Greenspace, Inflammation, Cardiovascular Health, and Cancer: A Review and Conceptual Framework for Greenspace in Cardio-Oncology Research
Abstract
:1. Introduction
1.1. Cardiovascular Disease Burden
1.2. Cardiovascular Disease Burden among Cancer Survivors
2. Inflammation and Cardiovascular Disease
3. Inflammation and Cancer
4. Anti-Inflammatory Pharmacological Interventions against MACE and Need for Other Innovative Interventions
5. Greenspace Interventions
5.1. Greenspace and Health Outcomes
5.2. Greenspace and Biopsychosocial Plausible Pathways to Positive Health Outcomes
5.3. Greenspace and CV Health
5.3.1. Ecological Studies of Greenspace and CV Health
5.3.2. Multilevel Studies of Greenspace and CV Health
5.3.3. Experimental Studies of Greenspace and CV Health
5.4. Greenspace and Inflammation
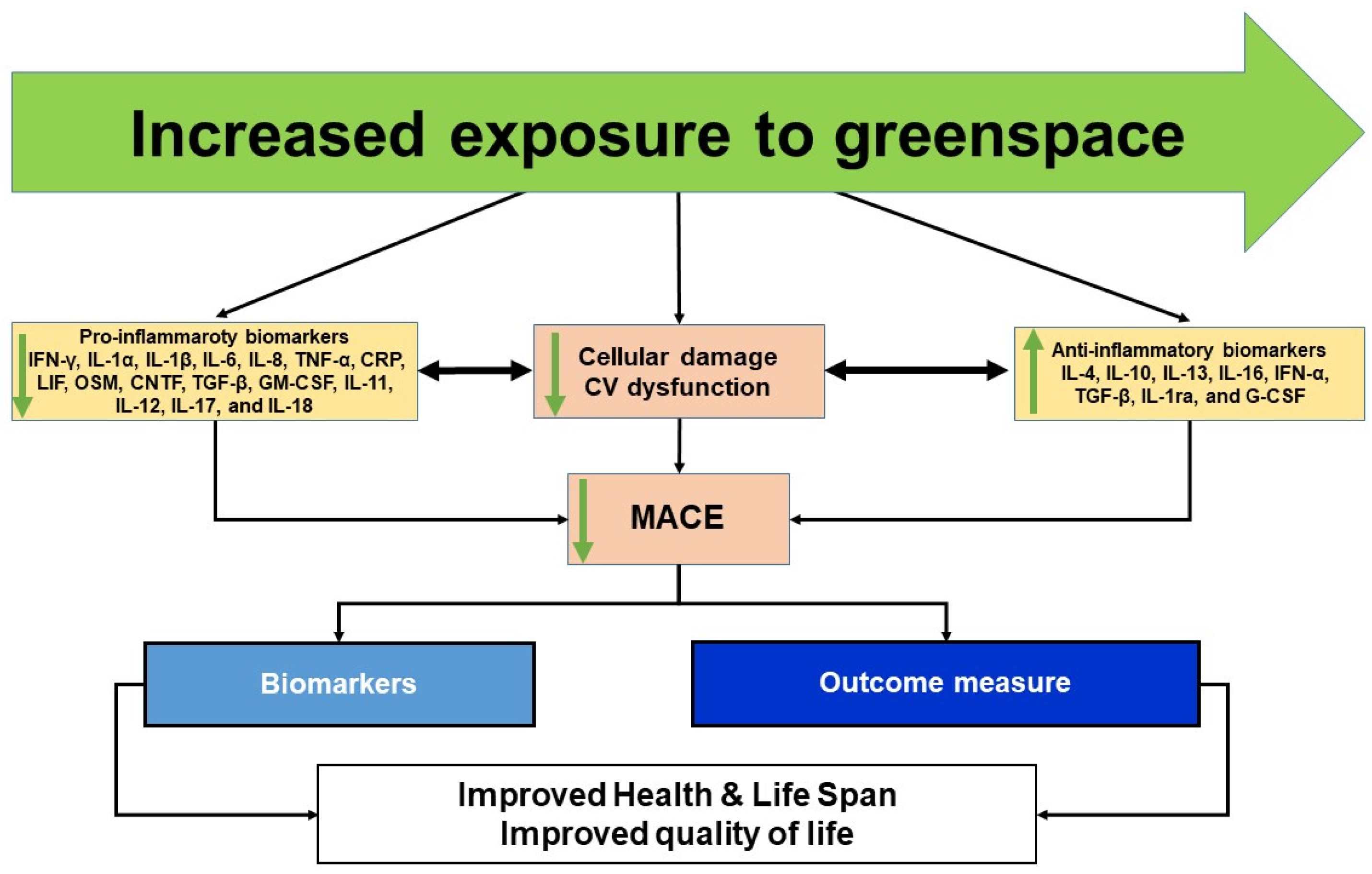
6. Proposed Conceptual Framework
7. Way Forward and Implications for Translational Science and Future Research
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Tarride, J.-E.; Lim, M.; DesMeules, M.; Luo, W.; Burke, N.; O’Reilly, D.; Bowen, J.; Goeree, R. A review of the cost of cardiovascular disease. Can. J. Cardiol. 2009, 25, e195–e202. [Google Scholar] [CrossRef] [Green Version]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- World Health Organization (WHO) Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 28 August 2020).
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; Floyd, J.; Fornage, M.; Gillespie, C.; Isasi, C.R.; et al. Heart Disease and Stroke Statistics—2017 Update: A Report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA. Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Dunbar, S.B.; Khavjou, O.A.; Bakas, T.; Hunt, G.; Kirch, R.A.; Leib, A.R.; Morrison, R.S.; Poehler, D.C.; Roger, V.L.; Whitsel, L.P. Projected Costs of Informal Caregiving for Cardiovascular Disease: 2015 to 2035: A Policy Statement from the American Heart Association. Circulation 2018, 137, e558–e577. [Google Scholar] [CrossRef]
- Bradshaw, P.T.; Stevens, J.; Khankari, N.; Teitelbaum, S.L.; Neugut, A.I.; Gammon, M.D. Cardiovascular Disease Mortality among Breast Cancer Survivors. Epidemiology 2016, 27, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Bertero, E.; Ameri, P.; Maack, C. Bidirectional Relationship between Cancer and Heart Failure: Old and New Issues in Cardio-oncology. Card. Fail. Rev. 2019, 5, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Barac, A.; Murtagh, G.; Carver, J.R.; Chen, M.H.; Freeman, A.M.; Herrmann, J.; Iliescu, C.; Ky, B.; Mayer, E.L.; Okwuosa, T.M.; et al. Cardiovascular health of patients with cancer and cancer survivors: A roadmap to the next level. J. Am. Coll. Cardiol. 2015, 65, 2739–2746. [Google Scholar] [CrossRef] [Green Version]
- Aleman, B.M.P.; Moser, E.C.; Nuver, J.; Suter, T.M.; Maraldo, M.V.; Specht, L.; Vrieling, C.; Darby, S.C. Cardiovascular disease after cancer therapy. Eur. J. Cancer Suppl. 2014. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.C.; Caan, B.J.; Prado, C.M.; Weltzien, E.; Xiao, J.; Cespedes Feliciano, E.M.; Kroenke, C.H.; Meyerhardt, J.A. Body Composition and Cardiovascular Events in Patients with Colorectal Cancer: A Population-Based Retrospective Cohort Study. JAMA Oncol. 2019, 5, 967–972. [Google Scholar] [CrossRef]
- Weiner, S.D.; Ahmed, H.N.; Jin, Z.; Cushman, M.; Herrington, D.M.; Nelson, J.C.; Di Tullio, M.R.; Homma, S. Systemic inflammation and brachial artery endothelial function in the Multi-Ethnic Study of Atherosclerosis (MESA). Heart 2014, 100, 862–866. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N. Engl. J. Med. 2018, 380, 752–762. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Micha, R.; Imamura, F.; Wyler von Ballmoos, M.; Solomon, D.H.; Hernán, M.A.; Ridker, P.M.; Mozaffarian, D. Systematic Review and Meta-Analysis of Methotrexate Use and Risk of Cardiovascular Disease. Am. J. Cardiol. 2011, 108, 1362–1370. [Google Scholar] [CrossRef] [Green Version]
- Bikomeye, J.C.; Namin, S.; Anyanwu, C.; Rublee, C.S.; Ferschinger, J.; Leinbach, K.; Lindquist, P.; Hoppe, A.; Hoffman, L.; Hegarty, J.; et al. Resilience and Equity in a Time of Crises: Investing in Public Urban Greenspace Is Now More Essential Than Ever in the US and Beyond. Int. J. Environ. Res. Public Health 2021, 18, 8420. [Google Scholar] [CrossRef]
- Bouabdallaoui, N.; Tardif, J.-C.; Waters, D.D.; Pinto, F.J.; Maggioni, A.P.; Diaz, R.; Berry, C.; Koenig, W.; Lopez-Sendon, J.; Gamra, H.; et al. Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur. Heart J. 2020, 41, 4092–4099. [Google Scholar] [CrossRef]
- Johnson, C.B.; Davis, M.K.; Law, A.; Sulpher, J. Shared risk factors for cardiovascular disease and cancer: Implications for preventive health and clinical care in oncology patients. Can. J. Cardiol. 2016, 32, 900–907. [Google Scholar] [CrossRef]
- Mehta, L.S.; Watson, K.E.; Barac, A.; Beckie, T.M.; Bittner, V.; Cruz-Flores, S.; Dent, S.; Kondapalli, L.; Ky, B.; Okwuosa, T.; et al. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement from the American Heart Association. Circulation 2018, 137, e30–e66. [Google Scholar] [CrossRef]
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.B.; Ewer, M.; et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef]
- Dent, S.F.; Kikuchi, R.; Kondapalli, L.; Ismail-Khan, R.; Brezden-Masley, C.; Barac, A.; Fradley, M. Optimizing Cardiovascular Health in Patients with Cancer: A Practical Review of Risk Assessment, Monitoring, and Prevention of Cancer Treatment–Related Cardiovascular Toxicity. Am. Soc. Clin. Oncol. Educ. B. 2020, 40, 501–515. [Google Scholar] [CrossRef]
- Perez, I.E.; Taveras Alam, S.; Hernandez, G.A.; Sancassani, R. Cancer therapy-related cardiac dysfunction: An overview for the clinician. Clin. Med. Insights Cardiol. 2019, 13, 13. [Google Scholar] [CrossRef] [Green Version]
- Accordino, M.K.; Neugut, A.I.; Hershman, D.L. Cardiac effects of anticancer therapy in the elderly. J. Clin. Oncol. 2014, 32, 2654. [Google Scholar] [CrossRef] [Green Version]
- Koelwyn, G.J.; Newman, A.A.C.; Afonso, M.S.; van Solingen, C.; Corr, E.M.; Brown, E.J.; Albers, K.B.; Yamaguchi, N.; Narke, D.; Schlegel, M.; et al. Myocardial infarction accelerates breast cancer via innate immune reprogramming. Nat. Med. 2020, 26, 1452–1458. [Google Scholar] [CrossRef]
- Hasin, T.; Gerber, Y.; McNallan, S.M.; Weston, S.A.; Kushwaha, S.S.; Nelson, T.J.; Cerhan, J.R.; Roger, V.L. Patients with heart failure have an increased risk of incident cancer. J. Am. Coll. Cardiol. 2013, 62, 881–886. [Google Scholar] [CrossRef] [Green Version]
- Hasin, T.; Gerber, Y.; Weston, S.A.; Jiang, R.; Killian, J.M.; Manemann, S.M.; Cerhan, J.R.; Roger, V.L. Heart Failure After Myocardial Infarction Is Associated with Increased Risk of Cancer. J. Am. Coll. Cardiol. 2016, 68, 265–271. [Google Scholar] [CrossRef]
- Weaver, K.E.; Foraker, R.E.; Alfano, C.M.; Rowland, J.H.; Arora, N.K.; Bellizzi, K.M.; Hamilton, A.S.; Oakley-Girvan, I.; Keel, G.; Aziz, N.M. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: A gap in survivorship care? J. Cancer Surviv. 2013, 7, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Gernaat, S.A.M.; Ho, P.J.; Rijnberg, N.; Emaus, M.J.; Baak, L.M.; Hartman, M.; Grobbee, D.E.; Verkooijen, H.M. Risk of death from cardiovascular disease following breast cancer: A systematic review. Breast Cancer Res. Treat. 2017, 164, 537–555. [Google Scholar] [CrossRef] [Green Version]
- Faber, J.; Wingerter, A.; Neu, M.-A.; Henninger, N.; Eckerle, S.; Münzel, T.; Lackner, K.J.; Beutel, M.E.; Blettner, M.; Rathmann, W. Burden of cardiovascular risk factors and cardiovascular disease in childhood cancer survivors: Data from the German CVSS-study. Eur. Heart J. 2018, 39, 1555–1562. [Google Scholar] [CrossRef]
- Mertens, A.C.; Liu, Q.; Neglia, J.P.; Wasilewski, K.; Leisenring, W.; Armstrong, G.T.; Robison, L.L.; Yasui, Y. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2008, 100, 1368–1379. [Google Scholar] [CrossRef]
- American Cancer Society How Chemotherapy Drugs Work? Available online: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/chemotherapy/how-chemotherapy-drugs-work.html (accessed on 3 February 2021).
- Saleh, Y.; Abdelkarim, O.; Herzallah, K.; Abela, G.S. Anthracycline-induced cardiotoxicity: Mechanisms of action, incidence, risk factors, prevention, and treatment. Heart Fail. Rev. 2021, 26, 1159–1173. [Google Scholar] [CrossRef]
- Aubel-Sadron, G.; Londos-Gagliardi, D. Daunorubicin and doxorubicin, anthracycline antibiotics, a physicochemical and biological review. Biochimie 1984, 66, 333–352. [Google Scholar] [CrossRef]
- Quryshi, N.; Norwood Toro, L.E.; Ait-Aissa, K.; Kong, A.; Beyer, A.M. Chemotherapeutic-Induced Cardiovascular Dysfunction: Physiological Effects, Early Detection—The Role of Telomerase to Counteract Mitochondrial Defects and Oxidative Stress. Int. J. Mol. Sci. 2018, 19, 797. [Google Scholar] [CrossRef] [Green Version]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef] [Green Version]
- Bjornsti, M.-A.; Kaufmann, S.H. Topoisomerases and cancer chemotherapy: Recent advances and unanswered questions. F1000Research 2019, 8, 1704. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Adams, M.J.; Colan, S.D.; Constine, L.S.; Herman, E.H.; Hsu, D.T.; Hudson, M.M.; Kremer, L.C.; Landy, D.C.; Miller, T.L.; et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation 2013, 128, 1927–1955. [Google Scholar] [CrossRef] [Green Version]
- van Nimwegen, F.A.; Schaapveld, M.; Janus, C.P.M.; Krol, A.D.G.; Petersen, E.J.; Raemaekers, J.M.M.; Kok, W.E.M.; Aleman, B.M.P.; van Leeuwen, F.E. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern. Med. 2015, 175, 1007–1017. [Google Scholar] [CrossRef]
- Madeddu, C.; Deidda, M.; Piras, A.; Cadeddu, C.; Demurtas, L.; Puzzoni, M.; Piscopo, G.; Scartozzi, M.; Mercuro, G. Pathophysiology of cardiotoxicity induced by nonanthracycline chemotherapy. J. Cardiovasc. Med. 2016, 17, S12–S18. [Google Scholar] [CrossRef]
- Braverman, A.C.; Antin, J.H.; Plappert, M.T.; Cook, E.F.; Lee, R.T. Cyclophosphamide cardiotoxicity in bone marrow transplantation: A prospective evaluation of new dosing regimens. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1991, 9, 1215–1223. [Google Scholar] [CrossRef]
- Yeh, E.T.H.; Bickford, C.L. Cardiovascular complications of cancer therapy: Incidence, pathogenesis, diagnosis, and management. J. Am. Coll. Cardiol. 2009, 53, 2231–2247. [Google Scholar] [CrossRef] [Green Version]
- Focaccetti, C.; Bruno, A.; Magnani, E.; Bartolini, D.; Principi, E.; Dallaglio, K.; Bucci, E.O.; Finzi, G.; Sessa, F.; Noonan, D.M. Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ROS production in endothelial cells and cardiomyocytes. PLoS ONE 2015, 10, e0115686. [Google Scholar] [CrossRef] [PubMed]
- Lown, J.W.; Chen, H.-H.; Plambeck, J.A.; Acton, E.M. Further studies on the generation of reactive oxygen species from activated anthracyclines and the relationship to cytotoxic action and cardiotoxic effects. Biochem. Pharmacol. 1982, 31, 575–581. [Google Scholar] [CrossRef]
- Gouspillou, G.; Scheede-Bergdahl, C.; Spendiff, S.; Vuda, M.; Meehan, B.; Mlynarski, H.; Archer-Lahlou, E.; Sgarioto, N.; Purves-Smith, F.M.; Konokhova, Y. Anthracycline-containing chemotherapy causes long-term impairment of mitochondrial respiration and increased reactive oxygen species release in skeletal muscle. Sci. Rep. 2015, 5, srep08717. [Google Scholar] [CrossRef]
- Asnani, A.; Moslehi, J.J.; Adhikari, B.B.; Baik, A.H.; Beyer, A.M.; de Boer, R.A.; Ghigo, A.; Grumbach, I.M.; Jain, S.; Zhu, H.; et al. Preclinical Models of Cancer Therapy–Associated Cardiovascular Toxicity: A Scientific Statement from the American Heart Association. Circ. Res. 2021, 129, e21–e34. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.M.; Bouchier-Hayes, D.J.; Harmey, J.H. Vascular endothelial growth factor (VEGF) and its role in non-endothelial cells: Autocrine signalling by VEGF. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Schmidinger, M.; Zielinski, C.C.; Vogl, U.M.; Bojic, A.; Bojic, M.; Schukro, C.; Ruhsam, M.; Hejna, M.; Schmidinger, H. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2008, 26, 5204–5212. [Google Scholar] [CrossRef]
- Elice, F.; Jacoub, J.; Rickles, F.R.; Falanga, A.; Rodeghiero, F. Hemostatic complications of angiogenesis inhibitors in cancer patients. Am. J. Hematol. 2008, 83, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Shak, S.D.J.L.-J.B. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783792. [Google Scholar] [CrossRef]
- Zaha, V.G.; Meijers, W.C.; Moslehi, J. Cardio-Immuno-Oncology. Circulation 2020, 141, 87–89. [Google Scholar] [CrossRef]
- Salem, J.-E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.-P.; Balko, J.M.; Bonaca, M.P. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Chen, M.H.; Colan, S.D.; Diller, L. Cardiovascular disease: Cause of morbidity and mortality in adult survivors of childhood cancers. Circ. Res. 2011, 108, 619–628. [Google Scholar] [CrossRef] [Green Version]
- Patnaik, J.L.; Byers, T.; DiGuiseppi, C.; Dabelea, D.; Denberg, T.D. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: A retrospective cohort study. Breast Cancer Res. 2011, 13, R64. [Google Scholar] [CrossRef] [Green Version]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Inflammation in atherosclerosis: From pathophysiology to practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvard Health Publishing Understanding Acute and Chronic Inflammation. Available online: https://www.health.harvard.edu/staying-healthy/understanding-acute-and-chronic-inflammation (accessed on 4 February 2021).
- Ansar, W.; Ghosh, S. Inflammation and inflammatory diseases, markers, and mediators: Role of CRP in some inflammatory diseases. In Biology of C Reactive Protein in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2016; pp. 67–107. [Google Scholar]
- Rankin, J.A. Biological mediators of acute inflammation. AACN Adv. Crit. Care 2004, 15, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Ariel, A.; Serhan, C.N. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007, 28, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef]
- Prasada, S.; Rivera, A.; Nishtala, A.; Pawlowski, A.E.; Sinha, A.; Bundy, J.D.; Chadha, S.A.; Ahmad, F.S.; Khan, S.S.; Achenbach, C.; et al. Differential Associations of Chronic Inflammatory Diseases with Incident Heart Failure. JACC. Heart Fail. 2020, 8, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef]
- Sanz, J.; Fayad, Z.A. Imaging of atherosclerotic cardiovascular disease. Nature 2008, 451, 953–957. [Google Scholar] [CrossRef]
- Seidman, M.A.; Mitchell, R.N.; Stone, J.R. Chapter 12—Pathophysiology of Atherosclerosis; Willis, M.S., Homeister, J.W., Stone, Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 221–237. ISBN 978-0-12-405206-2. [Google Scholar]
- Ridker, P.M.; Koenig, W.; Kastelein, J.J.; Mach, F.; Lüscher, T.F. Has the time finally come to measure hsCRP universally in primary and secondary cardiovascular prevention? Eur. Heart J. 2018, 39, 4109–4111. [Google Scholar] [CrossRef]
- van’t Klooster, C.C.; Ridker, P.M.; Hjortnaes, J.; van Der Graaf, Y.; Asselbergs, F.W.; Westerink, J.; Aerts, J.G.J.V.; Visseren, F.L.J. The relation between systemic inflammation and incident cancer in patients with stable cardiovascular disease: A cohort study. Eur. Heart J. 2019, 40, 3901–3909. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef]
- Crawford, A.A.; Söderberg, S.; Kirschbaum, C.; Murphy, L.; Eliasson, M.; Ebrahim, S.; Smith, G.D.; Olsson, T.; Sattar, N.; Lawlor, D.A. Morning plasma cortisol as a cardiovascular risk factor: Findings from prospective cohort and Mendelian randomization studies. Eur. J. Endocrinol. 2019, 181, 429–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, L.B.; Clopton, P.; Laughlin, G.A.; Maisel, A.S.; Barrett-Connor, E. Growth-differentiation factor-15 is a robust, independent predictor of 11-year mortality risk in community-dwelling older adults: The Rancho Bernardo Study. Circulation 2011, 123, 2101–2110. [Google Scholar] [CrossRef] [Green Version]
- Danesh, J.; Lewington, S.; Thompson, S.G.; Lowe, G.D.O.; Collins, R.; Kostis, J.B.; Wilson, A.C.; Folsom, A.R.; Wu, K.; Benderly, M.; et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: An individual participant meta-analysis. JAMA 2005, 294, 1799–1809. [Google Scholar] [CrossRef]
- Kleber, M.E.; Delgado, G.; Grammer, T.B.; Silbernagel, G.; Huang, J.; Krämer, B.K.; Ritz, E.; März, W. Uric acid and cardiovascular events: A Mendelian randomization study. J. Am. Soc. Nephrol. 2015, 26, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, J.G. Role of toll-like receptors in cardiovascular diseases. Clin. Sci. (Lond). 2011, 121, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaraj, S.; Xu, D.Y.; Jialal, I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: Implications for the metabolic syndrome and atherothrombosis. Circulation 2003, 107, 398–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.B.; Blumenthal, J.A.; Lee, S.Y.; Georgiades, A. Association of cortisol and the metabolic syndrome in Korean men and women. J. Korean Med. Sci. 2011, 26, 914–918. [Google Scholar] [CrossRef] [Green Version]
- Lind, L.; Wallentin, L.; Kempf, T.; Tapken, H.; Quint, A.; Lindahl, B.; Olofsson, S.; Venge, P.; Larsson, A.; Hulthe, J. Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: Results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) Study. Eur. Heart J. 2009, 30, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Eggers, K.M.; Kempf, T.; Larsson, A.; Lindahl, B.; Venge, P.; Wallentin, L.; Wollert, K.C.; Lind, L. Evaluation of temporal changes in cardiovascular biomarker concentrations improves risk prediction in an elderly population from the community. Clin. Chem. 2016, 62, 485–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotter, G.; Voors, A.A.; Prescott, M.F.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Pang, P.S.; Ponikowski, P.; Milo, O.; Hua, T.A. Growth differentiation factor 15 (GDF-15) in patients admitted for acute heart failure: Results from the RELAX-AHF study. Eur. J. Heart Fail. 2015, 17, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Fenech, G.; Rajzbaum, G.; Mazighi, M.; Blacher, J. Serum uric acid and cardiovascular risk: State of the art and perspectives. Jt. Bone Spine 2014, 81, 392–397. [Google Scholar] [CrossRef]
- Zhao, G.; Huang, L.; Song, M.; Song, Y. Baseline serum uric acid level as a predictor of cardiovascular disease related mortality and all-cause mortality: A meta-analysis of prospective studies. Atherosclerosis 2013, 231, 61–68. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Little, P.J.; Downey, L.; Afroz, R.; Wu, Y.; Ta, H.T.; Xu, S.; Kamato, D. The role of toll-like receptors in atherothrombotic cardiovascular disease. ACS Pharmacol. Transl. Sci. 2020, 3, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Moghimpour Bijani, F.; Vallejo, J.G.; Rezaei, N. Toll-like receptor signaling pathways in cardiovascular diseases: Challenges and opportunities. Int. Rev. Immunol. 2012, 31, 379–395. [Google Scholar] [CrossRef]
- Liu, F.-Y.; Fan, D.; Yang, Z.; Tang, N.; Guo, Z.; Ma, S.-Q.; Ma, Z.-G.; Wu, H.-M.; Deng, W.; Tang, Q.-Z. TLR9 is essential for HMGB1-mediated post-myocardial infarction tissue repair through affecting apoptosis, cardiac healing, and angiogenesis. Cell Death Dis. 2019, 10, 480. [Google Scholar] [CrossRef]
- Falck-Hansen, M.; Kassiteridi, C.; Monaco, C. Toll-like receptors in atherosclerosis. Int. J. Mol. Sci. 2013, 14, 14008–14023. [Google Scholar] [CrossRef] [Green Version]
- Ashayeri Ahmadabad, R.; Khaleghi Ghadiri, M.; Gorji, A. The role of Toll-like receptor signaling pathways in cerebrovascular disorders: The impact of spreading depolarization. J. Neuroinflamm. 2020, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Frantz, S.; Ertl, G.; Bauersachs, J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Shanmugam, M.K.; Ramachandran, L.; Kumar, A.P.; Tergaonkar, V. Multifaceted link between cancer and inflammation. Biosci. Rep. 2012, 32, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnoli, C.; Grioni, S.; Pala, V.; Allione, A.; Matullo, G.; Di Gaetano, C.; Tagliabue, G.; Sieri, S.; Krogh, V. Biomarkers of inflammation and breast cancer risk: A case-control study nested in the EPIC-Varese cohort. Sci. Rep. 2017, 7, 12708. [Google Scholar] [CrossRef] [Green Version]
- Allen, M.D.; Jones, L.J. The role of inflammation in progression of breast cancer: Friend or foe? (Review). Int. J. Oncol. 2015, 47, 797–805. [Google Scholar] [CrossRef]
- Mills, R.C. Breast Cancer Survivors, Common Markers of Inflammation, and Exercise: A Narrative Review. Breast Cancer Basic Clin. Res. 2017, 11, 11. [Google Scholar] [CrossRef]
- Seruga, B.; Zhang, H.; Bernstein, L.J.; Tannock, I.F. Cytokines and their relationship to the symptoms and outcome of cancer. Nat. Rev. Cancer 2008, 8, 887–899. [Google Scholar] [CrossRef]
- Van Der Willik, K.D.; Koppelmans, V.; Hauptmann, M.; Compter, A.; Ikram, M.A.; Schagen, S.B. Inflammation markers and cognitive performance in breast cancer survivors 20 years after completion of chemotherapy: A cohort study. Breast Cancer Res. 2018, 20, 135. [Google Scholar] [CrossRef] [Green Version]
- Bower, J.E.; Ganz, P.A.; Irwin, M.R.; Kwan, L.; Breen, E.C.; Cole, S.W. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? J. Clin. Oncol. 2011, 29, 3517–3522. [Google Scholar] [CrossRef] [Green Version]
- Tromp, J.; Boerman, L.M.; Sama, I.E.; Maass, S.W.M.C.; Maduro, J.H.; Hummel, Y.M.; Berger, M.Y.; de Bock, G.H.; Gietema, J.A.; Berendsen, A.J.; et al. Long-term survivors of early breast cancer treated with chemotherapy are characterized by a pro-inflammatory biomarker profile compared to matched controls. Eur. J. Heart Fail. 2020, 22, 1239–1246. [Google Scholar] [CrossRef] [Green Version]
- Westlake, S.L.; Colebatch, A.N.; Baird, J.; Kiely, P.; Quinn, M.; Choy, E.; Ostor, A.J.K.; Edwards, C.J. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: A systematic literature review. Rheumatology 2010, 49, 295–307. [Google Scholar] [CrossRef] [Green Version]
- Reiner, Ž.; Sirtori, C.R.; Banach, M.; Ruscica, M.; Sahebkar, A. Methotrexate for Cardiovascular Risk Reduction: The Right Choice? Angiology 2019, 71, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Howard, C.P.; Walter, V.; Everett, B.; Libby, P.; Hensen, J.; Thuren, T. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: A phase IIb randomized, placebo-controlled trial. Circulation 2012, 126, 2739–2748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikomeye, J.C.; Rublee, C.S.; Beyer, K.M.M. Positive Externalities of Climate Change Mitigation and Adaptation for Human Health: A Review and Conceptual Framework for Public Health Research. Int. J. Environ. Res. Public Health 2021, 18, 2481. [Google Scholar] [CrossRef]
- US Environmental Protection Agency What Is Open Space/Green Space?|Urban Environmental Program in New England. Available online: https://www3.epa.gov/region1/eco/uep/openspace.html (accessed on 25 February 2021).
- Gianfredi, V.; Buffoli, M.; Rebecchi, A.; Croci, R.; Oradini-Alacreu, A.; Stirparo, G.; Marino, A.; Odone, A.; Capolongo, S.; Signorelli, C. Association between Urban Greenspace and Health: A Systematic Review of Literature. Int. J. Environ. Res. Public Health 2021, 18, 5137. [Google Scholar] [CrossRef]
- Twohig-Bennett, C.; Jones, A. The health benefits of the great outdoors: A systematic review and meta-analysis of greenspace exposure and health outcomes. Environ. Res. 2018, 166, 628–637. [Google Scholar] [CrossRef]
- Rigolon, A.; Browning, M.H.E.M.; McAnirlin, O.; Yoon, H.V. Green Space and Health Equity: A Systematic Review on the Potential of Green Space to Reduce Health Disparities. Int. J. Environ. Res. Public Health 2021, 18, 2563. [Google Scholar] [CrossRef]
- Houlden, V.; Weich, S.; Porto de Albuquerque, J.; Jarvis, S.; Rees, K. The relationship between greenspace and the mental wellbeing of adults: A systematic review. PLoS ONE 2018, 13, e0203000. [Google Scholar] [CrossRef] [Green Version]
- Bikomeye, J.; Balza, J.; Beyer, K. The Impact of Schoolyard Greening on Children’s Physical Activity and Socioemotional Health: A Systematic Review of Experimental Studies. Int. J. Environ. Res. Public Health 2021, 18, 535. [Google Scholar] [CrossRef]
- Beyer, K.M.M.; Kaltenbach, A.; Szabo, A.; Bogar, S.; Nieto, F.J.; Malecki, K.M. Exposure to neighborhood green space and mental health: Evidence from the survey of the health of Wisconsin. Int. J. Environ. Res. Public Health 2014, 11, 3453–3472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadvand, P.; Nieuwenhuijsen, M.J.; Esnaola, M.; Forns, J.; Basagaña, X.; Alvarez-Pedrerol, M.; Rivas, I.; López-Vicente, M.; De Castro Pascual, M.; Su, J.; et al. Green spaces and cognitive development in primary schoolchildren. Proc. Natl. Acad. Sci. USA 2015, 112, 7937–7942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mygind, L.; Kjeldsted, E.; Hartmeyer, R.; Mygind, E.; Stevenson, M.P.; Quintana, D.S.; Bentsen, P. Effects of public green space on acute psychophysiological stress response: A systematic review and meta-analysis of the experimental and quasi-experimental evidence. Environ. Behav. 2021, 53, 184–226. [Google Scholar] [CrossRef]
- Porcherie, M.; Linn, N.; Le Gall, A.R.; Thomas, M.-F.; Faure, E.; Rican, S.; Simos, J.; Cantoreggi, N.; Vaillant, Z.; Cambon, L. Relationship between Urban Green Spaces and Cancer: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 1751. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M. How might contact with nature promote human health? Promising mechanisms and a possible central pathway. Front. Psychol. 2015, 6, 1093. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Kawada, T.; Park, B.J.; et al. Effect of phytoncide from trees on human natural killer cell function. Int. J. Immunopathol. Pharmacol. 2009, 22, 951–959. [Google Scholar] [CrossRef]
- Li, Q.; Otsuka, T.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Li, Y.; Hirata, K.; Shimizu, T. Acute effects of walking in forest environments on cardiovascular and metabolic parameters. Eur. J. Appl. Physiol. 2011, 111, 2845–2853. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [Green Version]
- Yeager, R.; Riggs, D.W.; DeJarnett, N.; Tollerud, D.J.; Wilson, J.; Conklin, D.J.; O’Toole, T.E.; McCracken, J.; Lorkiewicz, P.; Xie, Z.; et al. Association between residential greenness and cardiovascular disease risk. J. Am. Heart Assoc. 2018, 7, e009117. [Google Scholar] [CrossRef]
- Luo, Y.-N.; Huang, W.-Z.; Liu, X.-X.; Markevych, I.; Bloom, M.S.; Zhao, T.; Heinrich, J.; Yang, B.-Y.; Dong, G.-H. Greenspace with overweight and obesity: A systematic review and meta-analysis of epidemiological studies up to 2020. Obes. Rev. 2020, 21, e13078. [Google Scholar] [CrossRef]
- Bhatnagar, A. Environmental Determinants of Cardiovascular Disease. Circ. Res. 2017, 121, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Gascon, M.; Triguero-Mas, M.; Martínez, D.; Dadvand, P.; Rojas-Rueda, D.; Plasència, A.; Nieuwenhuijsen, M.J.; Martinez, D.; Dadvand, P.; Rojas-Rueda, D.; et al. Residential green spaces and mortality: A systematic review. Environ. Int. 2016, 86, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aerts, R.; Nemery, B.; Bauwelinck, M.; Trabelsi, S.; Deboosere, P.; Van Nieuwenhuyse, A.; Nawrot, T.S.; Casas, L. Residential green space, air pollution, socioeconomic deprivation and cardiovascular medication sales in Belgium: A nationwide ecological study. Sci. Total Environ. 2020, 712, 136426. [Google Scholar] [CrossRef] [PubMed]
- Leng, H.; Li, S.; Yan, S.; An, X. Exploring the Relationship between Green Space in a Neighbourhood and Cardiovascular Health in the Winter City of China: A Study Using a Health Survey for Harbin. Int. J. Environ. Res. Public Health 2020, 17, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silveira, I.H.; Junger, W.L. Green spaces and mortality due to cardiovascular diseases in the city of Rio de Janeiro. Rev. Saude Publica 2018, 52, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Komar, P.; Bauwelinck, M.; Zijlema, W.; Bartoll, X.; Cirach, M.; Vandenheede, H.; Nieuwenhuijsen, M.; Borrell, C.; Dadvand, P. Greenspace and cardiovascular morbidity: A comparative study in two European cities. Environ. Epidemiol. 2019, 3, 25. [Google Scholar]
- Crouse, D.L.; Pinault, L.; Balram, A.; Hystad, P.; Peters, P.A.; Chen, H.; van Donkelaar, A.; Martin, R.V.; Ménard, R.; Robichaud, A. Urban greenness and mortality in Canada’s largest cities: A national cohort study. Lancet Planet. Health 2017, 1, e289–e297. [Google Scholar] [CrossRef]
- Orioli, R.; Antonucci, C.; Scortichini, M.; Cerza, F.; Marando, F.; Ancona, C.; Manes, F.; Davoli, M.; Michelozzi, P.; Forastiere, F. Exposure to residential greenness as a predictor of cause-specific mortality and stroke incidence in the Rome longitudinal study. Environ. Health Perspect. 2019, 127, 27002. [Google Scholar] [CrossRef] [Green Version]
- Astell-Burt, T.; Feng, X. Urban green space, tree canopy and prevention of cardiometabolic diseases: A multilevel longitudinal study of 46 786 Australians. Int. J. Epidemiol. 2019, 926–933. [Google Scholar] [CrossRef] [Green Version]
- Dalton, A.M.; Jones, A.P. Residential neighbourhood greenspace is associated with reduced risk of cardiovascular disease: A prospective cohort study. PLoS ONE 2020, 15, e0226524. [Google Scholar] [CrossRef] [Green Version]
- Seo, S.; Choi, S.; Kim, K.; Kim, S.M.; Park, S.M. Association between urban green space and the risk of cardiovascular disease: A longitudinal study in seven Korean metropolitan areas. Environ. Int. 2019, 125, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Tamosiunas, A.; Grazuleviciene, R.; Luksiene, D.; Dedele, A.; Reklaitiene, R.; Baceviciene, M.; Vencloviene, J.; Bernotiene, G.; Radisauskas, R.; Malinauskiene, V. Accessibility and use of urban green spaces, and cardiovascular health: Findings from a Kaunas cohort study. Environ. Health 2014, 13, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanki, T.; Siponen, T.; Ojala, A.; Korpela, K.; Pennanen, A.; Tiittanen, P.; Tsunetsugu, Y.; Kagawa, T.; Tyrväinen, L. Acute effects of visits to urban green environments on cardiovascular physiology in women: A field experiment. Environ. Res. 2017, 159, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Donovan, G.H.; Michael, Y.L.; Gatziolis, D.; Prestemon, J.P.; Whitsel, E.A. Is tree loss associated with cardiovascular-disease risk in the Women’s Health Initiative? A natural experiment. Health Place 2015, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Donovan, G.H.; Butry, D.T.; Michael, Y.L.; Prestemon, J.P.; Liebhold, A.M.; Gatziolis, D.; Mao, M.Y. The relationship between trees and human health: Evidence from the spread of the emerald ash borer. Am. J. Prev. Med. 2013, 44, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Tsunetsugu, Y.; Takayama, N.; Park, B.-J.; Li, Q.; Song, C.; Komatsu, M.; Ikei, H.; Tyrväinen, L.; Kagawa, T.; et al. Influence of Forest Therapy on Cardiovascular Relaxation in Young Adults. Evid.-Based Complement. Altern. Med. 2014, 2014, 834360. [Google Scholar] [CrossRef]
- Jia, B.B.; Yang, Z.X.; Mao, G.X.; Lyu, Y.D.; Wen, X.L.; Xu, W.H.; Lyu, X.L.; Cao, Y.B.; WANG, G.F. Health Effect of Forest Bathing Trip on Elderly Patients with Chronic Obstructive Pulmonary Disease. Biomed. Environ. Sci. 2016, 29, 212–218. [Google Scholar] [CrossRef]
- Miyazaki, Y. Shinrin Yoku: The Japanese art of Forest Bathing; Timber Press: Portand, OR, USA, 2018; ISBN 1604698799. [Google Scholar]
- Mao, G.-X.; Cao, Y.-B.; Lan, X.-G.; He, Z.-H.; Chen, Z.-M.; Wang, Y.-Z.; Hu, X.-L.; Lv, Y.-D.; Wang, G.-F.; Yan, J. Therapeutic effect of forest bathing on human hypertension in the elderly. J. Cardiol. 2012, 60, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Im, S.G.; Choi, H.; Jeon, Y.-H.; Song, M.-K.; Kim, W.; Woo, J.-M. Comparison of effect of two-hour exposure to forest and urban environments on cytokine, anti-oxidant, and stress levels in young adults. Int. J. Environ. Res. Public Health 2016, 13, 625. [Google Scholar] [CrossRef] [Green Version]
- Mao, G.X.; Lan, X.G.; Cao, Y.B.; Chen, Z.M.; He, Z.H.; Lv, Y.D.; Wang, Y.Z.; Hu, X.L.; Wang, G.F.; Yan, J. Effects of Short-Term Forest Bathing on Human Health in a Broad-Leaved Evergreen Forest in Zhejiang Province, China. Biomed. Environ. Sci. 2012, 25, 317–324. [Google Scholar] [CrossRef]
- Antonelli, M.; Barbieri, G.; Donelli, D. Effects of forest bathing (shinrin-yoku) on levels of cortisol as a stress biomarker: A systematic review and meta-analysis. Int. J. Biometeorol. 2019, 63, 1117–1134. [Google Scholar] [CrossRef]
- Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Li, Y.J.; Wakayama, Y. A forest bathing trip increases human natural killer activity and expression of anti-cancer proteins in female subjects. J. Biol. Regul. Homeost Agents 2008, 22, 45–55. [Google Scholar] [PubMed]
- Li, Q. Effect of forest bathing trips on human immune function. Environ. Health Prev. Med. 2010, 15, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Suzuki, H.; Li, Y.J.; Wakayama, Y. Visiting a forest, but not a city, increases human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 2008, 21, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Demark-Wahnefried, W.; Cases, M.G.; Cantor, A.B.; Fruge, A.D.; Smith, K.P.; Locher, J.; Cohen, H.J.; Tsuruta, Y.; Daniel, M.; Kala, R.; et al. Pilot Randomized Controlled Trial of a Home Vegetable Gardening Intervention among Older Cancer Survivors Shows Feasibility, Satisfaction, and Promise in Improving Vegetable and Fruit Consumption, Reassurance of Worth, and the Trajectory of Central Adipos. J. Acad. Nutr. Diet. 2018, 118, 689–704. [Google Scholar] [CrossRef]
- Wu, Q.; Ye, B.; Lv, X.; Mao, G.; Wang, S.; Chen, Z.; Wang, G. Adjunctive therapeutic effects of cinnamomum camphora forest environment on elderly patients with hypertension. Int. J. Gerontol. 2020, 14, 327–331. [Google Scholar]
- Egorov, A.I.; Griffin, S.M.; Converse, R.R.; Styles, J.N.; Sams, E.A.; Wilson, A.; Jackson, L.E.; Wade, T.J. Vegetated land cover near residence is associated with reduced allostatic load and improved biomarkers of neuroendocrine, metabolic and immune functions. Environ. Res. 2017, 158, 508–521. [Google Scholar] [CrossRef]
- Mao, G.; Cao, Y.; Wang, B.; Wang, S.; Chen, Z.; Wang, J.; Xing, W.; Ren, X.; Lv, X.; Dong, J.; et al. The Salutary Influence of Forest Bathing on Elderly Patients with Chronic Heart Failure. Int. J. Environ. Res. Public Health Electron. Resour. 2017, 14, 368. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Kobayashi, M.; Kumeda, S.; Ochiai, T.; Miura, T.; Kagawa, T.; Imai, M.; Wang, Z.; Otsuka, T.; Kawada, T. Effects of Forest Bathing on Cardiovascular and Metabolic Parameters in Middle-Aged Males. Evid.-Based Complement. Altern. Med. eCAM 2016, 2016, 2587381. [Google Scholar] [CrossRef]
- Ochiai, H.; Ikei, H.; Song, C.; Kobayashi, M.; Takamatsu, A.; Miura, T.; Kagawa, T.; Li, Q.; Kumeda, S.; Imai, M.; et al. Physiological and psychological effects of forest therapy on middle-aged males with high-normal blood pressure. Int. J. Environ. Res. Public Health Electron. Resour. 2015, 12, 2532–2542. [Google Scholar] [CrossRef] [Green Version]
- Grazuleviciene, R.; Vencloviene, J.; Kubilius, R.; Grizas, V.; Danileviciute, A.; Dedele, A.; Andrusaityte, S.; Vitkauskiene, A.; Steponaviciute, R.; Nieuwenhuijsen, M.J. Tracking Restoration of Park and Urban Street Settings in Coronary Artery Disease Patients. Int. J. Environ. Res. Public Health Electron. Resour. 2016, 13, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.A.; Lee, A.Y.; Park, H.G.; Son, K.C.; Kim, D.S.; Lee, W.L. Gardening intervention as a low- to moderate-intensity physical activity for improving blood lipid profiles, blood pressure, inflammation, and oxidative stress in women over the age of 70: A pilot study. HortScience 2017, 52, 200–205. [Google Scholar] [CrossRef] [Green Version]
- Hansen, M.M.; Jones, R.; Tocchini, K. Shinrin-Yoku (Forest Bathing) and Nature Therapy: A State-of-the-Art Review. Int. J. Environ. Res. Public Health 2017, 14, 851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midouhas, E.; Kokosi, T.; Flouri, E. Neighbourhood-level air pollution and greenspace and inflammation in adults. Heal. Place 2019, 58, 102167. [Google Scholar] [CrossRef]
- Zupancic, T.; Westmacott, C.; Bulthuis, M. The Impact of Green Space on Heat and Air Pollution in Urban Communities: A Meta-narrative Systematic Review; David Suzuki Foundation: Vancouver, BC Canada, 2015. [Google Scholar]
- Mensah, G.A.; Dunbar, S.B. A framework for addressing disparities in cardiovascular health. J. Cardiovasc. Nurs. 2006, 21, 451–456. [Google Scholar] [CrossRef] [Green Version]
- National Park Trust Kids to Parks Day. Available online: https://parktrust.org/kids-to-parks-day/ (accessed on 7 February 2022).
- Litleskare, S.; MacIntyre, T.E.; Calogiuri, G. Enable, reconnect and augment: A new era of virtual nature research and application. Int. J. Environ. Res. Public Health 2020, 17, 1738. [Google Scholar] [CrossRef] [Green Version]
- Larson, L.R.; Szczytko, R.; Bowers, E.P.; Stephens, L.E.; Stevenson, K.T.; Floyd, M.F. Outdoor time, screen time, and connection to nature: Troubling trends among rural youth? Environ. Behav. 2019, 51, 966–991. [Google Scholar] [CrossRef]
- Battisto, D.; Vincent, E.; Dye, C.J. 5-Technological Supports to Increase Nature Contact for Older Adults; Pak, R., McLaughlin Technology and Health, Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 113–133. ISBN 978-0-12-811272-4. [Google Scholar]
- Levi, D.; Kocher, S. Virtual nature: The future effects of information technology on our relationship to nature. Environ. Behav. 1999, 31, 203–226. [Google Scholar] [CrossRef]
- Calogiuri, G.; Litleskare, S.; MacIntyre, T.E. Future-thinking through technological nature. Phys. Act. Nat. Settings Green Blue Exerc. 2019, 256. [Google Scholar]
- Browning, M.H.E.M.; Mimnaugh, K.J.; van Riper, C.J.; Laurent, H.K.; LaValle, S.M. Can simulated nature support mental health? Comparing short, single-doses of 360-degree nature videos in virtual reality with the outdoors. Front. Psychol. 2019, 10, 2667. [Google Scholar] [CrossRef] [Green Version]
- Valtchanov, D.; Barton, K.R.; Ellard, C. Restorative effects of virtual nature settings. Cyberpsychol. Behav. Soc. Netw. 2010, 13, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, A.; Papastavrou, E.; Avraamides, M.N.; Charalambous, A. Virtual Reality and Symptoms Management of Anxiety, Depression, Fatigue, and Pain: A Systematic Review. SAGE Open Nurs. 2020, 6, 2377960820936163. [Google Scholar] [CrossRef] [PubMed]
- Vincent, E.A. Therapeutic Benefits of Nature Images on Health, Clemson University. 2009. Available online: https://tigerprints.clemson.edu/cgi/viewcontent.cgi?referer=&httpsredir=1&article=1432&context=all_dissertations (accessed on 7 January 2022).
- Kjellgren, A.; Buhrkall, H. A comparison of the restorative effect of a natural environment with that of a simulated natural environment. J. Environ. Psychol. 2010, 30, 464–472. [Google Scholar] [CrossRef]
- Chirico, A.; Lucidi, F.; De Laurentiis, M.; Milanese, C.; Napoli, A.; Giordano, A. Virtual reality in health system: Beyond entertainment. a mini-review on the efficacy of VR during cancer treatment. J. Cell. Physiol. 2016, 231, 275–287. [Google Scholar] [CrossRef] [PubMed]
- White, M.P.; Yeo, N.L.; Vassiljev, P.; Lundstedt, R.; Wallergård, M.; Albin, M.; Lõhmus, M. A prescription for “nature”—The potential of using virtual nature in therapeutics. Neuropsychiatr. Dis. Treat. 2018, 14, 3001–3013. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| No. | Sample Studies | Greenspace Exposure | Measured Biomarkers and Outcomes Observed |
|---|---|---|---|
| 1 | Yeager et al. (2018) [116] | Residential greenness | Reduction in oxidative stress biomarkers:
No significant change in norepinephrine or other catecholamines and monoamines Improvement in circulating angiogenic cell profile |
| 2 | Mao et al. 2012 [138] | Forest bathing | Reduction in pro-inflammatory biomarkers levels:
Reduction in other biomarkers including oxidative stress:
Reduction in stress biomarker:
|
| 3 | Demark-Wahnefried et al. 2018 [143] | Vegetable gardening | Significant decrease in telomerase activity No significant change in cortisol and IL-6 Cancer survivorship implication:
|
| 4 | Wu et al. 2020 [144] | Forest bathing | Reduction in proinflammatory biomarker levels:
|
| 5 | Egorov et al. 2017 [145] | Vegetated land cover near residence | Improvement in all measured biomarkers:
|
| 6 | Antonelli et al. 2019 [139] | Forest bathing | Reduction in stress biomarker:
|
| 7 | Mao et al. 2017 [146] | Forest bathing | Reduction in proinflammatory biomarker levels:
Reduction in other CVD pathological factors including brain natriuretic peptide (BNP), a biomarker of heart failure, and constituents of the renin angiotensin system (RAS):
Improvement in oxidative stress biomarkers:
|
| 8 | Mao et al. 2012 [136] | Forest bathing | Reduction in proinflammatory biomarkers:
No significant change in TNF-α |
| 9 | Li et al. 2016 [147] | Forest bathing | Increased anti-inflammatory biomarkers:
|
| 10 | Ochiai et al. 2015 [148] | Forest therapy | Reduction in stress biomarkers:
|
| 11 | Grazuleviciene et al. 2016 [149] | Green exercise | Reduction in stress biomarkers:
|
| 12 | Park et al. 2017 [150] | Vegetable gardening | Reduction in proinflammatory biomarkers levels:
|
| 13 | Jia et al. 2016 [134] | Forest bathing | Reduction in proinflammatory biomarker levels:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bikomeye, J.C.; Beyer, A.M.; Kwarteng, J.L.; Beyer, K.M.M. Greenspace, Inflammation, Cardiovascular Health, and Cancer: A Review and Conceptual Framework for Greenspace in Cardio-Oncology Research. Int. J. Environ. Res. Public Health 2022, 19, 2426. https://doi.org/10.3390/ijerph19042426
Bikomeye JC, Beyer AM, Kwarteng JL, Beyer KMM. Greenspace, Inflammation, Cardiovascular Health, and Cancer: A Review and Conceptual Framework for Greenspace in Cardio-Oncology Research. International Journal of Environmental Research and Public Health. 2022; 19(4):2426. https://doi.org/10.3390/ijerph19042426
Chicago/Turabian StyleBikomeye, Jean C., Andreas M. Beyer, Jamila L. Kwarteng, and Kirsten M. M. Beyer. 2022. "Greenspace, Inflammation, Cardiovascular Health, and Cancer: A Review and Conceptual Framework for Greenspace in Cardio-Oncology Research" International Journal of Environmental Research and Public Health 19, no. 4: 2426. https://doi.org/10.3390/ijerph19042426
APA StyleBikomeye, J. C., Beyer, A. M., Kwarteng, J. L., & Beyer, K. M. M. (2022). Greenspace, Inflammation, Cardiovascular Health, and Cancer: A Review and Conceptual Framework for Greenspace in Cardio-Oncology Research. International Journal of Environmental Research and Public Health, 19(4), 2426. https://doi.org/10.3390/ijerph19042426









