Water as a Source of Indoor Air Contamination with Potentially Pathogenic Aeromonas hydrophila in Aquaculture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
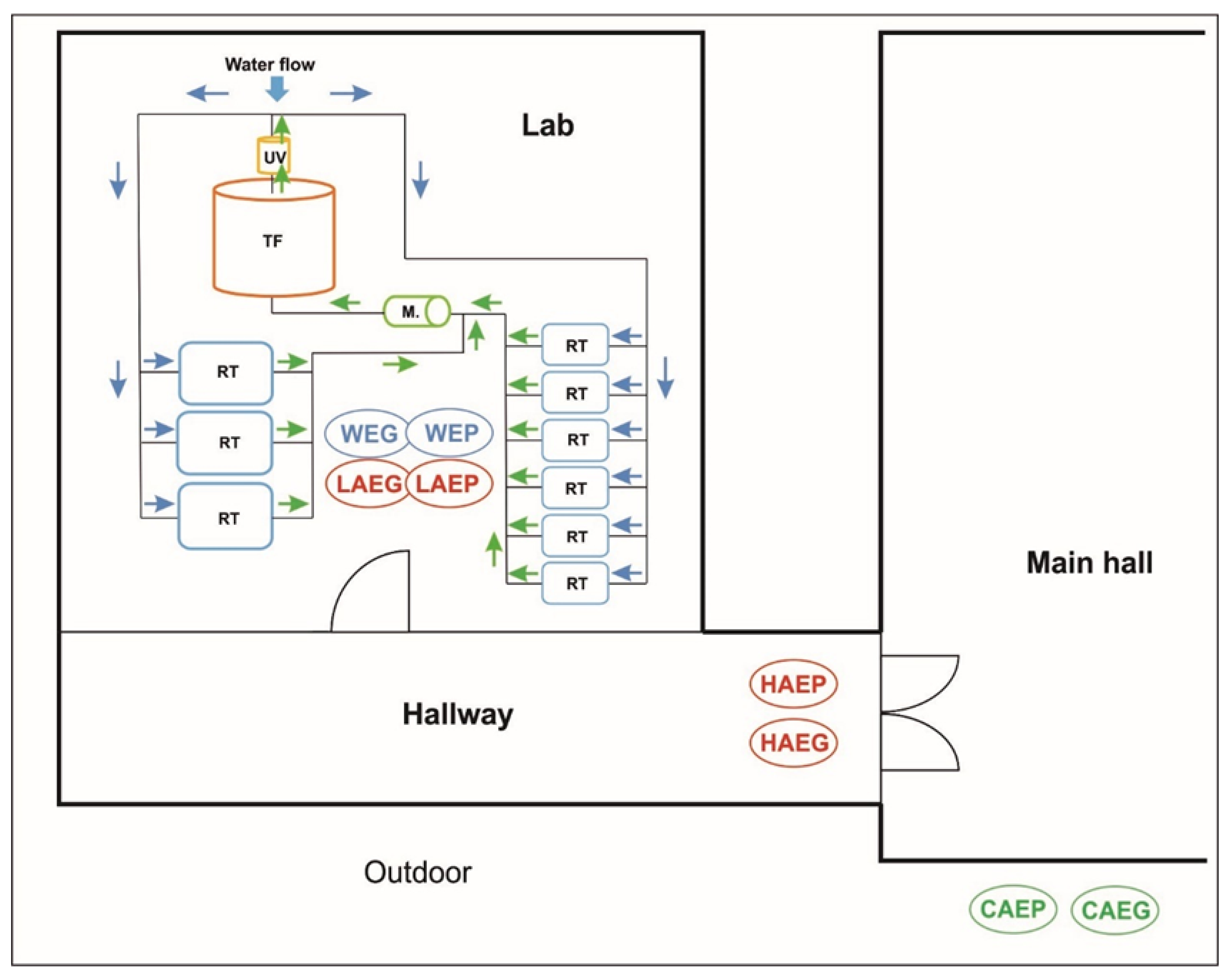
2.2. Research Premises and Sample Collection
2.2.1. Sampling Periods
2.2.2. Air Sampling
2.2.3. Water Sampling
2.3. Microbiological Analyses
2.3.1. Aeromonas hydrophila Counts in the Samples of Indoor Air and Tank Water
2.3.2. Antibiotic Resistance of PPAH in Samples of Indoor Air and Water
2.4. Physical Parameters of Air and Water Samples
2.5. Statistical Analysis
3. Results
3.1. Aeromonas hydrophila Counts in Air and Water Samples Collected during Fish Rearing Experiments
3.2. Physical Parameters and Their Influence on the Counts of Potentially Pathogenic A. hydrophila in Samples of Indoor Air
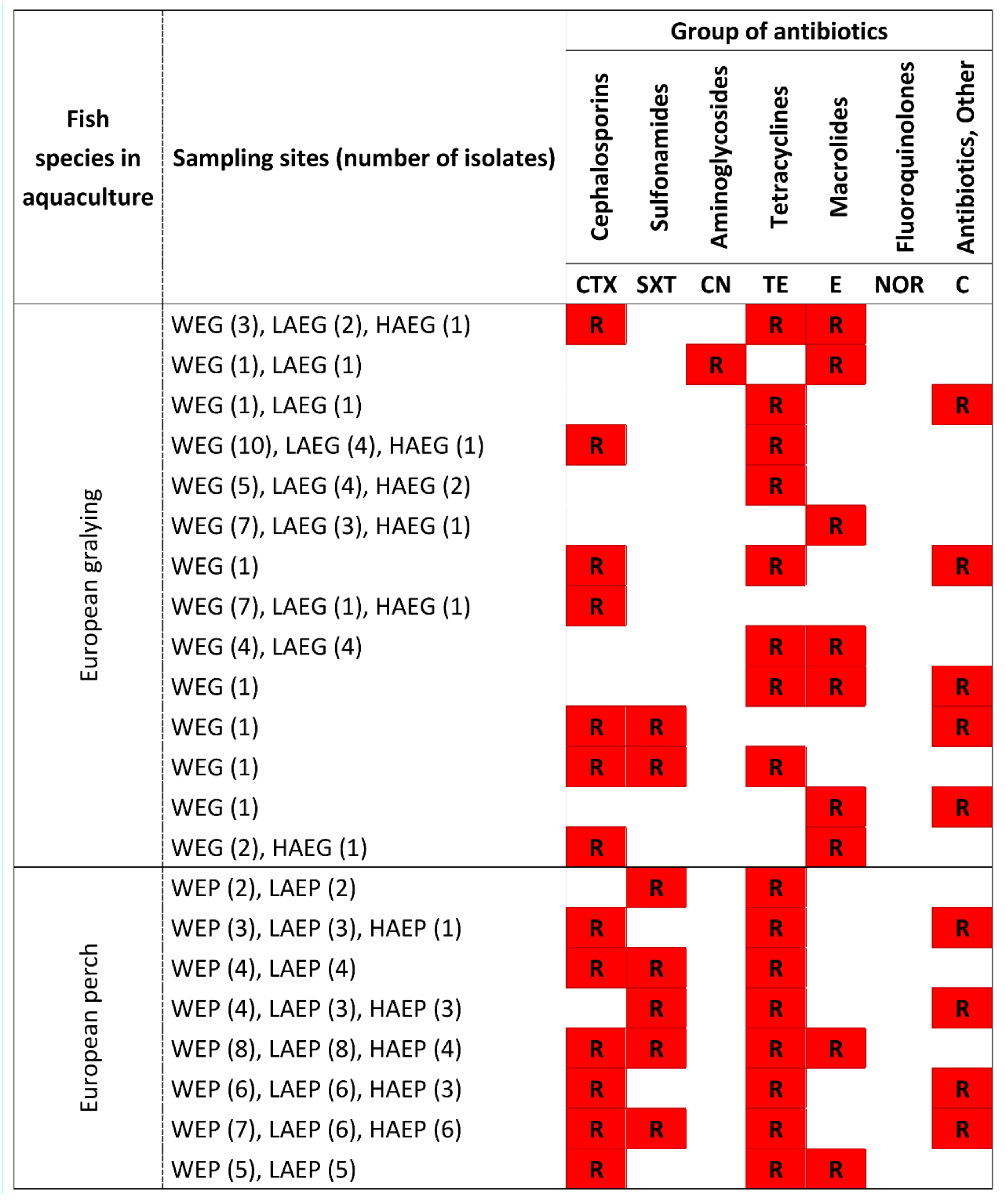
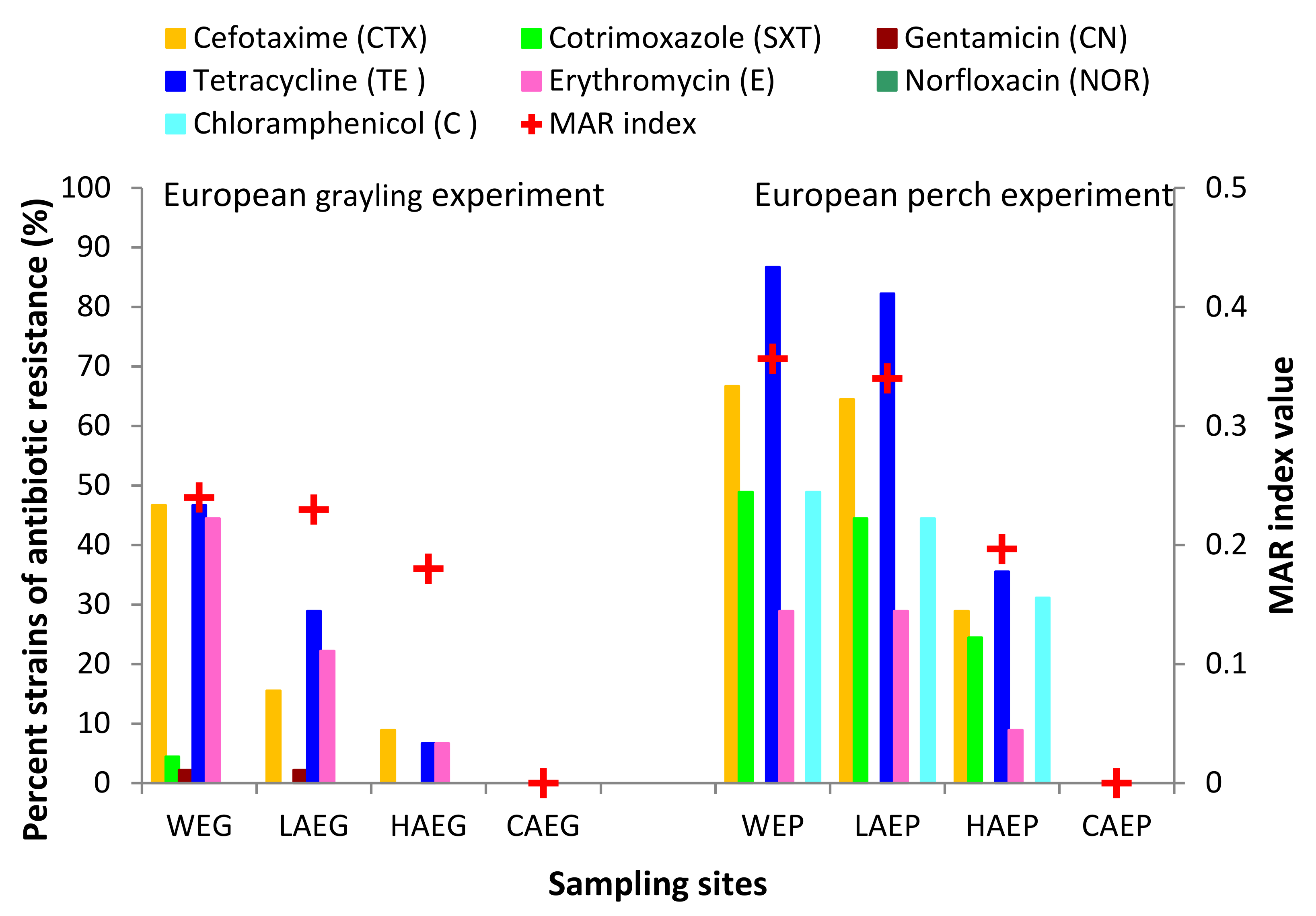
3.3. Antibiotic Resistance of Potentially Pathogenic A. hydrophila
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood, R.A.; Burchett, M.D.; Orwell, R.A.; Tarran, J.; Torpy, F. Plant/Soil Capacities to Remove Harmful Substances from Polluted Indoor Air. Plants and Environmental Quality Group, Centre for Ecotoxicology, UTS, Australia. 2002. Available online: https://greenplantsforgreenbuildings.org/wpcontent/uploads/2014/01/HarmfulSubstancesPlantSoil.pdf (accessed on 1 January 2002).
- Mandal, J.; Brandl, H. Bioaerosols in indoor environment—A review with special reference to residential and occupational locations. Open Environ. Biol. Monit. J. 2011, 4, 83–96. [Google Scholar] [CrossRef]
- Gawrońska, H.; Bakera, B. Phytoremediation of particulate matter from indoor air by Chlorophytum comosum L. plants. Air Qual. Atmos. Health 2015, 8, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gizaw, Z.; Gebrehiwot, M.; Yenew, C. High bacterial load of indoor air in hospital wards: The case of University of Gondar teaching hospital, Northwest Ethiopia. Multidiscip. Respir. Med. 2016, 11, 24. [Google Scholar] [CrossRef] [Green Version]
- Pastuszka, J.S.; Paw, U.K.T.; Lis, D.O.; Wlazlo, A.; Ulfig, K. Bacterial and fungal aerosol in indoor environment in Upper Silesia, Poland. Atmos. Environ. 2000, 26, 2149–2162. [Google Scholar] [CrossRef]
- Górny, R.L.; Dutkiewicz, J. Bacterial and fungal aerosols in indoor environment in Central and Eastern European countries. Ann. Agric. Environ. Med. 2002, 9, 17–23. [Google Scholar] [PubMed]
- Brągoszewska, E.; Biedroń, I.; Kozielska, B.; Pastuszka, J.S. Microbiological indoor air quality in an office building in Gliwice, Poland: Analysis of the case study. Air Qual. Atmos. Health 2018, 11, 729–740. [Google Scholar] [CrossRef] [Green Version]
- Tsai, F.C.; Macher, J.M. Concentrations of airborne culturable bacteria in 100 large US office buildings from the BASE study. Indoor Air 2005, 15, 71–81. [Google Scholar] [CrossRef]
- Giulio, M.D.; Grande, R.; Campli, E.D.; Bartolomeo, S.D.; Cellini, L. Indoor air quality in university environments. Environ. Monit. Assess 2010, 170, 509–517. [Google Scholar] [CrossRef]
- Godwin, C.; Batterman, S. Indoor air quality in Michigan schools. Indoor Air 2007, 17, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, Y.; Veillette, M.; Duchaine, C. Airborne bacteria and antibiotic resistance genes in hospital rooms. Aerobiologia 2010, 26, 185–194. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kim, C.N.; Kim, D. Distribution characteristics of airborne bacteria and fungi in the general hospitals of Korea. Ind. Health 2010, 48, 236–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.; Xiong, J.; Yao, L.; Gou, L.; Zhang, W. Determination of dust and microorganism accumulation in different designs of AHU system in Shaanxi History Museum. Build Environ. 2016, 104, 232–242. [Google Scholar] [CrossRef] [Green Version]
- Błaszczyk, E.; Rogula-Kozłowska, W.; Klejnowski, K.; Kubiesa, P.; Fulara, I.; Mielżyńska-Švach, D. Indoor air quality in urban and rural kindergartens: Short-term studies in Silesia, Poland. Air Qual. Atmos. Health 2017, 10, 1207–1220. [Google Scholar] [CrossRef] [Green Version]
- EPA (United States Environmental Protection Agency). Aeromonas: Human Health Criteria Document. Washington, DC 20460. 2006. Available online: https://nepis.epa.gov/Exe/tiff2png.exe/901Q0C00.PNG?r+75+g+7+D%3A%5CZYFILES%5CINDEX%20DATA%5C06THRU10%5CTIFF%5C00000076%5C901Q0C00.TIF (accessed on 6 March 2006).
- Kawahara, N.; Shigematsu, K.; Miyadai, T.; Kondo, R. Comparison of bacterial communities in fish farm sediments along organic enrichment gradient. Aquaculture 2009, 287, 107–113. [Google Scholar] [CrossRef]
- Miyagi, K.; Hirai, I.; Sano, K. Distribution of Aeromonas species in environmental water used in daily life in Okinawa Prefecture. Jpn. Environ. Health Prev. Med. 2016, 21, 287–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fannin, K.F.; Vana, S.C.; Jakubowski, W. Effect of an activated sludge wastewater treatment plant on ambient air densities of aerosols containing bacteria and viruses. Appl. Environ. Microbiol. 1985, 49, 1191–1196. [Google Scholar] [CrossRef] [Green Version]
- Sobsey, M.D.; Khatib, L.A.; Hill, V.R.; Alocilja, E.; Pillai, S. Pathogens in animal wastes and the impacts of waste management practices on their survival, transport and fate. In Animal Agriculture and the Environment: National Centre for Manure and Animal Waste Management White Papers; Rice, J.M., Caldwell, D.F., Humenik, F.J., Eds.; ASABE: St. Joseph, MO, USA, 2006; pp. 609–666. [Google Scholar]
- Lehane, L.; Rawlin, G.T. Topically acquired bacterial zoonoses from fish: A review. Med. J. Aust. 2000, 173, 256–259. [Google Scholar] [CrossRef]
- Martins, L.M.; Marquez, R.F.; Yano, T. Incidence of toxic Aeromonas isolated from food and human infection. FEMS Immunol Med. Microbiol. 2002, 32, 237–342. [Google Scholar] [CrossRef]
- Ji, Y.; Li, J.; Qin, Z.; Li, A.; Gu, Z.; Liu, X.; Lin, L.; Zhou, Y. Contribution of nuclease to the pathogenesis of Aeromonas hydrophila. Virulence 2015, 6, 515–522. [Google Scholar] [CrossRef] [Green Version]
- ASHRAE. Handbook–HVAC Applications. Chapter 14: Laboratories; American Society of Heating, Refrigerating and Air-Conditioning Engineers: Atlanta, GA, USA, 2007. [Google Scholar]
- Gołaś, I.; Szmyt, M.; Potorski, J.; Łopata, M.; Gotkowska-Płachta, A.; Glińska-Lewczuk, K. Distribution of Pseudomonas fluorescens and Aeromonas hydrophila bacteria in a recirculating aquaculture system during farming of European Grayling (Thymallus thymallus L.) broodstock. Water 2019, 11, 376. [Google Scholar] [CrossRef] [Green Version]
- Terech-Majewska, E.; Grudniewska, J.; Siwicki, A.K. Disinfection with the most effective biocides as a prophylactic method to supplement the treatment of fish diseases. Komun. Ryb. 2010, 2, 11–16. (In Polish) [Google Scholar]
- Franke-Whittle, I.; Klammer, S.; Insam, H. Design and application of an oligonucleotide microarray for the investigation of compost microbial communities. J. Microbiol. Methods 2005, 62, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.I.; Liu, W.Y.; Shyu, C.Z. Use of prawn blood agar hemolysis to screen for bacteria opportunistic to cultured tiger prawns Penaeus monodon. Dis. Aquat. Organ. 2000, 43, 153–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, T.C.; Waltman, W.D.; Shotts, E.B. Correlation of extracellular enzymatic activity and biochemical characteristics with regard to virulence of Aeromonas hydrophila. Develop. Biol. Stand. 1981, 49, 101–111. [Google Scholar]
- Bauer, A.W.; Kirby, W.N.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. An. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Susceptibility Tests, Approved Standard, 11th ed.; M02-A11; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Yucel, N.; Aslam, B.; Beyatli, Y. Prevalence and resistance to antibiotics for Aeromonas species isolated from retail fish in Turkey. J. Food Qual. 2005, 28, 313–324. [Google Scholar] [CrossRef]
- Krumperman, P.H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of food. Appl. Environ. Microb. 1983, 46, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.L.; Bezbaruah, R.L.; Roy, M.K.; Ghosh, A.C. Multiple antibiotic resistance index and its reversion in Pseudomonas aeruginosa. Lett. Appl. Microbiol. 1997, 24, 169–171. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide, Software for Canonical Community Ordination; Version 4.5; Microcomputer Power: New York, NY, USA, 2002; 500p. [Google Scholar]
- Sneath, P.H.A. Evaluation of clustering methods. In Numerical Taxonomy; Cole, A.J., Ed.; Academic Press Inc.: New York, NY, USA, 1969; pp. 257–267. [Google Scholar]
- Commission of European Communities. Biological Particles in Indoor Environments, European Collaborative Action–Indoor Air Quality and Its Impact on Man; Report No. 12; CEC: Luxembourg, 1993. [Google Scholar]
- Burkowska, A.; Kalwasińska, A.; Walczak, M. Airborne mesophilic bacteria at the Ciechocinek Health Resort. Pol. J. Environ. Stud. 2012, 21, 307–312. [Google Scholar]
- Martin-Carnahan, A.; Joseph, S.W. Aeromonas. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Krieg, B., Garrity, S., Eds.; Williams and Wilkins: New York, NY, USA, 2005; Volume 2. [Google Scholar]
- Gołaś, I.; Korzekwa, K.; Harnisz, M.; Zmysłowska, I.; Teodorowicz, M.; Terech-Majewska, E.; Rodziewicz, W.; Bieńkowska, M. Influence of fishery management and environmental factors on occurrence of heterotrophic, hemolytic and mesophilic bacteria and Aeromonas hydrophila in waters of Drwęca River, Poland. Arch. Environ. Prot. 2009, 35, 27–40. [Google Scholar]
- American Industrial Hygiene Association (AIHA). The practitioner’s approach to IAQ investigations. In Proceedings of the Indoor Air Quality International Symposium, St. Louis, MO, USA, 23 May 1989; Weekes, D.M., Gammage, R.B., Eds.; AIHA: St. Louis, MO, USA, 1989; pp. 43–66. [Google Scholar]
- Kalogerakis, N.; Paschali, D.; Lekaditis, V.; Pantidou, A.; Eleftheriadis, K.; Lazaridis, M. Indoor air quality—Bioaerosol measurements in domestic and office premises. J. Aerosol Sci. 2005, 36, 751–761. [Google Scholar] [CrossRef]
- Solomon, F.B.; Wadilo, F.W.; Arota, A.A.; Abraham, Y.L. Antibiotic resistant airborne bacteria and their multidrug resistance pattern at University teaching referral Hospital in South Ethiopia. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Jankowiak, E.; Kubera, Ł.; Małecka-Adamowicz, M.; Dembowska, E. Microbiological air quality in pharmacies and an antibiotic resistance profile of staphylococci species. Aerobiologia 2020, 36, 551–563. [Google Scholar] [CrossRef]
- Monir, M.S.; Bagum, N.; Kabir, S.M.L.; Borty, S.C.; Ud-Doulah, M.A. Isolation, molecular identification and characterization of Aeromonas hydrophila from infected air-breathing catfish Magur (Clarias batrachus) cultured in Mymensingh, Bangladesh. Asian Aust. J. Food Saf. Secur. 2017, 1, 17–24. [Google Scholar] [CrossRef]
- Belém-Costa, A.; Cyrino, J.E.P. Antibiotic resistance of Aeromonas hydrophila isolated from Piaractus mesopotamicus (Holmberg, 1887) and Oreochromis niloticus (Linnaeus, 1758). Sci. Agric. 2006, 63, 281–284. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhang, L.; Tiu, L.; Wang, H.H. Characterization of antibiotic resistance in commensal bacteria from an aquaculture ecosystem. Front. Microbiol. 2015, 6, 914. [Google Scholar] [CrossRef] [Green Version]
- Patil, H.J.; Benet-Perelberg, A.; Naor, A.; Smirnov, M.; Ofek, T.; Nasser, A.; Minz, D.; Cytryn, E. Evidence of increased antibiotic resistance in phylogenetically-diverse Aeromonas isolates from semi-intensive fish ponds treated with antibiotics. Front. Microbiol. 2016, 7, 1875. [Google Scholar] [CrossRef] [Green Version]
- Pepi, M.; Focardi, S. Antibiotic-resistant bacteria in aquaculture and climate change: A challenge for health in the Mediterranean Area. Int. J. Environ. Res. Public Health 2021, 18, 5723. [Google Scholar] [CrossRef]
- Piotrowska, M.; Popowska, M. Insight into the mobilome of Aeromonas strains. Front. Microbiol. 2015, 6, 494. [Google Scholar] [CrossRef] [Green Version]



| Sample | Bacterial Counts (cfu/m3) | Indoor/Outdoor Ratio (I/O) | ||
|---|---|---|---|---|
| TCMAH 1 | PPAH 2 | TCMAH | PPAH | |
| CAEG 3 | 0.8 × 101 ± 0.3 × 101 | 0.2 × 101 ± 0.1 × 101 | na 11 | na |
| LAEG 4 | 5.3 × 102 ± 3.0 × 102 | 4.3 × 101 ± 1.5 × 101 | 16.98 | 38.33 |
| HAEG 5 | 3.2 × 101 ± 0.1 × 101 | 0.5 × 101 ± 0.3 × 101 | 3.35 | 4.56 |
| CAEP 6 | 0.3 × 101 ± 0.3 × 101 | 0.1 × 101 ± 0.1 × 101 | na | na |
| LAEP 7 | 1.4 × 103 ± 0.3 × 103 | 4.7 × 102 ± 2.2 × 102 | 481.38 | 400.00 |
| HAEP 8 | 9.2 × 101 ± 6.5 × 101 | 4.0 × 101 ± 1.0 × 101 | 65.26 | 35.00 |
| WEG 9 | 3.3 × 109 ± 2.0 × 109 | 0.9 × 109 ± 0.5 × 109 | na | na |
| WEP 10 | 7.0 × 109 ± 1.5 × 109 | 2.8 × 109 ± 1.1 × 109 | na | na |
| Sample | Temperature (°C) | Relative Humidity (%) |
|---|---|---|
| CAEG 1 | 20.9 ± 3.5 | 30.1 ± 2.3 |
| LAEG 2 | 12.5 ± 0.3 | 41.9 ± 0.5 |
| HAEG 3 | 21.9 ± 0.5 | 32.7 ± 2.1 |
| CAEP 4 | 21.5 ± 4.3 | 31.0 ± 0.8 |
| LAEP 5 | 17.9 ± 0.4 | 48.3 ± 0.9 |
| HAEP 6 | 20.2 ± 0.2 | 33.0 ± 0.5 |
| WEG 7 | 11.0 ± 0.2 | na 9 |
| WEP 8 | 18.0 ± 0.5 | na |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gołaś, I.; Szmyt, M.; Glińska-Lewczuk, K. Water as a Source of Indoor Air Contamination with Potentially Pathogenic Aeromonas hydrophila in Aquaculture. Int. J. Environ. Res. Public Health 2022, 19, 2379. https://doi.org/10.3390/ijerph19042379
Gołaś I, Szmyt M, Glińska-Lewczuk K. Water as a Source of Indoor Air Contamination with Potentially Pathogenic Aeromonas hydrophila in Aquaculture. International Journal of Environmental Research and Public Health. 2022; 19(4):2379. https://doi.org/10.3390/ijerph19042379
Chicago/Turabian StyleGołaś, Iwona, Mariusz Szmyt, and Katarzyna Glińska-Lewczuk. 2022. "Water as a Source of Indoor Air Contamination with Potentially Pathogenic Aeromonas hydrophila in Aquaculture" International Journal of Environmental Research and Public Health 19, no. 4: 2379. https://doi.org/10.3390/ijerph19042379






