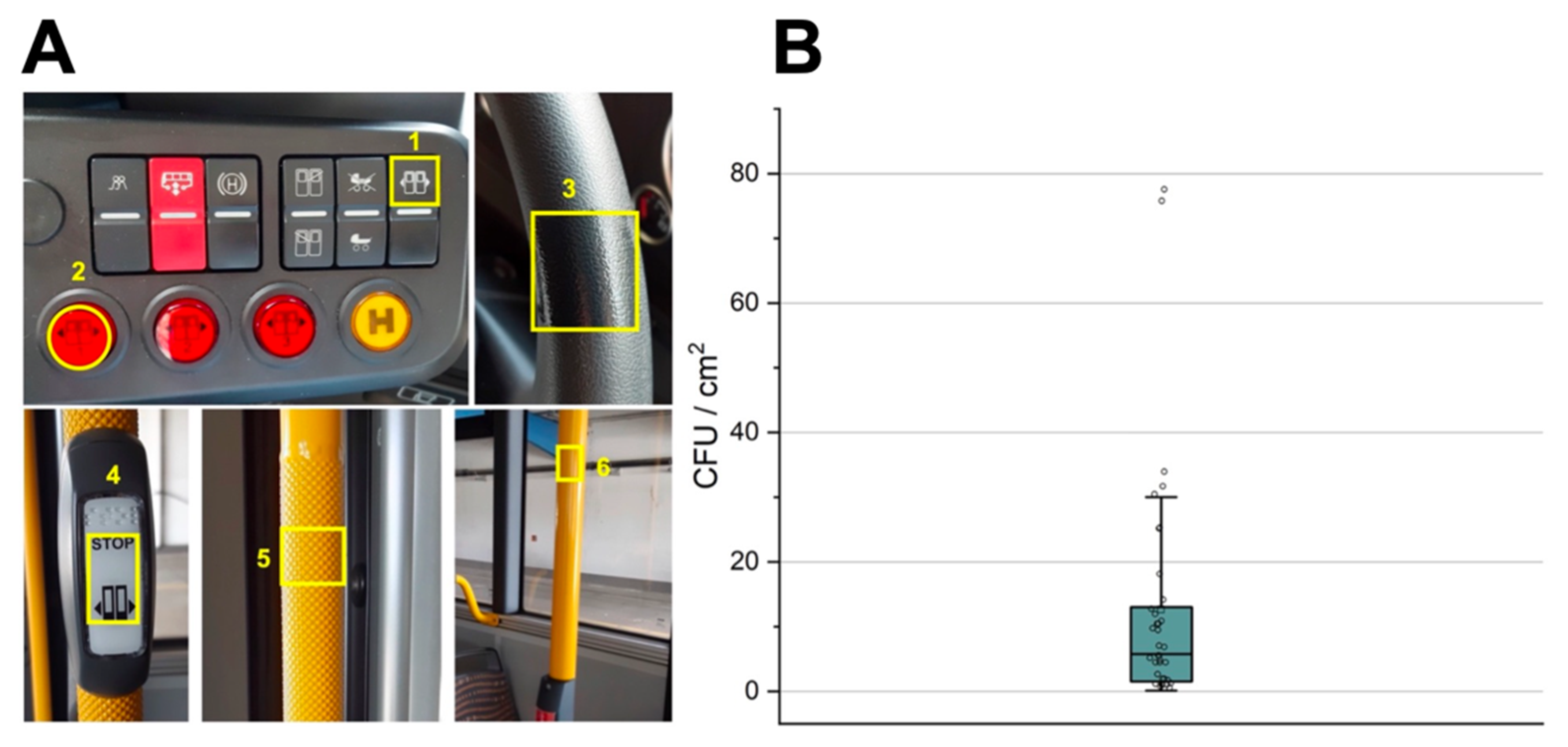
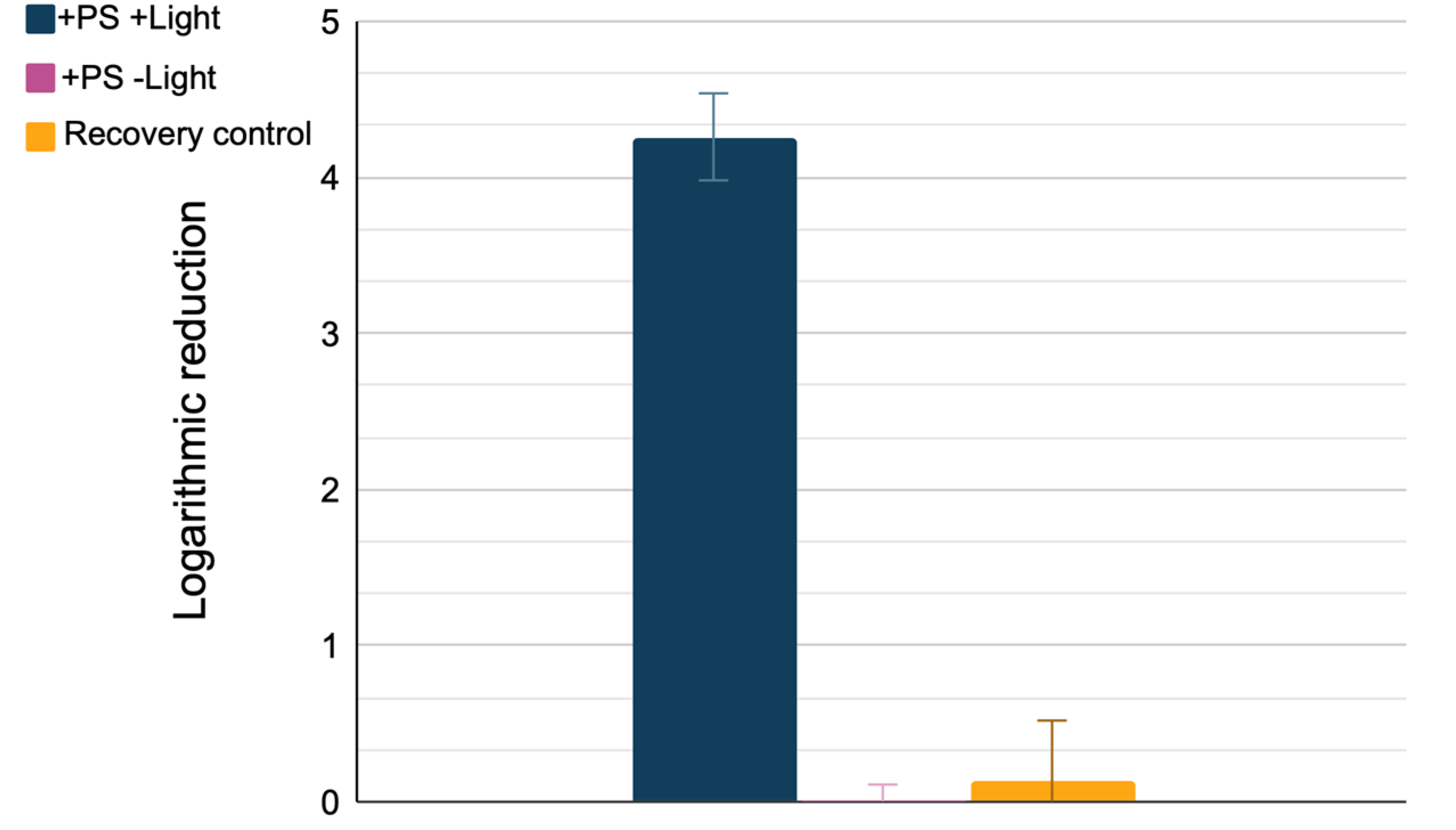
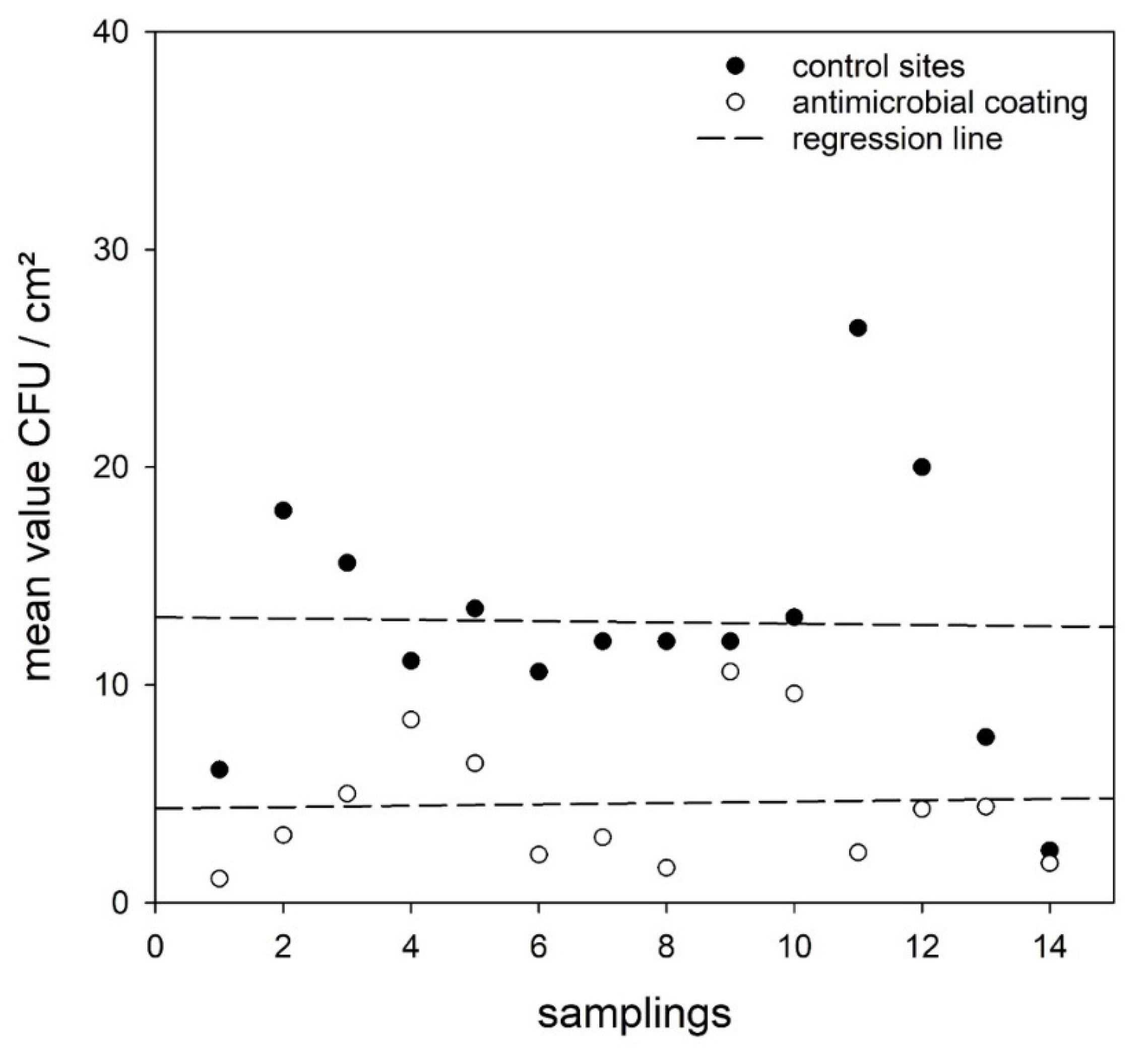
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehrenberg, R. Urban microbes come out of the shadows. Nat. News 2015, 522, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afshinnekoo, E.; Meydan, C.; Chowdhury, S.; Jaroudi, D.; Boyer, C.; Bernstein, N.; Maritz, J.M.; Reeves, D.; Gandara, J.; Chhangawala, S. Geospatial resolution of human and bacterial diversity with city-scale metagenomics. Cell Syst. 2015, 1, 72–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danko, D.; Bezdan, D.; Afshin, E.E.; Ahsanuddin, S.; Bhattacharya, C.; Butler, D.J.; Chng, K.R.; Donnellan, D.; Hecht, J.; Jackson, K. A global metagenomic map of urban microbiomes and antimicrobial resistance. Cell 2021, 184, 3376–3393.e17. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Ni, Y.; Li, J.; Imamovic, L.; Sarkar, C.; Kobler, M.D.; Heshiki, Y.; Zheng, T.; Kumari, S.; Wong, J.C.Y. The environmental exposures and inner-and intercity traffic flows of the metro system may contribute to the skin microbiome and resistome. Cell Rep. 2018, 24, 1190–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costello, C. Are “Bad” Microbes Getting a Free Ride on Your Transit System? Available online: https://www.metro-magazine.com/10111889/are-bad-microbes-getting-a-free-ride-on-your-transit-system (accessed on 18 January 2022).
- Otter, J.A.; French, G.L. Bacterial contamination on touch surfaces in the public transport system and in public areas of a hospital in London. Lett. Appl. Microbiol. 2009, 49, 803–805. [Google Scholar] [CrossRef]
- Patel, K.V.; Bailey, C.L.; Harding, A.; Biggin, M.; Crook, B. Background levels of micro-organisms in the busy urban environment of transport hubs. J. Appl. Microbiol. 2018, 125, 1541–1551. [Google Scholar] [CrossRef]
- Chowdhury, T.; Mahmud, A.; Barua, A.; Khalil, M.D.I.; Chowdhury, R.; Ahamed, F.; Dhar, K. Bacterial contamination on hand touch surfaces of public buses in Chittagong city, Bangladesh. J. Environ. Sci. Toxicol. Food Technol. 2016, 10, 48–55. [Google Scholar]
- Hernández, A.M.; Vargas-Robles, D.; Alcaraz, L.D.; Peimbert, M. Station and train surface microbiomes of Mexico City’s metro (subway/underground). Sci. Rep. 2020, 10, 8798. [Google Scholar] [CrossRef]
- Triadó-Margarit, X.; Veillette, M.; Duchaine, C.; Talbot, M.; Amato, F.; Minguillón, M.C.; Martins, V.; de Miguel, E.; Casamayor, E.O.; Moreno, T. Bioaerosols in the Barcelona subway system. Indoor Air 2017, 27, 564–575. [Google Scholar] [CrossRef] [Green Version]
- Lutz, J.K. Methicillin-Resistant Staphylococcus aureus on Public Transportation Vehicles: Sampler Performance, Prevalence, and Epidemiology. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2011. [Google Scholar]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Kanamori, H.; Rutala, W.A.; Weber, D.J. The role of patient care items as a fomite in healthcare-associated outbreaks and infection prevention. Clin. Infect. Dis. 2017, 65, 1412–1419. [Google Scholar] [CrossRef]
- Correa-Martinez, C.L.; Tönnies, H.; Froböse, N.J.; Mellmann, A.; Kampmeier, S. Transmission of vancomycin-resistant enterococci in the hospital setting: Uncovering the patient-environment interplay. Microorganisms 2020, 8, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bäumler, W.; Eckl, D.; Holzmann, T.; Schneider-Brachert, W. Antimicrobial coatings for environmental surfaces in hospitals: A potential new pillar for prevention strategies in hygiene. Crit. Rev. Microbiol. 2021, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Mariello, M.; Guido, F.; Mastronardi, V.M.; Giannuzzi, R.; Algieri, L.; Qualteri, A.; Maffezzoli, A.; De Vittorio, M. Reliability of Protective Coatings for Flexible Piezoelectric Transducers in Aqueous Environments. Micromachines 2019, 10, 739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Pittol, M.; Tomacheski, D.; Simões, D.N.; Ribeiro, V.F.; Santana, R.M.C. Antimicrobial performance of thermoplastic elastomers containing zinc pyrithione and silver nanoparticles. Mater. Res. 2017, 20, 1266–1273. [Google Scholar] [CrossRef] [Green Version]
- Martinaga Pintarić, L.; Somogi Škoc, M.; Ljoljić Bilić, V.; Pokrovac, I.; Kosalec, I.; Rezić, I. Synthesis, modification and characterization of antimicrobial textile surface containing ZnO nanoparticles. Polymers 2020, 12, 1210. [Google Scholar] [CrossRef] [PubMed]
- Thukkaram, M.; Vaidulych, M.; Kylián, O.; Rigole, P.; Aliakbarshirazi, S.; Asadian, M.; Nikiforov, A.; Biederman, H.; Coenye, T.; Du Laing, G. Biological activity and antimicrobial property of Cu/aC: H nanocomposites and nanolayered coatings on titanium substrates. Mater. Sci. Eng. C 2021, 119, 111513. [Google Scholar] [CrossRef]
- Nakano, R.; Hara, M.; Ishiguro, H.; Yao, Y.; Ochiai, T.; Nakata, K.; Murakami, T.; Kajioka, J.; Sunada, K.; Hashimoto, K. Broad spectrum microbicidal activity of photocatalysis by TiO2. Catalysts 2013, 3, 310–323. [Google Scholar] [CrossRef] [Green Version]
- Fisher, L.; Ostovapour, S.; Kelly, P.; Whitehead, K.A.; Cooke, K.; Storgårds, E.; Verran, J. Molybdenum doped titanium dioxide photocatalytic coatings for use as hygienic surfaces: The effect of soiling on antimicrobial activity. Biofouling 2014, 30, 911–919. [Google Scholar] [CrossRef]
- Li, R.; Jin, T.Z.; Liu, Z.; Liu, L. Antimicrobial double-layer coating prepared from pure or doped-titanium dioxide and binders. Coatings 2018, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Varghese, S.; Elfakhri, S.; Sheel, D.W.; Sheel, P.; Bolton, F.J.; Foster, H.A. Novel antibacterial silver-silica surface coatings prepared by chemical vapour deposition for infection control. J. Appl. Microbiol. 2013, 115, 1107–1116. [Google Scholar] [CrossRef]
- Scuri, S.; Petrelli, F.; Grappasonni, I.; Idemudia, L.; Marchetti, F.; Di Nicola, C. Evaluation of the antimicrobial activity of novel composite plastics containing two silver (I) additives, acyl pyrazolonate and acyl pyrazolone. Acta Bio Med. Atenei Parm. 2019, 90, 370. [Google Scholar]
- Fay, F.; Linossier, I.; Langlois, V.; Vallee-Rehel, K.; Krasko, M.Y.; Domb, A.J. Protecting biodegradable coatings releasing antimicrobial agents. J. Appl. Polym. Sci. 2007, 106, 3768–3777. [Google Scholar] [CrossRef]
- Olsen, S.M.; Pedersen, L.T.; Laursen, M.H.; Kiil, S.; Dam-Johansen, K. Enzyme-based antifouling coatings: A review. Biofouling 2007, 23, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Druvari, D.; Koromilas, N.D.; Lainioti, G.C.; Bokias, G.; Vasilopoulos, G.; Vantarakis, A.; Baras, I.; Dourala, N.; Kallitsis, J.K. Polymeric quaternary ammonium-containing coatings with potential dual contact-based and release-based antimicrobial activity. ACS Appl. Mater. Interfaces 2016, 8, 35593–35605. [Google Scholar] [CrossRef]
- Bieser, A.M.; Tiller, J.C. Mechanistic considerations on contact-active antimicrobial surfaces with controlled functional group densities. Macromol. Biosci. 2011, 11, 526–534. [Google Scholar] [CrossRef]
- Felgenträger, A.; Maisch, T.; Späth, A.; Schröder, J.A.; Bäumler, W. Singlet oxygen generation in porphyrin-doped polymeric surface coating enables antimicrobial effects on Staphylococcus aureus. Phys. Chem. Chem. Phys. 2014, 16, 20598–20607. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.-K.; Song, S.; Jung, S.-J.; Hwang, J.-W.; Kim, M.-G.; Kim, J.-H.; Sung, J.; Lee, J.-K.; Kim, Y.-R. Lifetime and diffusion distance of singlet oxygen in air under everyday atmospheric conditions. Phys. Chem. Chem. Phys. 2020, 22, 21664–21671. [Google Scholar] [CrossRef]
- Eichner, A.; Holzmann, T.; Eckl, D.B.; Zeman, F.; Koller, M.; Huber, M.; Pemmerl, S.; Schneider-Brachert, W.; Bäumler, W. Novel photodynamic coating reduces the bioburden on near-patient surfaces thereby reducing the risk for onward pathogen transmission: A field study in two hospitals. J. Hosp. Infect. 2020, 104, 85–91. [Google Scholar] [CrossRef]
- DIN EN 13697:2019-10; Chemische Desinfektionsmittel und Antiseptika—Quantitativer Oberflächen-Versuch zur Bestimmung der bakteriziden und/oder fungiziden Wirkung chemischer Desinfektionsmittel auf nicht porösen Oberflächen in den Bereichen Lebensmittel, Industrie, Haushalt u. Deutsches Institut für Normung: Muggensturm, Germany, 2019. [CrossRef]
- Dancer, S.J. Controlling hospital-acquired infection: Focus on the role of the environment and new technologies for decontamination. Clin. Microbiol. Rev. 2014, 27, 665–690. [Google Scholar] [CrossRef] [Green Version]
- White, L.F.; Dancer, S.J.; Robertson, C.; McDonald, J. Are hygiene standards useful in assessing infection risk? Am. J. Infect. Control 2008, 36, 381–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dancer, S.J. How do we assess hospital cleaning? A proposal for microbiological standards for surface hygiene in hospitals. J. Hosp. Infect. 2004, 56, 10–15. [Google Scholar] [CrossRef]
- Kramer, A.; Assadian, O. Survival of microorganisms on inanimate surfaces. In Use of Biocidal Surfaces for Reduction of Healthcare Acquired Infections; Springer: Berlin/Heidelberg, Germany, 2014; pp. 7–26. [Google Scholar]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Dhand, R.; Li, J. Coughs and sneezes: Their role in transmission of respiratory viral infections, including SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020, 202, 651–659. [Google Scholar] [CrossRef]
- Chowdhury, D.; Tahir, S.; Legge, M.; Hu, H.; Prvan, T.; Johani, K.; Whiteley, G.S.; Glasbey, T.O.; Deva, A.K.; Vickery, K. Transfer of dry surface biofilm in the healthcare environment: The role of healthcare workers’ hands as vehicles. J. Hosp. Infect. 2018, 100, e85–e90. [Google Scholar] [CrossRef] [PubMed]
- Suleyman, G.; Alangaden, G.; Bardossy, A.C. The role of environmental contamination in the transmission of nosocomial pathogens and healthcare-associated infections. Curr. Infect. Dis. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Carling, P.C.; Parry, M.M.; Rupp, M.E.; Po, J.L.; Dick, B.; Von Beheren, S.; Group, H.E.H.S. Improving cleaning of the environment surrounding patients in 36 acute care hospitals. Infect. Control Hosp. Epidemiol. 2008, 29, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.; Delungahawatta, T.; Dunne, C.P. Hand hygiene-related clinical trials reported between 2014 and 2020: A comprehensive systematic review. J. Hosp. Infect. 2021, 111, 6–26. [Google Scholar] [CrossRef]
- Shaban, R.Z.; Sotomayor-Castillo, C.F.; Malik, J.; Li, C. Global commercial passenger airlines and travel health information regarding infection control and the prevention of infectious disease: What’s in a website? Travel Med. Infect. Dis. 2020, 33, 101528. [Google Scholar] [CrossRef]
- Lawson, A.; Vaganay-Miller, M.; Cameron, R. An Investigation of the General Population’s Self-Reported Hand Hygiene Behaviour and Compliance in a Cross-European Setting. Int. J. Environ. Res. Public Health 2021, 18, 2402. [Google Scholar] [CrossRef] [PubMed]
- Green, L.R.; Selman, C.A.; Radke, V.; Ripley, D.; Mack, J.C.; Reimann, D.W.; Stigger, T.; Motsinger, M.; Bushnell, L. Food worker hand washing practices: An observation study. J. Food Prot. 2006, 69, 2417–2423. [Google Scholar] [CrossRef]
- Cairncross, S.; Hunt, C.; Boisson, S.; Bostoen, K.; Curtis, V.; Fung, I.C.H.; Schmidt, W.-P. Water, sanitation and hygiene for the prevention of diarrhoea. Int. J. Epidemiol. 2010, 39, i193–i205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, M.C.; Stocks, M.E.; Cumming, O.; Jeandron, A.; Higgins, J.P.T.; Wolf, J.; Prüss-Ustün, A.; Bonjour, S.; Hunter, P.R.; Fewtrell, L. Systematic review: Hygiene and health: Systematic review of handwashing practices worldwide and update of health effects. Trop. Med. Int. Health 2014, 19, 906–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mardiko, A.A.; von Lengerke, T. When, how, and how long do adults in Germany self-reportedly wash their hands? Compliance indices based on handwashing frequency, technique, and duration from a cross-sectional representative survey. Int. J. Hyg. Environ. Health 2020, 230, 113590. [Google Scholar] [CrossRef] [PubMed]
- Czeisler, M.É.; Garcia-Williams, A.G.; Molinari, N.-A.; Gharpure, R.; Li, Y.; Barrett, C.E.; Robbins, R.; Facer-Childs, E.R.; Barger, L.K.; Czeisler, C.A. Demographic Characteristics, Experiences, and Beliefs Associated with Hand Hygiene Among Adults During the COVID-19 Pandemic—United States, June 24–30, 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1485. [Google Scholar] [CrossRef]
- Makhni, S.; Umscheid, C.A.; Soo, J.; Chu, V.; Bartlett, A.; Landon, E.; Marrs, R. Hand Hygiene Compliance Rate During the COVID-19 Pandemic. JAMA Intern. Med. 2021, 181, 1006–1008. [Google Scholar] [CrossRef]
- ISO 22196:2011-08; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. International Organization for Standardization: Geneva, Switzerland, 2011.
- ISO 21702:2019-05; Measurement of Antiviral Activity on Plastics and Other Non-Porous Surfaces. International Organization for Standardization: Geneva, Switzerland, 2019.
- Michels, H.T.; Noyce, J.O.; Keevil, C.W. Effects of temperature and humidity on the efficacy of methicillin-resistant Staphylococcus aureus challenged antimicrobial materials containing silver and copper. Lett. Appl. Microbiol. 2009, 49, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Alquethamy, S.F.; Adams, F.G.; Naidu, V.; Khorvash, M.; Pederick, V.G.; Zang, M.; Paton, J.C.; Paulsen, I.T.; Hassan, K.A.; Cain, A.K. The role of zinc efflux during Acinetobacter baumannii infection. ACS Infect. Dis. 2019, 6, 150–158. [Google Scholar] [CrossRef]
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and emerging topical antibacterials and antiseptics: Agents, action, and resistance patterns. Clin. Microbiol. Rev. 2017, 30, 827–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EMA. Reflection Paper on Antimicrobial Resistance in the Environment. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-antimicrobial-resistance-environment-considerations-current-future-risk-assessment_en.pdf (accessed on 18 January 2022).
- Pal, C.; Asiani, K.; Arya, S.; Rensing, C.; Stekel, D.J.; Larsson, D.G.J.; Hobman, J.L. Metal resistance and its association with antibiotic resistance. Adv. Microb. Physiol. 2017, 70, 261–313. [Google Scholar] [PubMed]
- Zhang, X.; Ma, J.; Chen, M.; Wu, Z.; Wang, Z. Microbial responses to transient shock loads of quaternary ammonium compounds with different length of alkyl chain in a membrane bioreactor. AMB Express 2018, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Chemicals Strategy for Sustainability towards a Toxic-Free Environment. Available online: https://ec.europa.eu/environment/pdf/chemicals/2020/10/Strategy.pdf (accessed on 18 January 2022).
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer—A Review of the Current Clinical Status. Front. Chem. 2021, 9, 608. [Google Scholar] [CrossRef]
- Nicolò, M.; Ferro Desideri, L.; Vagge, A.; Traverso, C.E. Current pharmacological treatment options for central serous chorioretinopathy: A review. Pharmaceuticals 2020, 13, 264. [Google Scholar] [CrossRef]

| Benchmarks | Uncoated (n = 168) | Antimicrobial Coating (n = 168) | ||
|---|---|---|---|---|
| Number | Percent | Number | Percent | |
| cfu/cm2 ≤ 2.5 | 68 | 40.5% | 107 | 63.7% |
| cfu/cm2 > 2.5 | 100 | 59.5% | 61 | 36.3% |
| cfu/cm2 ≤ 5 | 93 | 55.4% | 131 | 78.0% |
| cfu/cm2 > 5 | 75 | 44.6% | 37 | 22.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalb, L.; Bäßler, P.; Schneider-Brachert, W.; Eckl, D.B. Antimicrobial Photodynamic Coatings Reduce the Microbial Burden on Environmental Surfaces in Public Transportation—A Field Study in Buses. Int. J. Environ. Res. Public Health 2022, 19, 2325. https://doi.org/10.3390/ijerph19042325
Kalb L, Bäßler P, Schneider-Brachert W, Eckl DB. Antimicrobial Photodynamic Coatings Reduce the Microbial Burden on Environmental Surfaces in Public Transportation—A Field Study in Buses. International Journal of Environmental Research and Public Health. 2022; 19(4):2325. https://doi.org/10.3390/ijerph19042325
Chicago/Turabian StyleKalb, Larissa, Pauline Bäßler, Wulf Schneider-Brachert, and Daniel Bernhard Eckl. 2022. "Antimicrobial Photodynamic Coatings Reduce the Microbial Burden on Environmental Surfaces in Public Transportation—A Field Study in Buses" International Journal of Environmental Research and Public Health 19, no. 4: 2325. https://doi.org/10.3390/ijerph19042325
APA StyleKalb, L., Bäßler, P., Schneider-Brachert, W., & Eckl, D. B. (2022). Antimicrobial Photodynamic Coatings Reduce the Microbial Burden on Environmental Surfaces in Public Transportation—A Field Study in Buses. International Journal of Environmental Research and Public Health, 19(4), 2325. https://doi.org/10.3390/ijerph19042325





