The Impact of Treated Wastewater Irrigation on the Metabolism of Barley Grown in Arid and Semi-Arid Regions
Abstract
1. Introduction
2. Materials and Methods
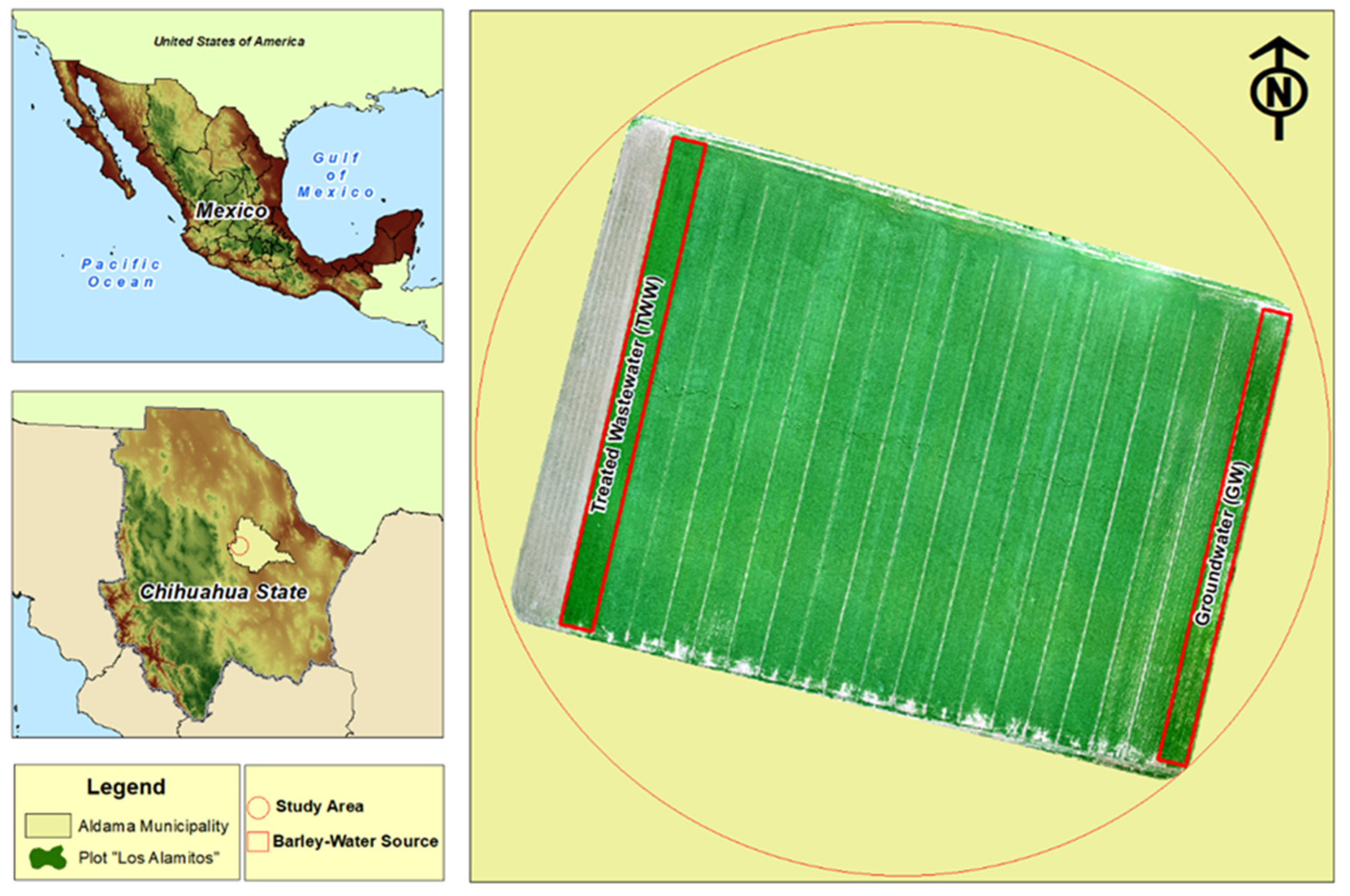
2.1. Study Area and Experimental Setup
2.2. Agronomic Attributes
2.3. Photosynthetic Efficiency
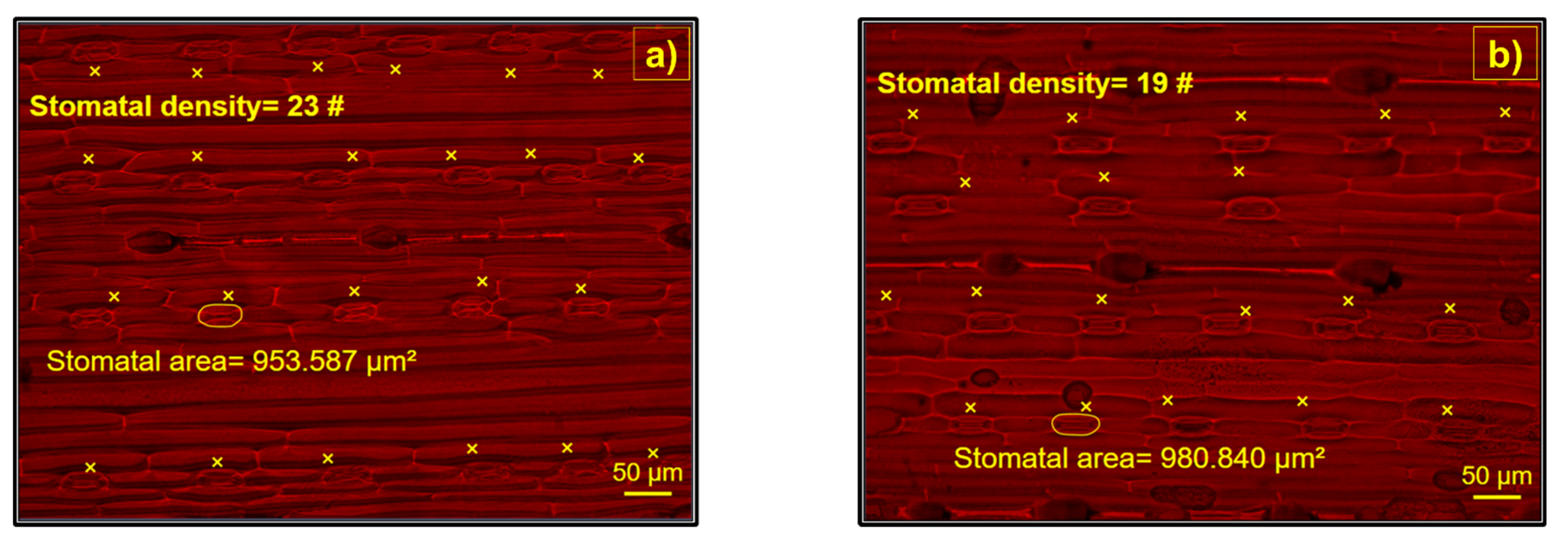
2.4. Stomatal Characteristics
2.5. Analysis of Nutritional Components
2.6. Statistical Analysis
3. Results and Discussion
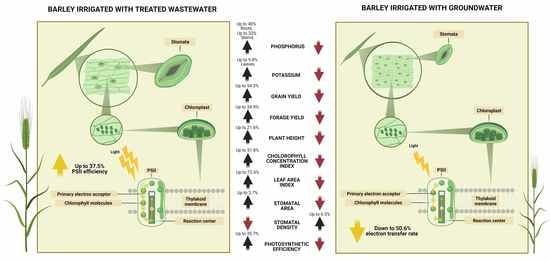
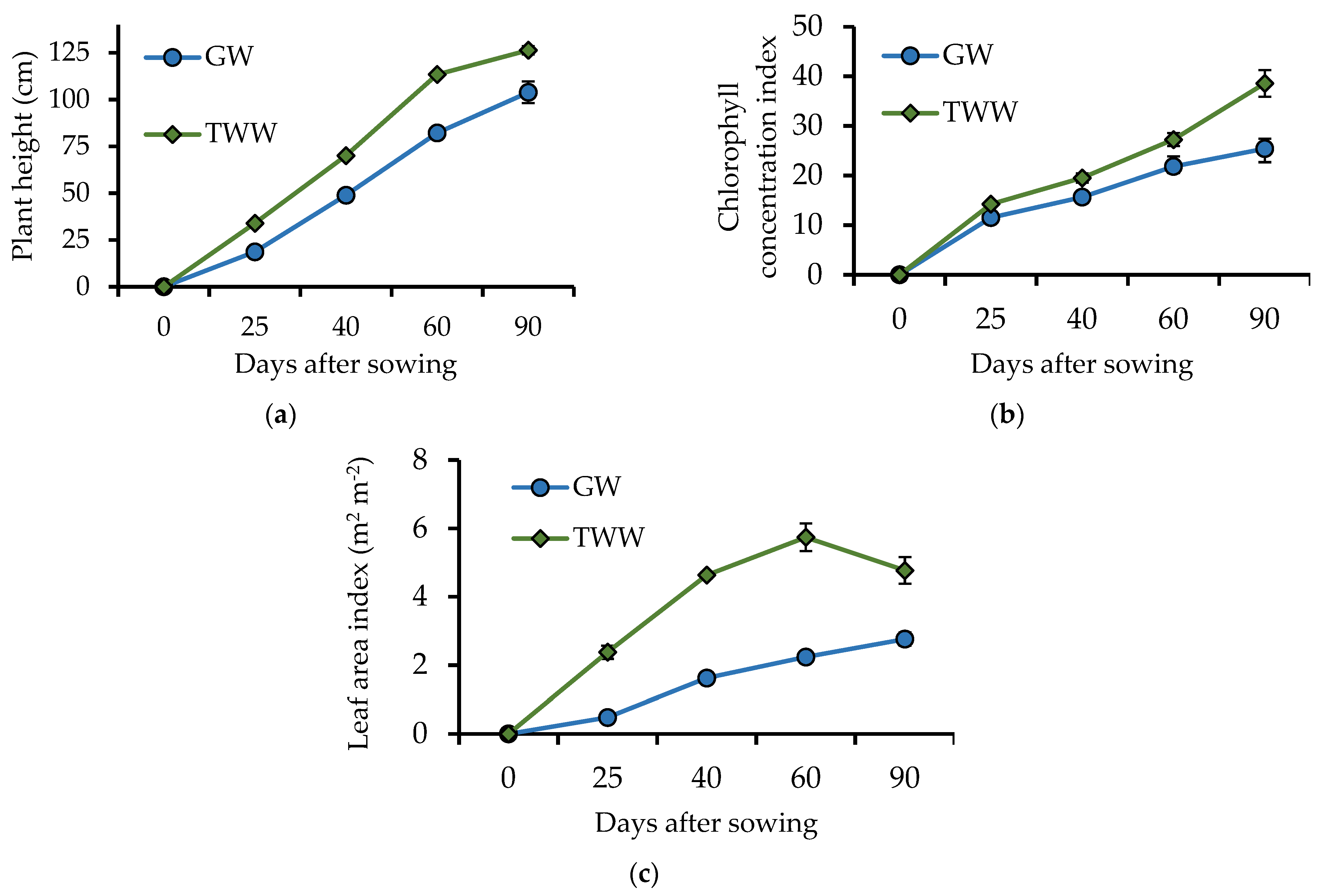
3.1. Treated Wastewater Improve Agronomic Traits on Barley
3.2. Treated Wastewater Enhanced the Photosynthetic Efficiency of Barley Plants
3.3. Treated Wastewater Produced Changes in Stomatal Density and Area of Barley Plants
3.4. Treated Wastewater Changed the Nutritional Composition of Barley Plants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qadir, M.; Drechsel, P.; Jiménez Cisneros, B.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Global and regional potential of wastewater as a water, nutrient and energy source. Nat. Resour. Forum. 2020, 44, 40–51. [Google Scholar] [CrossRef]
- Lado, M.; Ben-Hur, M. Treated domestic sewage irrigation effects on soil hydraulic properties in arid and semiarid zones: A review. J. Soil. Tillage Research 2009, 106, 152–163. [Google Scholar] [CrossRef]
- AQUASTAT. FAO Global Information System on Water and Agriculture. Wastewater Section. Available online: http://www.fao.org/nr/water/aquastat/wastewater/index.stm (accessed on 14 May 2021).
- Zang, J.; Kumar, M.; Werner, D. Real-world sustainability analysis of an innovative decentralized water system with rainwater harvesting and wastewater reclamation. J. Environ. Manag. 2021, 280, 111639. [Google Scholar] [CrossRef] [PubMed]
- Mahfooz, Y.; Yasar, A.; Guijian, L.; Islam, Q.U.; Akhtar, A.B.T.; Rasheed, R.; Irshad, S.; Naeem, U. Critical risk analysis of metals toxicity in wastewater irrigated soil and crops: A study of a semi-arid developing region. Sci. Rep. 2020, 10, 12845. [Google Scholar] [CrossRef] [PubMed]
- Connor, R.; Renata, A.; Ortigara, C.; Koncagül, E.; Uhlenbrook, S.; Lamizana-Diallo, B.M.; Zadeh, S.M.; Qadir, M.; Kjellén, M.; Sjödin, J. The United Nations World Water Development Report 2017. Wastewater: The Untapped Resource; The United Nations Educational, Scientific and Cultural Organization: Paris, France, 2017. [Google Scholar]
- Jaramillo, M.F.; Restrepo, I. Wastewater reuse in agriculture: A review about its limitations and benefits. Sustainability 2017, 9, 1734. [Google Scholar] [CrossRef]
- Ofori, S.; Puskacova, A.; Ruzickova, I.; Wanner, J. Treated wastewater reuse for irrigation: Pros and cons. Sci. Total Environ. 2021, 760, 144026. [Google Scholar] [CrossRef]
- Al-Rashed, M.F.; Sherif, M.M. Water resources in the GCC countries: An overview. Water Resour. Manag. 2000, 14, 59–75. [Google Scholar] [CrossRef]
- Mohammad, M.J.; Mazahreh, N. Changes in soil fertility parameters in response to irrigation of forage crops with secondary treated wastewater. Commun. Soil Sci. Plant Anal. 2003, 34, 1281–1294. [Google Scholar] [CrossRef]
- Mendoza-Espinosa, L.G.; Cabello-Pasini, A.; Macias-Carranza, V.; Daessle-Heuser, W.; Orozco-Borbon, M.V.; Quintanilla-Montoya, A.L. The effect of reclaimed wastewater on the quality and growth of grapevines. Water Sci. Technol. 2008, 57, 1445–1450. [Google Scholar] [CrossRef]
- Tahtouh, J.; Mohtar, R.; Assi, A.; Schwab, P.; Jantrania, A.; Deng, Y.; Munster, C. Impact of brackish groundwater and treated wastewater on soil chemical and mineralogical properties. Sci. Total Environ. 2019, 647, 99–109. [Google Scholar] [CrossRef]
- Lahlou, F.-Z.; Mackey, H.R.; Al-Ansari, T. Wastewater reuse for livestock feed irrigation as a sustainable practice: A socio-environmental-economic review. J. Clean. Prod. 2021, 294, 126331. [Google Scholar] [CrossRef]
- Rusan, M.J.M.; Hinnawi, S.; Rousan, L. Long term effect of wastewater irrigation of forage crops on soil and plant quality parameters. Desalination 2007, 215, 143–152. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Bibi, I.; Sarwar, T.; Shah, A.H.; Niazi, N.K. A review of environmental contamination and health risk assessment of wastewater use for crop irrigation with a focus on low and high-income countries. Int. J. Environ. Res. Public Health 2018, 15, 895. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, H.; Caucci, S.; Ardakanian, R. The golden example of nexus approach. In Safe Use of Wastewater in Agriculture; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–11. [Google Scholar]
- Inyinbor, A.A.; Bello, O.S.; Oluyori, A.P.; Inyinbor, H.E.; Fadiji, A.E. Wastewater conservation and reuse in quality vegetable cultivation: Overview, challenges and future prospects. Food Control 2019, 98, 489–500. [Google Scholar] [CrossRef]
- Elfanssi, S.; Ouazzani, N.; Mandi, L. Soil properties and agro-physiological responses of alfalfa (Medicago sativa L.) irrigated by treated domestic wastewater. Agric. Water Manag. 2018, 202, 231–240. [Google Scholar] [CrossRef]
- Shahrivar, A.A.; Rahman, M.M.; Hagare, D.; Maheshwari, B. Variation in kikuyu grass yield in response to irrigation with secondary and advanced treated wastewaters. Agric. Water Manag. 2019, 222, 375–385. [Google Scholar] [CrossRef]
- Tekaya, M.; Mechri, B.; Dabbaghi, O.; Mahjoub, Z.; Laamari, S.; Chihaoui, B.; Boujnah, D.; Hammami, M.; Chehab, H. Changes in key photosynthetic parameters of olive trees following soil tillage and wastewater irrigation, modified olive oil quality. Agric. Water Manag. 2016, 178, 180–188. [Google Scholar] [CrossRef]
- Samarah, N.H.; Bashabsheh, K.Y.; Mazahrih, N.T. Treated wastewater outperformed freshwater for barley irrigation in arid lands. Ital. J. Agron. 2020, 15, 183–193. [Google Scholar] [CrossRef]
- Langridge, P. Economic and Academic Importance of Barley. In The Barley Genome; Springer: Cham, Switzerland; New York, NY, USA, 2018; pp. 1–10. [Google Scholar]
- INEGI. Instituto Nacional de Estadística, Geografía e Historia. Pronatuario de información Geográfica Municipal de los Estados Unidos Mexicanos. Aldama, Chihuahua. Available online: https://www.inegi.org.mx/contenidos/app/mexicocifras/datos_geograficos/08/08002.pdf (accessed on 13 May 2021).
- CONAGUA-SMN. Comisión Nacional del Agua-Servicio Meteorológico Nacional. Información Climatológica por Estado. Available online: https://smn.conagua.gob.mx/es/informacion-climatologica-por-estado?estado=chih (accessed on 13 May 2021).
- Baird, R.B. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Water Environment Federation, American Public Health Association, American Water Works Association, 2017; Available online: https://connect.wef.org/s/store#/store/browse/detail/a2n1S000000KVk1 (accessed on 6 February 2022).
- CONAGUA. Ley Federal de Derechos. Disposiciones Aplicables en Materia de Aguas Nacionales 2020. Available online: https://www.gob.mx/cms/uploads/attachment/file/555838/CGRF-1-20_LFD.pdf (accessed on 11 May 2021).
- Ayers, R.; Westcott, D. Water Quality for Agriculture; No. 29; Food and Agriculture Organization of the United Nations, 1985; Available online: https://www.fao.org/3/t0234e/t0234e00.htm (accessed on 6 February 2022).
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta -Biomembr. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Miller, J.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry; Pearson Education: Harlow, UK, 2018. [Google Scholar]
- Bru, I.P.; Lozano, L.L.; Bru, A.P.; Fernández, M.A.; González-Estecha, M. Procedimiento de validación de un método para cuantificar cromo en suero por espectroscopía de absorción atómica con atomización electrotérmica. Rev. Lab. Clin. 2015, 8, 52–57. [Google Scholar] [CrossRef]
- EPA. Methods for Chemical Analysis of Water and Wastes. Available online: https://www.wbdg.org/FFC/EPA/EPACRIT/epa600_4_79_020.pdf (accessed on 10 May 2021).
- Alkhamisi, S.A.; Abdelrahman, H.; Ahmed, M.; Goosen, M. Assessment of reclaimed water irrigation on growth, yield, and water-use efficiency of forage crops. Appl. Water Sci. 2011, 1, 57–65. [Google Scholar] [CrossRef]
- Aghtape, A.A.; Ghanbari, A.; Sirousmehr, A.; Siahsar, B.; Asgharipour, M.; Tavssoli, A. Effect of irrigation with wastewater and foliar fertilizer application on some forage characteristics of foxtail millet (Setaria italica). Int. J. Plant Physiol. Biochem. 2011, 3, 34–42. [Google Scholar] [CrossRef]
- Ramírez-Novoa, U.; Rodríguez-Guillén, A.; Morán-Vázquez, N.; Cervantes-Ortíz, F.; Mendoza-Elos, M.; Rangel-Lucio, J. Manejo agronómico de cebada maltera. Rendimiento de semilla y componentes. Cienc y Tecnol. Agrop. México 2014, 2, 24–29. [Google Scholar]
- Tambussi, E.; Nogues, S.; Ferrio, P.; Voltas, J.; Araus, J. Does higher yield potential improve barley performance in Mediterranean conditions?: A case study. Field Crops Res. 2005, 91, 149–160. [Google Scholar] [CrossRef]
- Palliotti, A.; Silvestroni, O.; Petoumenou, D.; Viticulture. Photosynthetic and photoinhibition behavior of two field-grown grapevine cultivars under multiple summer stresses. AJEV 2009, 60, 189–198. [Google Scholar]
- Hailemichael, G.; Catalina, A.; González, M.; Martin, P. Relationships between water status, leaf chlorophyll content and photosynthetic performance in Tempranillo vineyards. SAJEV 2016, 37, 149–156. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, M.; Cheng, F.; Liu, S.; Liang, Y. Effects of LED photoperiods and light qualities on in vitro growth and chlorophyll fluorescence of Cunninghamia lanceolata. BMC Plant Biol. 2020, 20, 269. [Google Scholar] [CrossRef]
- Osmond, C.B.; Chow, W.S.; Robinson, S.A. Inhibition of non-photochemical quenching increases functional absorption cross-section of photosystem II as excitation from closed reaction centres is transferred to open centres, facilitating earlier light saturation of photosynthetic electron transport. Funct. Plant Biol. 2021. [Google Scholar] [CrossRef]
- Lu, T.; Meng, Z.; Zhang, G.; Qi, M.; Sun, Z.; Liu, Y.; Li, T. Sub-high Temperature and High Light Intensity Induced Irreversible Inhibition on Photosynthesis System of Tomato Plant (Solanum lycopersicum L.). Front. Plant Sci. 2017, 8, 365. [Google Scholar] [CrossRef]
- Webb, J.A.; Fletcher, R. Paclobutrazol protects wheat seedlings from injury due to waterlogging. Plant Growth Regul. 1996, 18, 201–206. [Google Scholar] [CrossRef]
- Odasz-Albrigtsen, A.M.; Tømmervik, H.; Murphy, P. Decreased photosynthetic efficiency in plant species exposed to multiple airborne pollutants along the Russian-Norwegian border. Can. J. Bot. 2000, 78, 1021–1033. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M. PSII Fluorescence Techniques for Measurement of Drought and High Temperature Stress Signal in Crop Plants: Protocols and Applications. In Molecular Stress Physiology of Plants; Springer: Berlin/Heidelberg, Germany, 2013; pp. 87–131. [Google Scholar]
- Shu, S.; Yuan, L.-Y.; Guo, S.-R.; Sun, J.; Liu, C.-J. Effects of exogenous spermidine on photosynthesis, xanthophyll cycle and endogenous polyamines in cucumber seedlings exposed to salinity. Afr. J. Biotechnol. 2012, 11, 6064–6074. [Google Scholar] [CrossRef]
- Marriboina, S.; Attipalli, R.R. Hydrophobic cell-wall barriers and vacuolar sequestration of Na+ ions are among the key mechanisms conferring high salinity tolerance in a biofuel tree species, Pongamia pinnata L. pierre. EEB 2020, 171, 103949. [Google Scholar] [CrossRef]
- Song, L.; Yue, L.; Zhao, H.; Hou, M. Protection effect of nitric oxide on photosynthesis in rice under heat stress. Acta Physiol. Plant 2013, 35, 3323–3333. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Xu, P.; Tian, L.; Kloz, M.; Croce, R. Molecular insights into Zeaxanthin-dependent quenching in higher plants. Sci. Rep. 2015, 5, 13679. [Google Scholar] [CrossRef]
- Dlouhý, O.; Kurasová, I.; Karlický, V.; Javornik, U.; Šket, P.; Petrova, N.Z.; Krumova, S.B.; Plavec, J.; Ughy, B.; Špunda, V. Modulation of non-bilayer lipid phases and the structure and functions of thylakoid membranes: Effects on the water-soluble enzyme violaxanthin de-epoxidase. Sci. Rep. 2020, 10, 11959. [Google Scholar] [CrossRef]
- Samson, G.; Bonin, L.; Maire, V. Dynamics of regulated YNPQ and non-regulated YNO energy dissipation in sunflower leaves exposed to sinusoidal lights. Photosynth. Res. 2019, 141, 315–330. [Google Scholar] [CrossRef]
- Xue, D.W.; Jiang, H.; Hu, J.; Zhang, X.Q.; Guo, L.B.; Zeng, D.L.; Dong, G.J.; Sun, G.C.; Qian, Q. Characterization of physiological response and identification of associated genes under heat stress in rice seedlings. Plant Physiol. Biochem. 2012, 61, 46–53. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Boyle, D.; Welti, R.; Jagadish, S.; Prasad, P. Decreased photosynthetic rate under high temperature in wheat is due to lipid desaturation, oxidation, acylation, and damage of organelles. BMC Plant Biol. 2018, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H. Heat stress in wheat during reproductive and grain-filling phases. Crit. Rev. Plant Sci. 2011, 30, 491–507. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Simkin, A.J.; Kelly, G.; Granot, D. Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol. 2014, 203, 1064–1081. [Google Scholar] [CrossRef]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of Stomatal Density and Morphology on Water-Use Efficiency in a Changing World. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef]
- Franks, P.J.W.; Doheny-Adams, T.; Britton-Harper, Z.J.; Gray, J.E. Increasing water-use efficiency directly through genetic manipulation of stomatal density. New Phytol. 2015, 207, 188–195. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Wei, X.; Zhang, X.; Xue, R.; Zhao, Y.; Zhao, H. Transcriptome analysis of drought-responsive genes regulated by hydrogen sulfide in wheat (Triticum aestivum L.) leaves. Mol. Genet. Genomics 2017, 292, 1091–1110. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef]
- Raven, J.A. Speedy small stomata? J. Exp. Bot. 2014, 65, 1415–1424. [Google Scholar] [CrossRef]
- Fanourakis, D.; Giday, H.; Milla, R.; Pieruschka, R.; Kjaer, K.H.; Bolger, M.; Vasilevski, A.; Nunes-Nesi, A.; Fiorani, F.; Ottosen, C.-O. Pore size regulates operating stomatal conductance, while stomatal densities drive the partitioning of conductance between leaf sides. Ann. Bot. 2015, 115, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Hepworth, C.; Dutton, C.; Dunn, J.A.; Hunt, L.; Stephens, J.; Waugh, R.; Cameron, D.D.; Gray, J.E. Reducing stomatal density in barley improves drought tolerance without impacting on yield. Plant Physiol. 2017, 174, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.P.; Bonmati, A.; Esteller, L.J.; Brunn, S.; Jensen, L.S.; Meers, E.; Anton, A. Selection and application of agri-environmental indicators to assess potential technologies for nutrient recovery in agriculture. Ecol. Indic. 2022, 134, 108471. [Google Scholar] [CrossRef]
- Reyes, V.M.G.; Gutiérrez, M.; Haro, B.N.; López, D.N.; Herrera, M.T.A. Groundwater quality impacted by land use/land cover change in a semiarid region of Mexico. Groundw. Sustain. Dev. 2017, 5, 160–167. [Google Scholar] [CrossRef]
- Pilbeam, D.J. Handbook of Plant Nutrition; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of Nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Stuttgart, Germany, 2012; pp. 191–248. [Google Scholar]
- Bettenhausen, H.M.; Barr, L.; Broeckling, C.D.; Chaparro, J.M.; Holbrook, C.; Sedin, D.; Heuberger, A.L. Influence of malt source on beer chemistry, flavor, and flavor stability. Food Res. Int. 2018, 113, 487–504. [Google Scholar] [CrossRef]
- Dos Santos, M.T.R.; de Mello, P.P.M.; Sérvulo, E.F.C. Nitrogen compounds in brewing wort and beer: A review. J. Brew. Distilling 2014, 5, 10–17. [Google Scholar] [CrossRef]
- Chan, S.S.; Khoo, K.S.; Chew, K.W.; Ling, T.C.; Show, P.L. Recent advances biodegradation and biosorption of organic compounds from wastewater: Microalgae-bacteria consortium-A review. Bioresour. Technol. 2022, 344, 126159. [Google Scholar] [CrossRef]
- Ravelo, S.G.M.; Reyes, F.G.; Cabriales, J.J.P.; Moreno, J.P.; Chávez, L.T. Evaluación de la recuperación del nitrógeno y fósforo de diferentes fuentes de fertilizantes por el cultivo de trigo irrigado con aguas residuales y de pozo. Acta Agron. 2014, 63, 25–30. [Google Scholar] [CrossRef]
- Márquez, L.V.; González, M.R.; Bayter, Y.O.; Sarabia, A.B.C. Caracterización microbiológica y fisicoquímica de aguas subterráneas de los municipios de La Paz y San Diego, Cesar, Colombia. RIAA 2012, 3, 27–35. [Google Scholar] [CrossRef][Green Version]
- Egle, L.; Rechberger, H.; Zessner, M. Overview and description of technologies for recovering phosphorus from municipal wastewater. Resour. Conserv. Recy. 2015, 105, 325–346. [Google Scholar] [CrossRef]
- Wilfert, P.; Kumar, P.S.; Korving, L.; Witkamp, G.J.; van Loosdrecht, M.C. The Relevance of Phosphorus and Iron Chemistry to the Recovery of Phosphorus from Wastewater: A Review. Environ. Sci. Technol. 2015, 49, 9400–9414. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Soranno, P.A.; Wagner, T. The role of phosphorus and nitrogen on chlorophyll a: Evidence from hundreds of lakes. Water Res. 2020, 185, 116236. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Reddy, V.; Fleisher, D.; Timlin, D. Relationship between photosynthetic pigments and chlorophyll fluorescence in soybean under varying phosphorus nutrition at ambient and elevated CO2. Photosynthetica 2017, 55, 421–433. [Google Scholar] [CrossRef]
- Dayal, J.; Goswami, C.; Munjal, R. Influence of phosphorus application on water relations, biochemical parameters and gum content in cluster bean under water deficit. Biol. Plant. 2004, 48, 445–448. [Google Scholar] [CrossRef]
- Jiang, H.M.; Yang, J.C.; Zhang, J.F. Effects of external phosphorus on the cell ultrastructure and the chlorophyll content of maize under cadmium and zinc stress. Environ. Pollut. 2007, 147, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Waraich, E.A.; Ahmad, R.; Shahbaz, M. Modulation in water relations, chlorophyll contents and antioxidants activity of maize by foliar phosphorus application under drought stress. Pak. J. Bot. 2017, 49, 11–19. [Google Scholar]
- Singh, S.K.; Reddy, V.R. Response of carbon assimilation and chlorophyll fluorescence to soybean leaf phosphorus across CO2: Alternative electron sink, nutrient efficiency and critical concentration. J. Photochem. Photobiol. B Biol. 2015, 151, 276–284. [Google Scholar] [CrossRef]
- Jones, J.J.B. Plant Nutrition and Soil Fertility manual; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Humble, G.D.; Raschke, K. Stomatal opening quantitatively related to potassium transport: Evidence from electron probe analysis. Plant Physiol. 1971, 48, 447–453. [Google Scholar] [CrossRef]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef]
- Shabala, S.; Hariadi, Y.; Jacobsen, S.-E. Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. Plant Physiol. 2013, 170, 906–914. [Google Scholar] [CrossRef]
- Benlloch-González, M.; Arquero, O.; Fournier, J.M.; Barranco, D.; Benlloch, M. K+ starvation inhibits water-stress-induced stomatal closure. Plant Physiol. 2008, 165, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fan, J.; Zhang, F.; Wang, H.; Yang, L.; Sun, X.; Cheng, M.; Cheng, H.; Li, Z. Optimizing irrigation amount and potassium rate to simultaneously improve tuber yield, water productivity and plant potassium accumulation of drip-fertigated potato in northwest China. Agric. Water Manag. 2022, 264, 107493. [Google Scholar] [CrossRef]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers; Prentice Hall: Newark, NJ, USA, 2016. [Google Scholar]
- Dung, D.D.; Godwin, I.; Nolan, J.V. Nutrient content and in sacco digestibility of barley grain and sprouted barley. J. Anim. Vet. Adv. 2010, 9, 2485–2492. [Google Scholar] [CrossRef]
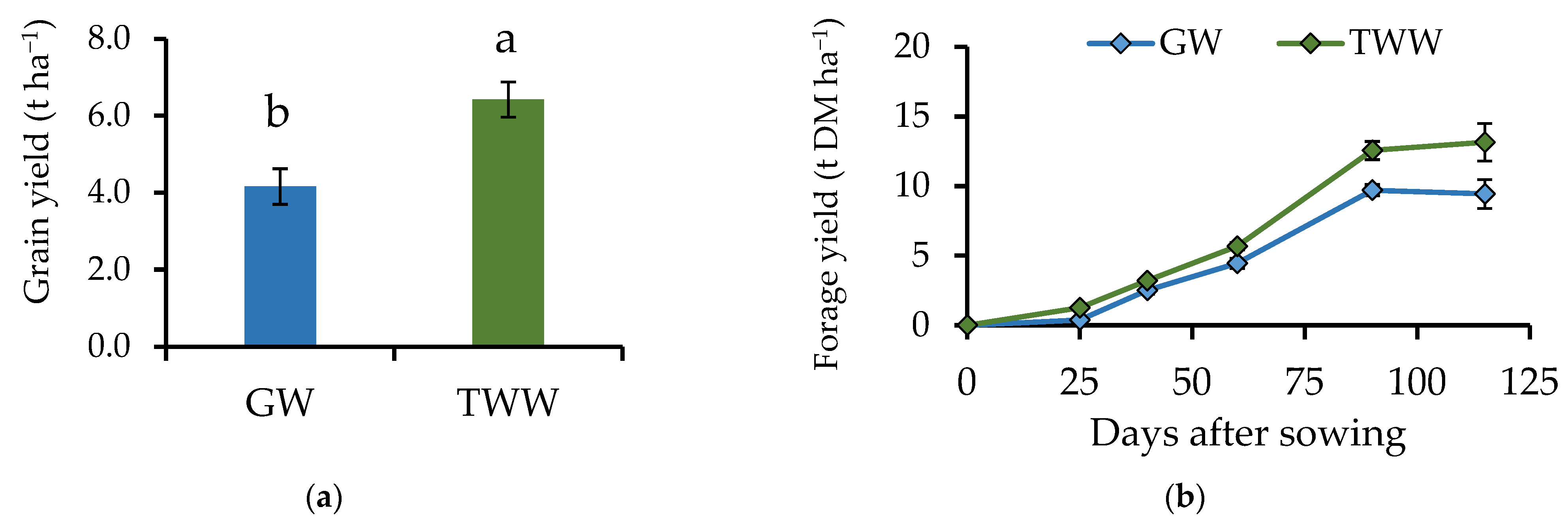


| Variable | Units | GW | TWW |
|---|---|---|---|
| pH | 8.51 | 8.44 | |
| Electrical Conductivity (EC) | dS m−1 | 1.17 | 1.01 |
| Texture | Clay loam | Clay loam | |
| Cation Exchange Capacity (CEC) | meq 100 g−1 | 36.01 | 32.71 |
| * Organic matter (OM) | % | 1.3 | 1.5 |
| Nitrogen (N-NO3−) | mg kg−1 | 29.11 | 9.49 |
| 1 Phosphosrus available (P) | 8.24 | 12.76 | |
| 2 Removable potassium (K) | 693.98 | 895.39 | |
| 3 Removable calcium (Ca) | 4102.53 | 4702.02 | |
| 4 Removable magnesium (Mg) | 449.26 | 388.48 | |
| * Copper (Cu) | 1.59 | 1.20 | |
| * Iron (Fe) | 0.6 | 1.76 | |
| * Manganese (Mn) | 2.66 | 2.30 | |
| * Zinc (Zn) | 2.01 | 3.31 |
| GW | TWW | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Units | Mean | Min | Max | Mean | Min | Max | Quality Standards |
| pH | 7.67 | 7.18 | 8.89 | 7.78 | 7.25 | 8.75 | 6.0–9.0 * | |
| Electrical Conductivity (EC) | mS cm−1 | 0.94 | 0.25 | 1.12 | 0.94 | 0.76 | 1.18 | NA |
| Temperature (°C) | °C | 22.34 | 17.10 | 31.10 | 21.46 | 14.30 | 30.40 | NA |
| Nitrate–nitrogen (NO3 as N) | mg L−1 | 33.63 | 0.00 | 42.49 | 31.85 | 0.00 | 60.76 | NA |
| Phosphate (PO₄3−) | mg L−1 | 0.00 | 0.00 | 0.00 | 6.80 | 0.00 | 8.75 | NA |
| Sulfate (SO₄2−) | mg L−1 | 137.80 | 95.89 | 168.36 | 97.85 | 0.00 | 123.86 | 250.00 * |
| Copper (Cu) | mg L−1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.20 * |
| Iron (Fe) | mg L−1 | 0.02 | 0.00 | 0.06 | 0.00 | 0.00 | 0.01 | 5.00 * |
| Manganese (Mn) | mg L−1 | 0.78 | 0.36 | 1.25 | 0.60 | 0.38 | 0.72 | 0.20 * |
| Molybdenum (Mo) | mg L−1 | 0.005 | 0.001 | 0.015 | 0.015 | 0.004 | 0.100 | NA |
| Nickel (Ni) | mg L−1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.20 * |
| Zinc (Zn) | mg L−1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.00 * |
| K | mg L−1 | 0.16 | 0.06 | 0.24 | 0.24 | 0.22 | 0.26 | 0–2 ** |
| Ca | meq L−1 | 4.06 | 2.16 | 5.10 | 4.93 | 4.93 | 5.73 | 0–20 ** |
| Mg | meq L−1 | 0.78 | 0.36 | 1.25 | 0.60 | 0.38 | 0.72 | 0–5 ** |
| Water Source | Nitrogen (N) mg kg−1 | Phosphorus (P) mg kg−1 | Potassium (K) mg kg−1 | Magnesium (Mg) mg kg−1 |
|---|---|---|---|---|
| Grains | ||||
| GW | 21,300 a | 3508 a | 7500 a | 1600 a |
| TWW | 11,450 b | 2800 a | 6300 b | 1400 a |
| Leaves | ||||
| GW | 11,320 a | 833 a | 8300 b | 2400 a |
| TWW | 11,020 b | 933 a | 9200 a | 2000 b |
| Stems | ||||
| GW | 7500 a | 458 b | 8200 a | 1500 a |
| TWW | 7100 a | 691 a | 8700 a | 1200 b |
| Roots | ||||
| GW | 5500 a | 933 b | 8300 a | 2600 a |
| TWW | 5200 a | 1566 a | 8400 a | 2000 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Holguin, A.; Sosa-Perez, G.; Ponce-Garcia, O.C.; Lara-Macias, C.R.; Villarreal-Guerrero, F.; Monzon-Burgos, C.G.; Ochoa-Rivero, J.M. The Impact of Treated Wastewater Irrigation on the Metabolism of Barley Grown in Arid and Semi-Arid Regions. Int. J. Environ. Res. Public Health 2022, 19, 2345. https://doi.org/10.3390/ijerph19042345
Alvarez-Holguin A, Sosa-Perez G, Ponce-Garcia OC, Lara-Macias CR, Villarreal-Guerrero F, Monzon-Burgos CG, Ochoa-Rivero JM. The Impact of Treated Wastewater Irrigation on the Metabolism of Barley Grown in Arid and Semi-Arid Regions. International Journal of Environmental Research and Public Health. 2022; 19(4):2345. https://doi.org/10.3390/ijerph19042345
Chicago/Turabian StyleAlvarez-Holguin, Alan, Gabriel Sosa-Perez, Omar Castor Ponce-Garcia, Carlos Rene Lara-Macias, Federico Villarreal-Guerrero, Carlos Gustavo Monzon-Burgos, and Jesus Manuel Ochoa-Rivero. 2022. "The Impact of Treated Wastewater Irrigation on the Metabolism of Barley Grown in Arid and Semi-Arid Regions" International Journal of Environmental Research and Public Health 19, no. 4: 2345. https://doi.org/10.3390/ijerph19042345
APA StyleAlvarez-Holguin, A., Sosa-Perez, G., Ponce-Garcia, O. C., Lara-Macias, C. R., Villarreal-Guerrero, F., Monzon-Burgos, C. G., & Ochoa-Rivero, J. M. (2022). The Impact of Treated Wastewater Irrigation on the Metabolism of Barley Grown in Arid and Semi-Arid Regions. International Journal of Environmental Research and Public Health, 19(4), 2345. https://doi.org/10.3390/ijerph19042345