Importance and Reality of TDM for Antibiotics Not Covered by Insurance in Japan
Abstract
1. Introduction
2. TDM of Ceftriaxone
2.1. Characteristics of Ceftriaxone and Significance of TDM
2.2. Report on the Measurement of Blood Levels and CSF of CTRX in Japan
3. TDM of Daptomycin
3.1. Characteristics of Daptomycin and Significance of Blood Level Measurement
3.2. Report on the Measurement of Blood Levels of Daptomycin in Japan
4. TDM of Linezolid
4.1. Characteristics of Linezolid and Significance of Blood Level Measurement
4.2. Report on the Measurement of Blood Levels of Linezolid in Japan
5. TDM of Tedizolid
5.1. Characteristics of Tedizolid and Significance of Blood Level Measurement
5.2. Report on the Measurement of Blood Levels of Tedizolid in Japan
6. The States of TDM for DAP, LZD, and TZD in Other Countries
7. Limitation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morikawa, G.; Sorimachi, M.; Tamura, K.; Moriiwa, Y.; Shoji, A.; Okazawa, K.; Yanagida, A. Development of a practical HPLC system for in-hospital analysis of blood concentration of various medicines. Bunseki Kagaku 2019, 68, 473–481. [Google Scholar] [CrossRef]
- Saito, T.; Tominaga, A.; Nozawa, M.; Unei, H.; Hatano, Y.; Fujita, Y.; Iseki, K.; Hori, Y. Committee on Toxicology Laboratories; Japanese Society for Clinical Toxicology [Survey of analytical works for drugs at emergency and critical care centers with high-performance instruments provided by the Ministry of Health and Welfare (at present: Ministry of Health, Labour, and Welfare) in fiscal 1998—Continuation of survey with 2008 survey results as point of reference]. Chudoku Kenkyu 2013, 26, 226–233. [Google Scholar] [PubMed]
- Otani, N.; Hifumi, T.; Kitamoto, T.; Kobayashi, K.; Nakaya, N.; Tomioka, J. Current State of Drug Analysis in Japanese Emergency Departments: A Nationwide Survey. Acute Med. Surg. 2020, 7, e566. [Google Scholar] [CrossRef] [PubMed]
- Practice Guidelines for Therapeutic Drug Monitoring of Antimicrobials in 2022. (Executive Summary)-the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. Available online: http://www.chemotherapy.or.jp/guideline/tdm2022.html (accessed on 16 February 2022).
- Klein, N.C.; Cunha, B.A. Third-Generation Cephalosporins. Med. Clin. N. Am. 1995, 79, 705–719. [Google Scholar] [CrossRef]
- Aronoff, G.R.; Bennett, W.M.; Berns, J.S.; Brier, M.E.; Kasbekar, N.; Mueller, B.A.; Pasko, D.A.; Smoyer, W.E. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults and Children, 5th ed.; American College of Physicians: Philadelphia, PA, USA, 2007; p. 153. [Google Scholar]
- Patel, I.H.; Sugihara, J.G.; Weinfeld, R.E.; Wong, E.G.; Siemsen, A.W.; Berman, S.J. Ceftriaxone Pharmacokinetics in Patients with Various Degrees of Renal Impairment. Antimicrob. Agents Chemother. 1984, 25, 438–442. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Darby, R.R.; Raibagkar, P.; Gonzalez Castro, L.N.; Berkowitz, A.L. Antibiotic-Associated Encephalopathy. Neurology 2016, 86, 963–971. [Google Scholar] [CrossRef]
- Lamoth, F.; Buclin, T.; Pascual, A.; Vora, S.; Bolay, S.; Decosterd, L.A.; Calandra, T.; Marchetti, O. High Cefepime Plasma Concentrations and Neurological Toxicity in Febrile Neutropenic Patients with Mild Impairment of Renal Function. Antimicrob. Agents Chemother. 2010, 54, 4360–4367. [Google Scholar] [CrossRef]
- Durand-Maugard, C.; Lemaire-Hurtel, A.-S.; Gras-Champel, V.; Hary, L.; Maizel, J.; Prud’homme-Bernardy, A.; Andréjak, C.; Andréjak, M. Blood and CSF Monitoring of Cefepime-Induced Neurotoxicity: Nine Case Reports. J. Antimicrob. Chemother. 2012, 67, 1297–1299. [Google Scholar] [CrossRef]
- Rhodes, N.J.; Kuti, J.L.; Nicolau, D.P.; Neely, M.N.; Nicasio, A.M.; Scheetz, M.H. An Exploratory Analysis of the Ability of a Cefepime Trough Concentration Greater than 22 Mg/L to Predict Neurotoxicity. J. Infect. Chemother. 2016, 22, 78–83. [Google Scholar] [CrossRef]
- Lacroix, C.; Kheloufi, F.; Montastruc, F.; Bennis, Y.; Pizzoglio, V.; Micallef, J. Serious Central Nervous System Side Effects of Cephalosporins: A National Analysis of Serious Reports Registered in the French Pharmacovigilance Database. J. Neurol. Sci. 2019, 398, 196–201. [Google Scholar] [CrossRef]
- Kim, K.B.; Kim, S.M.; Park, W.; Kim, J.S.; Kwon, S.K.; Kim, H.-Y. Ceftiaxone-Induced Neurotoxicity: Case Report, Pharmacokinetic Considerations, and Literature Review. J. Korean Med. Sci. 2012, 27, 1120–1123. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Hosokawa, N.; Kudo, T.; Goda, H.; Ito, K.; Suzuki, M.; Funakoshi, R. Chorea-like Symptoms and High Blood Concentration of Ceftriaxone in a Patient Undergoing Hemodialysis: A Case Report. J. Infect. Chemother. 2020, 26, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Doi, Y.; Arisato, T.; Sugioka, S.; Koga, K.; Nishioka, K.; Sugawara, A. Three Cases of Hemodialysis Patients Receiving High-Dose Ceftriaxone: Serum Concentrations and Its Neurotoxicity. Kidney Int. Rep. 2017, 2, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, C.; Bera-Jonville, A.-P.; Montastruc, F.; Velly, L.; Micallef, J.; Guilhaumou, R. Serious Neurological Adverse Events of Ceftriaxone. Antibiotics 2021, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- AMR Clinical Reference Center. Surveillance of Antibiotic Sales in Japan. Available online: https://amrcrc.ncgm.go.jp/surveillance/020/salestableDDD2017_2021.4.xlsx (accessed on 18 December 2021).
- Kotani, A.; Hirai, J.; Hamada, Y.; Fujita, J.; Hakamata, H. Determination of Ceftriaxone Concentration in Human Cerebrospinal Fluid by High-Performance Liquid Chromatography with UV Detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1124, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Oda, K. Development of Software for Antimicrobial PK/PD Simulation Incorporating Montecarlo Simulation Based on Microsoft®; Office Excel. Iryo Yakugaku 2011, 37, 335–344. [Google Scholar] [CrossRef][Green Version]
- Suzuki, S.; Naito, S.; Numasawa, Y.; Asada, M.; Shoji, N.; Zeniya, M.; Takahashi, D.; Sato, H.; Iimori, S.; Nomura, N.; et al. Encephalopathy Induced by High Plasma and Cerebrospinal Fluid Ceftriaxone Concentrations in a Hemodialysis Patient. Intern. Med. 2019, 58, 1775–1779. [Google Scholar] [CrossRef]
- Allegra, S.; Cardellino, C.S.; Fatiguso, G.; Cusato, J.; De Nicolò, A.; Avataneo, V.; Bonora, S.; D’Avolio, A.; Di Perri, G.; Calcagno, A. Effect of ABCC2 and ABCG2 Gene Polymorphisms and CSF-to-Serum Albumin Ratio on Ceftriaxone Plasma and Cerebrospinal Fluid Concentrations. J. Clin. Pharmacol. 2018, 58, 1550–1556. [Google Scholar] [CrossRef]
- Le Turnier, P.; Grégoire, M.; Garot, D.; Guimard, T.; Duval, X.; Bernard, L.; Boutoille, D.; Dailly, É.; Navas, D.; Asseray, N. CSF Concentration of Ceftriaxone Following High-Dose Administration: Pharmacological Data from Two French Cohorts. J. Antimicrob. Chemother. 2019, 74, 1753–1755. [Google Scholar] [CrossRef]
- Nau, R.; Prange, H.W.; Muth, P.; Mahr, G.; Menck, S.; Kolenda, H.; Sörgel, F. Passage of Cefotaxime and Ceftriaxone into Cerebrospinal Fluid of Patients with Uninflamed Meninges. Antimicrob. Agents Chemother. 1993, 37, 1518–1524. [Google Scholar] [CrossRef]
- Urakami, T.; Hamada, Y.; Oka, Y.; Okinaka, T.; Yamakuchi, H.; Magarifuchi, H.; Aoki, Y. Clinical Pharmacokinetic and Pharmacodynamic Analysis of Daptomycin and the Necessity of High-Dose Regimen in Japanese Adult Patients. J. Infect. Chemother. 2019, 25, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Ooi, Y.; Oda, K.; Shibata, Y.; Kawanishi, F.; Suzuki, K.; Nishihara, M.; Nakano, T.; Yoshida, M.; Uchida, T.; et al. Observational Study to Determine the Optimal Dose of Daptomycin Based on Pharmacokinetic/Pharmacodynamic Analysis. J. Infect. Chemother. 2020, 26, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Miyadera, Y.; Naito, T.; Yamada, T.; Kawakami, J. Simple LC-MS/MS Methods Using Core-Shell Octadecylsilyl Microparticulate for the Quantitation of Total and Free Daptomycin in Human Plasma. Ther. Drug Monit. 2018, 40, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Suzuki, Y.; Goto, K.; Yasuda, N.; Koga, H.; Kai, S.; Ohchi, Y.; Sato, Y.; Kitano, T.; Itoh, H. Development and Validation of Sensitive and Selective Quantification of Total and Free Daptomycin in Human Plasma Using Ultra-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2019, 165, 56–64. [Google Scholar] [CrossRef]
- Ando, M.; Nishioka, H.; Nakasako, S.; Kuramoto, E.; Ikemura, M.; Kamei, H.; Sono, Y.; Sugioka, N.; Fukushima, S.; Hashida, T. Observational Retrospective Single-Centre Study in Japan to Assess the Clinical Significance of Serum Daptomycin Levels in Creatinine Phosphokinase Elevation. J. Clin. Pharm. Ther. 2020, 45, 290–297. [Google Scholar] [CrossRef]
- Tsuji, Y.; Tashiro, M.; Ashizawa, N.; Ota, Y.; Obi, H.; Nagura, S.; Narukawa, M.; Fukahara, K.; Yoshimura, N.; To, H.; et al. Treatment of Mediastinitis Due to Methicillin-Resistant Staphylococcus Aureus in a Renal Dysfunction Patient Undergoing Adjustments to the Linezolid Dose. Intern. Med. 2015, 54, 235–239. [Google Scholar] [CrossRef]
- Matsuda, S.; Kimura, R.; Izumi, K.; Noguchi, A.; Imazu, T.; Kugaya, Y.; Shimokawa, F.; Maeda, Y. Two Cases for Prevention of Linezolid-Induced Thrombocytopenia by Therapeutic Drug Monitoring. J. Jpn. Soc. Hosp. Pharm. 2019, 55, 423–427. [Google Scholar]
- Ashizawa, N.; Tsuji, Y.; Kawago, K.; Higashi, Y.; Tashiro, M.; Nogami, M.; Gejo, R.; Narukawa, M.; Kimura, T.; Yamamoto, Y. Successful Treatment of Methicillin-Resistant Staphylococcus Aureus Osteomyelitis with Combination Therapy Using Linezolid and Rifampicin under Therapeutic Drug Monitoring. J. Infect. Chemother. 2016, 22, 331–334. [Google Scholar] [CrossRef]
- Tanaka, R.; Kai, M.; Goto, K.; Ohchi, Y.; Yasuda, N.; Tatsuta, R.; Kitano, T.; Itoh, H. High-Throughput and Wide-Range Simultaneous Determination of Linezolid, Daptomycin and Tedizolid in Human Plasma Using Ultra-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2021, 194, 113764. [Google Scholar] [CrossRef]
- Kai, M.; Tanaka, R.; Suzuki, Y.; Goto, K.; Ohchi, Y.; Yasuda, N.; Tatsuta, R.; Kitano, T.; Itoh, H. Simultaneous Quantification of Plasma Levels of 12 Antimicrobial Agents Including Carbapenem, Anti-Methicillin-Resistant Staphylococcus Aureus Agent, Quinolone and Azole Used in Intensive Care Unit Using UHPLC-MS/MS Method. Clin. Biochem. 2021, 90, 40–49. [Google Scholar] [CrossRef]
- Wootton, M.; MacGowan, A.P.; Walsh, T.R. Comparative Bactericidal Activities of Daptomycin and Vancomycin against Glycopeptide-Intermediate Staphylococcus Aureus (GISA) and Heterogeneous GISA Isolates. Antimicrob. Agents Chemother. 2006, 50, 4195–4197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dandekar, P.K.; Tessier, P.R.; Williams, P.; Nightingale, C.H.; Nicolau, D.P. Pharmacodynamic Profile of Daptomycin against Enterococcus Species and Methicillin-Resistant Staphylococcus Aureus in a Murine Thigh Infection Model. J. Antimicrob. Chemother. 2003, 52, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Dvorchik, B.H.; Brazier, D.; DeBruin, M.F.; Arbeit, R.D. Daptomycin Pharmacokinetics and Safety Following Administration of Escalating Doses Once Daily to Healthy Subjects. Antimicrob. Agents Chemother. 2003, 47, 1318–1323. [Google Scholar] [CrossRef]
- Cubist Pharmaceuticals. Cubicin Package Insert; Cubist Pharmaceuticals: Lexington, MA, USA, 2003. [Google Scholar]
- Hawkey, P.M. Pre-Clinical Experience with Daptomycin. J. Antimicrob. Chemother. 2008, 62 (Suppl. 3), iii7–iii14. [Google Scholar] [CrossRef] [PubMed]
- Safdar, N.; Andes, D.; Craig, W.A. In Vivo Pharmacodynamic Activity of Daptomycin. Antimicrob. Agents Chemother. 2004, 48, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Russo, A.; Cassetta, M.I.; Lappa, A.; Tritapepe, L.; d’Ettorre, G.; Fallani, S.; Novelli, A.; Venditti, M. Variability of Pharmacokinetic Parameters in Patients Receiving Different Dosages of Daptomycin: Is Therapeutic Drug Monitoring Necessary? J. Infect. Chemother. 2013, 19, 732–739. [Google Scholar] [CrossRef]
- Galar, A.; Muñoz, P.; Valerio, M.; Cercenado, E.; García-González, X.; Burillo, A.; Sánchez-Somolinos, M.; Juárez, M.; Verde, E.; Bouza, E. Current Use of Daptomycin and Systematic Therapeutic Drug Monitoring: Clinical Experience in a Tertiary Care Institution. Int. J. Antimicrob. Agents 2019, 53, 40–48. [Google Scholar] [CrossRef]
- Bhavnani, S.M.; Rubino, C.M.; Ambrose, P.G.; Drusano, G.L. Daptomycin Exposure and the Probability of Elevations in the Creatine Phosphokinase Level: Data from a Randomized Trial of Patients with Bacteremia and Endocarditis. Clin. Infect. Dis. 2010, 50, 1568–1574. [Google Scholar] [CrossRef]
- Kazory, A.; Dibadj, K.; Weiner, I.D. Rhabdomyolysis and Acute Renal Failure in a Patient Treated with Daptomycin. J. Antimicrob. Chemother. 2006, 57, 578–579. [Google Scholar] [CrossRef]
- Cojutti, P.G.; Candoni, A.; Ramos-Martin, V.; Lazzarotto, D.; Zannier, M.E.; Fanin, R.; Hope, W.; Pea, F. Population Pharmacokinetics and Dosing Considerations for the Use of Daptomycin in Adult Patients with Haematological Malignancies. J. Antimicrob. Chemother. 2017, 72, 2342–2350. [Google Scholar] [CrossRef]
- Carugati, M.; Bayer, A.S.; Miró, J.M.; Park, L.P.; Guimarães, A.C.; Skoutelis, A.; Fortes, C.Q.; Durante-Mangoni, E.; Hannan, M.M.; Nacinovich, F.; et al. High-Dose Daptomycin Therapy for Left-Sided Infective Endocarditis: A Prospective Study from the International Collaboration on Endocarditis. Antimicrob. Agents Chemother. 2013, 57, 6213–6222. [Google Scholar] [CrossRef] [PubMed]
- Seaton, R.A.; Menichetti, F.; Dalekos, G.; Beiras-Fernandez, A.; Nacinovich, F.; Pathan, R.; Hamed, K. Evaluation of Effectiveness and Safety of High-Dose Daptomycin: Results from Patients Included in the European Cubicin(®) Outcomes Registry and Experience. Adv. Ther. 2015, 32, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Reiber, C.; Senn, O.; Müller, D.; Kullak-Ublick, G.A.; Corti, N. Therapeutic Drug Monitoring of Daptomycin: A Retrospective Monocentric Analysis. Ther. Drug Monit. 2015, 37, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, A.; Tascini, C.; Polillo, M.; Gemignani, G.; Nielsen, E.I.; Bocci, G.; Karlsson, M.O.; Menichetti, F.; Danesi, R. Population Pharmacokinetics of Daptomycin in Patients Affected by Severe Gram-Positive Infections. Int. J. Antimicrob. Agents 2013, 42, 250–255. [Google Scholar] [CrossRef]
- Goutelle, S.; Roux, S.; Gagnieu, M.-C.; Valour, F.; Lustig, S.; Ader, F.; Laurent, F.; Chidiac, C.; Ferry, T. Pharmacokinetic Variability of Daptomycin during Prolonged Therapy for Bone and Joint Infections. Antimicrob. Agents Chemother. 2016, 60, 3148–3151. [Google Scholar] [CrossRef]
- Barreau, S.; Benaboud, S.; Kernéis, S.; Moachon, L.; Blanche, P.; Groh, M.; Massias, L.; Treluyer, J.-M.; Poyart, C.; Raymond, J. Staphylococcus Aureus Osteo-Articular Infection: Usefulness of the Determination of Daptomycin Serum Concentration to Explain a Treatment Failure. Int. J. Clin. Pharmacol. Ther. 2016, 54, 923–927. [Google Scholar] [CrossRef]
- Bozdogan, B.; Appelbaum, P.C. Oxazolidinones: Activity, Mode of Action, and Mechanism of Resistance. Int. J. Antimicrob. Agents 2004, 23, 113–119. [Google Scholar] [CrossRef]
- Mendes, R.E.; Deshpande, L.M.; Jones, R.N. Linezolid Update: Stable in Vitro Activity Following More than a Decade of Clinical Use and Summary of Associated Resistance Mechanisms. Drug Resist. Updates 2014, 17, 1–12. [Google Scholar] [CrossRef]
- Bassetti, M.; Baguneid, M.; Bouza, E.; Dryden, M.; Nathwani, D.; Wilcox, M. European Perspective and Update on the Management of Complicated Skin and Soft Tissue Infections Due to Methicillin-Resistant Staphylococcus Aureus after More than 10 Years of Experience with Linezolid. Clin. Microbiol. Infect. 2014, 20 (Suppl. 4), 3–18. [Google Scholar] [CrossRef]
- Gerson, S.L.; Kaplan, S.L.; Bruss, J.B.; Le, V.; Arellano, F.M.; Hafkin, B.; Kuter, D.J. Hematologic Effects of Linezolid: Summary of Clinical Experience. Antimicrob. Agents Chemother. 2002, 46, 2723–2726. [Google Scholar] [CrossRef]
- Brier, M.E.; Stalker, D.J.; Aronoff, G.R.; Batts, D.H.; Ryan, K.K.; O’Grady, M.; Hopkins, N.K.; Jungbluth, G.L. Pharmacokinetics of Linezolid in Subjects with Renal Dysfunction. Antimicrob. Agents Chemother. 2003, 47, 2775–2780. [Google Scholar] [CrossRef]
- Cattaneo, D.; Gervasoni, C.; Cozzi, V.; Castoldi, S.; Baldelli, S.; Clementi, E. Therapeutic Drug Management of Linezolid: A Missed Opportunity for Clinicians? Int. J. Antimicrob. Agents 2016, 48, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Rayner, C.R.; Forrest, A.; Meagher, A.K.; Birmingham, M.C.; Schentag, J.J. Clinical Pharmacodynamics of Linezolid in Seriously Ill Patients Treated in a Compassionate Use Programme. Clin. Pharmacokinet. 2003, 42, 1411–1423. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef] [PubMed]
- Pea, F.; Viale, P.; Cojutti, P.; Del Pin, B.; Zamparini, E.; Furlanut, M. Therapeutic Drug Monitoring May Improve Safety Outcomes of Long-Term Treatment with Linezolid in Adult Patients. J. Antimicrob. Chemother. 2012, 67, 2034–2042. [Google Scholar] [CrossRef]
- Matsumoto, K.; Takeshita, A.; Ikawa, K.; Shigemi, A.; Yaji, K.; Shimodozono, Y.; Morikawa, N.; Takeda, Y.; Yamada, K. Higher Linezolid Exposure and Higher Frequency of Thrombocytopenia in Patients with Renal Dysfunction. Int. J. Antimicrob. Agents 2010, 36, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Nukui, Y.; Hatakeyama, S.; Okamoto, K.; Yamamoto, T.; Hisaka, A.; Suzuki, H.; Yata, N.; Yotsuyanagi, H.; Moriya, K. High Plasma Linezolid Concentration and Impaired Renal Function Affect Development of Linezolid-Induced Thrombocytopenia. J. Antimicrob. Chemother. 2013, 68, 2128–2133. [Google Scholar] [CrossRef]
- Tsuji, Y.; Holford, N.H.G.; Kasai, H.; Ogami, C.; Heo, Y.-A.; Higashi, Y.; Mizoguchi, A.; To, H.; Yamamoto, Y. Population Pharmacokinetics and Pharmacodynamics of Linezolid-Induced Thrombocytopenia in Hospitalized Patients. Br. J. Clin. Pharmacol. 2017, 83, 1758–1772. [Google Scholar] [CrossRef]
- Im, W.B.; Choi, S.H.; Park, J.-Y.; Choi, S.H.; Finn, J.; Yoon, S.-H. Discovery of Torezolid as a Novel 5-Hydroxymethyl-Oxazolidinone Antibacterial Agent. Eur. J. Med. Chem. 2011, 46, 1027–1039. [Google Scholar] [CrossRef]
- Shaw, K.J.; Poppe, S.; Schaadt, R.; Brown-Driver, V.; Finn, J.; Pillar, C.M.; Shinabarger, D.; Zurenko, G. In Vitro Activity of TR-700, the Antibacterial Moiety of the Prodrug TR-701, against Linezolid-Resistant Strains. Antimicrob. Agents Chemother. 2008, 52, 4442–4447. [Google Scholar] [CrossRef]
- Prokocimer, P.; Bien, P.; Surber, J.; Mehra, P.; DeAnda, C.; Bulitta, J.B.; Corey, G.R. Phase 2, Randomized, Double-Blind, Dose-Ranging Study Evaluating the Safety, Tolerability, Population Pharmacokinetics, and Efficacy of Oral Torezolid Phosphate in Patients with Complicated Skin and Skin Structure Infections. Antimicrob. Agents Chemother. 2011, 55, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Schaadt, R.; Sweeney, D.; Shinabarger, D.; Zurenko, G. In Vitro Activity of TR-700, the Active Ingredient of the Antibacterial Prodrug TR-701, a Novel Oxazolidinone Antibacterial Agent. Antimicrob. Agents Chemother. 2009, 53, 3236–3239. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.J.; Barbachyn, M.R. The Oxazolidinones: Past, Present, and Future: The Oxazolidinones: Past, Present, and Future. Ann. N. Y. Acad. Sci. 2011, 1241, 48–70. [Google Scholar] [CrossRef] [PubMed]
- Grau, S.; Ferrández, O.; Urbina, O.; Espona, M.; Salas, E.; Ferrández, I. Potential Role of Tedizolid Phosphate in the Treatment of Acute Bacterial Skin Infections. Drug Des. Dev. Ther. 2013, 7, 243. [Google Scholar] [CrossRef]
- Flanagan, S.; Fang, E.; Muñoz, K.A.; Minassian, S.L.; Prokocimer, P.G. Single- and Multiple-Dose Pharmacokinetics and Absolute Bioavailability of Tedizolid. Pharmacotherapy 2014, 34, 891–900. [Google Scholar] [CrossRef]
- Housman, S.T.; Pope, J.S.; Russomanno, J.; Salerno, E.; Shore, E.; Kuti, J.L.; Nicolau, D.P. Pulmonary Disposition of Tedizolid Following Administration of Once-Daily Oral 200-Milligram Tedizolid Phosphate in Healthy Adult Volunteers. Antimicrob. Agents Chemother. 2012, 56, 2627–2634. [Google Scholar] [CrossRef]
- Sahre, M.; Sabarinath, S.; Grant, M.; Seubert, C.; DeAnda, C.; Prokocimer, P.; Derendorf, H. Skin and Soft Tissue Concentrations of Tedizolid (Formerly Torezolid), a Novel Oxazolidinone, Following a Single Oral Dose in Healthy Volunteers. Int. J. Antimicrob. Agents 2012, 40, 51–54. [Google Scholar] [CrossRef]
- Flanagan, S.D.; Bien, P.A.; Muñoz, K.A.; Minassian, S.L.; Prokocimer, P.G. Pharmacokinetics of Tedizolid Following Oral Administration: Single and Multiple Dose, Effect of Food, and Comparison of Two Solid Forms of the Prodrug. Pharmacotherapy 2014, 34, 240–250. [Google Scholar] [CrossRef]
- Roger, C.; Roberts, J.A.; Muller, L. Clinical Pharmacokinetics and Pharmacodynamics of Oxazolidinones. Clin. Pharm. 2018, 57, 559–575. [Google Scholar] [CrossRef]
- Flanagan, S.; Passarell, J.; Lu, Q.; Fiedler-Kelly, J.; Ludwig, E.; Prokocimer, P. Tedizolid Population Pharmacokinetics, Exposure Response, and Target Attainment. Antimicrob. Agents Chemother. 2014, 58, 6462–6470. [Google Scholar] [CrossRef]
- Flanagan, S.; Prokocimer, P. Reduction in Tedizolid Plasma Exposure among End-Stage Renal Disease Patients Undergoing Dialysis Is Explained by Variations in Ideal Body Weight. Antimicrob. Agents Chemother. 2016, 60, 3246–3247. [Google Scholar] [CrossRef]
- Lodise, T.P.; Bidell, M.R.; Flanagan, S.D.; Zasowski, E.J.; Minassian, S.L.; Prokocimer, P. Characterization of the Haematological Profile of 21 Days of Tedizolid in Healthy Subjects. J. Antimicrob. Chemother. 2016, 71, 2553–2558. [Google Scholar] [CrossRef] [PubMed]
- Ong, V.; Flanagan, S.; Fang, E.; Dreskin, H.J.; Locke, J.B.; Bartizal, K.; Prokocimer, P. Absorption, Distribution, Metabolism, and Excretion of the Novel Antibacterial Prodrug Tedizolid Phosphate. Drug Metab. Dispos. 2014, 42, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.S.; Flanagan, S.D.; Arrieta, A.C.; Jacobs, R.; Capparelli, E.; Prokocimer, P. Pharmacokinetics, Safety and Tolerability of Single Oral or Intravenous Administration of 200 Mg Tedizolid Phosphate in Adolescents. Pediatric Infect. Dis. J. 2016, 35, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Pan, C.; Xie, Q.; Zheng, Y.; Hu, Y.; Lin, Y. Simultaneous Determination of Tedizolid and Linezolid in Rat Plasma by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry and Its Application to a Pharmacokinetic Study. J. Chromatogr. B 2016, 1011, 94–98. [Google Scholar] [CrossRef]
- Deshpande, D.; Srivastava, S.; Pasipanodya, J.G.; Lee, P.S.; Gumbo, T. Tedizolid Is Highly Bactericidal in the Treatment of Pulmonary Mycobacterium Avium Complex Disease. J. Antimicrob. Chemother. 2017, 72, i30–i35. [Google Scholar] [CrossRef]
- Park, A.; Young, J.; Wang, J.; Jayne, J.; Fukushima, L.; Rao, A.P.; D’Argenio, D.Z.; Beringer, P.M. Pharmacokinetics of Tedizolid in Plasma and Sputum of Adults with Cystic Fibrosis. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Stainton, S.M.; Monogue, M.L.; Baummer-Carr, A.; Shepard, A.K.; Nugent, J.F.; Kuti, J.L.; Nicolau, D.P. Comparative Assessment of Tedizolid Pharmacokinetics and Tissue Penetration between Diabetic Patients with Wound Infections and Healthy Volunteers via In Vivo Microdialysis. Antimicrob. Agents Chemother. 2018, 62, e01880-17. [Google Scholar] [CrossRef]
- Dorn, C.; Schießer, S.; Wulkersdorfer, B.; Hitzenbichler, F.; Kees, M.G.; Zeitlinger, M. Determination of Free Clindamycin, Flucloxacillin or Tedizolid in Plasma: Pay Attention to Physiological Conditions When Using Ultrafiltration. Biomed. Chromatogr. 2020, 34, e4820. [Google Scholar] [CrossRef]
- Tsuji, Y.; Numajiri, M.; Ogami, C.; Kurosaki, F.; Miyamoto, A.; Aoyama, T.; Kawasuji, H.; Nagaoka, K.; Matsumoto, Y.; To, H.; et al. Development of a Simple Method for Measuring Tedizolid Concentration in Human Serum Using HPLC with a Fluorescent Detector. Medicine 2021, 100, e28127. [Google Scholar] [CrossRef]
- Sandaradura, I.; Alffenaar, J.-W.; Cotta, M.O.; Daveson, K.; Day, R.O.; Van Hal, S.; Lau, C.; Marriott, D.J.E.; Penm, J.; Roberts, J.A.; et al. Emerging Therapeutic Drug Monitoring of Anti-Infective Agents in Australian Hospitals: Availability, Performance and Barriers to Implementation. Br. J. Clin. Pharm. 2022, 88, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.C.P.; Muller, A.E.; Hunfeld, N.G.M.; de Winter, B.C.M.; Ewoldt, T.M.J.; Abdulla, A.; Endeman, H. Therapeutic Drug Monitoring of Antibiotics in Critically Ill Patients: Current Practice and Future Perspectives With a Focus on Clinical Outcome. Ther. Drug Monit. 2022, 44, 11–18. [Google Scholar] [CrossRef] [PubMed]
| Drug | Characteristics | Objective | Renal Function | Dose | Measurement System | Measurement Accuracy | Blood Concentration | CSF Concentration | Ref. |
|---|---|---|---|---|---|---|---|---|---|
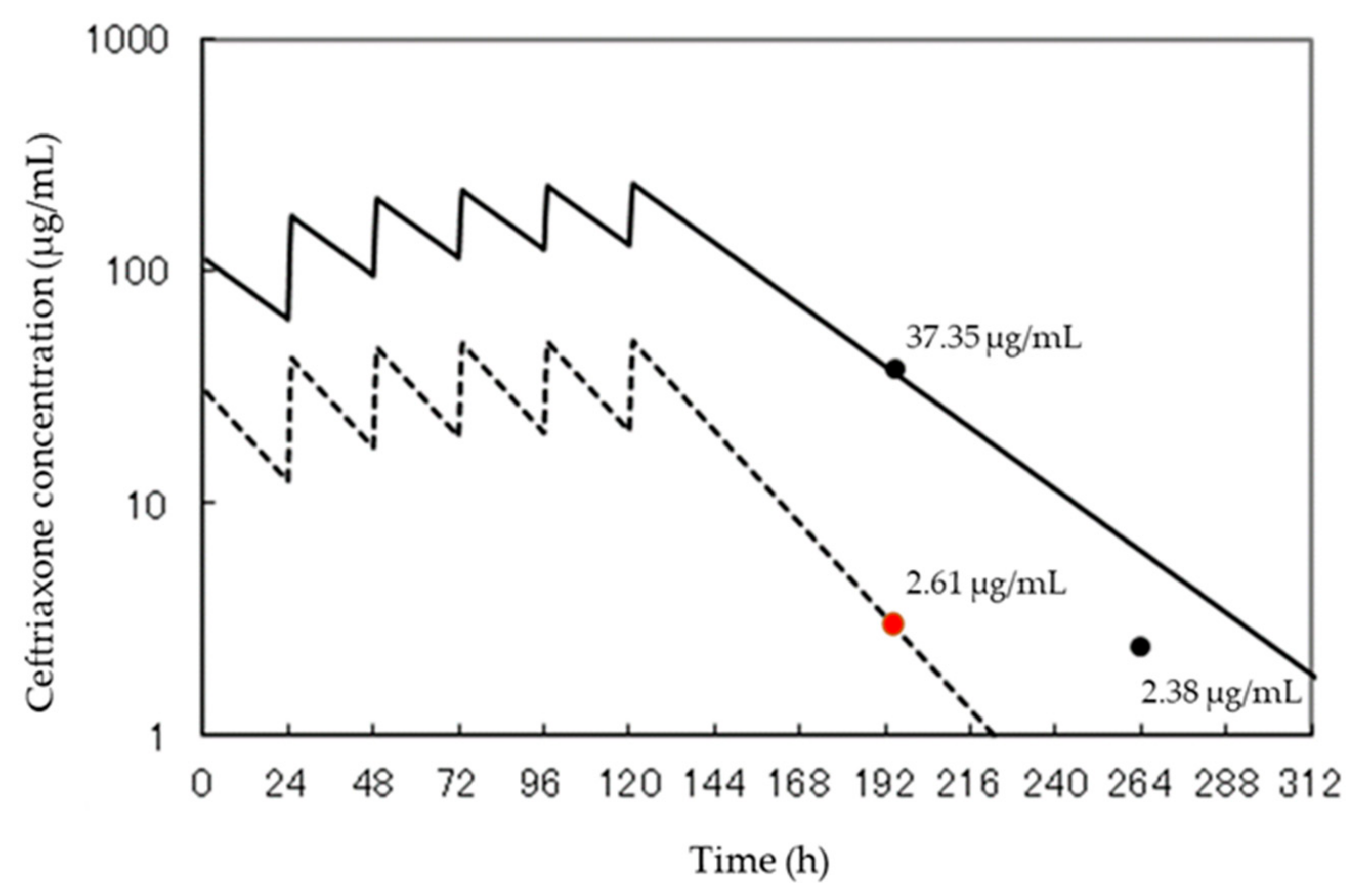
| CTRX | Age: 75 Sex: female | Development of HPLC method for accurate, precise, and selective determination of CTRX and its clinical application | peritoneal dialysis | 2 g/day | HPLC-UV | -Chromatographic peak heights of CTRX: 0.1–100 μg/mL (r = 0.999) -Detection limit of CTRX: 35 ng/mL -Repeatability (n = 6) of the chromatographic peak height for 4.0 μg/mL CTRX: 0.38% RSD.-Recovery rates of CTRX: >95.3%, and these RSDs were <5.8% | 37.35 μg/mL | 2.61 μg/mL | [18] |
| Age: 86 Sex: female | Report of encephalopathy associated with high levels of ceftriaxone in plasma and cerebrospinal fluid, investigation of the causal relationship between ceftriaxone administration and the development of encephalopathy | hemodialysis | 2 g/day | HPLC | nd | >100 μg/mL | 10.2 μg/mL | [20] | |
| Population: n = 43 patients Sex: male median age: 51.7 years (IQR 33.3–67.1) median BMI: 24.7 kg/m2 (IQR 22.4–27.7 kg/m2) | Determining the role of transporter genetic variation and blood-brain barrier permeability in predicting ceftriaxone exposure in the central nervous system | estimated creatinine clearance < 30 mL/min | 2 g twice a day | HPLC | -Detection limits: 0.24 mg/L in plasma and 0.5 mg/L in CSF -Accuracy: 5.2% for plasma, 7.2% for CSF -Intra- and inter-day coefficients of variation (CV%): 3.6% and 4.5% for plasma samples, and 7.2%, 7.8%, and 10.3% for CSF samples -Recovery rate: 86% (CV% = 3) for CSF samples and 82% (CV% = 8) for plasma samples. | Median Cmax: 157,193.00 ng/mL (IQR 105,164.0–184,852.0 ng/mL) | Median Cmax: 3512.0 ng/mL (IQR 2134.0–6193.0 ng/mL) | [21] | |
| Population: n = 16 patients | Evaluation of tolerability and pharmacokinetic parameters of high-dose ceftriaxone in adult patients treated for central nervous system infections: pharmacological data from two French cohorts | nd | 6.5 g/day (range 4–9 g) 97.5 mg/kg (range 77–131 mg/kg) | HPLC | nd | Median total plasma: 69.3 mg/L (range 21.6–201.3 mg/L; n =14) Median unbound plasma: 7.95 mg/L (range 0.8–43.7 mg/L; n = 8) | Median: 13.3 mg/L (range 0.9–91.2 mg/L) | [22] | |
| Population: n = 7 patients | Investigation of the pharmacokinetics ofboth antibiotics in patients with non- inflammatory obstructive hydrocephalus undergoing external ventricular surgery treated with cefotaxime or ceftriaxone for extracerebral infections | Scr < 1.5 mg/dL | 2 g single dose 30 min | HPLC-UV | -Quantification limits of ceftriaxone; 0.8 mg/L in serum and 0.08 mg/L in CSF. -Interday coefficients of variation; 2.0% 249.6; n = 6) at 99.7 and 6.8% at 1.55 mg/L inserum and 3.3% at 16.2 and 6.4% at 0.16 mg/L in CSF (n = 6). | Cmax: 172.2–271.7 mg/L (median = 249.6; n = 6) | Cmax: 0.18–1.04 mg/L (median = 0.43; n = 5), confirmed 1–16 h after injection (median = 12 h; n = 5). | [23] | |
| DAP | Population:16 patients (8 males and 8 females) Age: 70.0 ± 3.4 years weight: 47.6 ± 5.0 kg | Investigate the optimal dosing regimen for daptomycin and determine the need and appropriateness of a high-dose regimen in terms of PK / PD parameters using Monte Carlo Simulation and TDM in a Japanese clinical setting | CLcr 16.2–173.4 mL/min (n = 11) hemodialysis (n = 5) | single doses (6 mg/kg, 8 mg/kg, 10 mg/kg, and 12 mg/kg) and dosing intervals (24 h and 48 h) | HPLC-UV | -Lowest limit of quantification: 0.78 μg/mL | Cmin: 0.13–49.4 μg/mL Cpeak: 34.2–130.0 μg/mL | nd | [24] |
| Population: n = 20 patients | Investigate associations between DAP Cmin and creatine phosphokinase elevation via logistic regression analysis (E/R analysis), and to analyze DAP PPK via adaptation of a one-compartment model in Japanese patients to determine optimal DAP doses for minimizing adverse effects and maximizing treatment success by E/R analysis. | CLcr 22.4–213.8 mL/min, | 2.8–8.6 mg/kg | HPLC-UV | -Lowest limit of quantitation: 1.0 μg/mL -within-day and between-day coefficients of variation of <5.0%. | Cmin: 2.8–92.4 μg/mL Cpeak: 30.4–76.7 μg/mL | nd | [25] | |
| Population: n = 15 patients | Development of an assay method for the determination of total and free daptomycin in human plasma | nd | 4–8 mg/kg once over a 24-hour period. | LC-MS/MS | -Concentration ranges: 1.0–100 μg/mL in total daptomycin and 0.1–10 μg/mL in free daptomycin - Limits of quantitation: 1.0 μg/mL(total daptomycin) and 0.1 μg/mL(free daptomycin) -Recovery rate: total daptomycin measurements ranged from 106.1% and free daptomycin measurements ranged from 98.2% | The plasma concentration ranges of total and free daptomycin in 15 infected patients were 3.01–34.1 and 0.39–3.64 μg/mL | nd | [26] | |
| Population: two patients admitted to intensive care unit (2 males) Weight: 61.1 kg, 59.0 kg | Development of a new assay for measuring total and free concentrations of daptomycin in plasma with potential clinical applications | CLcr 17.5 mL/min CLcr 140.5 mL/min | -every 48 h of 350 mg (CLcr < 30 mL/min)-once-daily dose of 350 mg (CLcr ≥ 30 mL/min) | UPLC-MS / MS | -Concentration ranges: 0.5–200 μg/mL in total daptomycin and 0.04–40 μg/mL in free daptomycin-Recovery rate: approximately 100% of free daptomycin from ultrafiltration -Limits of quantitation: 0.5 μg/mL (total daptomycin) and 0.04 μg/mL (free daptomycin) -Recovery rate: total daptomycin measurements ranged from 57.1 to 67.4% and free daptomycin measurements ranged from 54.6 to 62.3% | -Patient with low renal function: Cmax of free drug: 2.85 µg/mL (Day 3), 4.2 µg/mL (Day 5) Ctrough of free drug: 0.29 µg/mL (Day 3), 0.86 µg/mL (Day 5) -Patient with normal renal function: Median unbound plasma: Cmax of free drug: 2.69 µg/mL (Day 3), 2.77 µg/mL (Day 5) Ctrough of free drug: 0.77 µg/mL (Day 3), 0.34 µg/mL (Day 5) | nd | [27] | |
| Population: n = 53 patients Sex: Male (n = 33), female (n = 19) | Examine serum daptomycin levels, creatinine phosphokinase levels, and the incidence of other adverse effects | CLcr ≥ 80 mL/min: n = 15 30 ≤ CLcr < 80 mL/min: n = 23 CLcr < 30 mL/min: n = 14 haemodialysis: n = 8 | 4.0 < dose ≤5.0 mg/kg: n = 7 5.0 < dose ≤6.0 mg/kg: n = 19 6.0 < dose ≤7.0 mg/kg: n = 17 ≤7.0 mg/kg: n = 4 | HPLC-PDA | -Response at the lowest concentration (3.5 μg/mL) was significantly more than 5 times higher than that of the blank serum -Interday coefficient of variation for the lowest and highest concentration (200 μg/mL) samples was within 15%. | Cmax: 172.2–271.7 mg/L (median = 249.6; n = 6) | nd | [28] | |
| LZD | Age: 78 Sex: male weight: 48.2 kg | Treatment of mediastinitis with TDM of serum and wound exudate concentrations of linezolid in renal function impaired patients. | Scr: 5.6 mg/dL glomerular filtration rate: 8.6 mL/min/1.73 m2 | 600 mg every 24 h After that, 300 mg every 24 h | HPLC | -Lower limit: 0.1 μg/mL -Intra/interday precision below 5.0% | Cmin: 11.5 μg/mL (Day 21) Cmin: 5.5 μg/mL (Day 55) | nd | [29] |
| Age: 77 Sex: female weight: 55 kg | TDM was effective in preventing thrombocytopenia with linezolid: a case report | CLcr 29.9 mL/min | 600 mg twice a day After that, 600 mg every 24 h | HPLC | -Lower limit: 0.25 μg/mL -Intra/interday precision below 5.0% | 39.4 µg/mL (Day 9) | nd | [30] | |
| Age: 79 Sex: female weight: 58.5 kg | Successful combination therapy with linezolid and rifampicin with appropriate management of linezolid TDM in MRSA osteomyelitis: a case report | Scr 0.4 mg/dL | 600 mg twice a day Thereafter, 300 mg twice a day At the time rifampicin is combined, 600 mg twice a day | HPLC | -Lower limit: 0.1 μg/mL -Intra/interday precision below 5.0% | Cmin: 15.1 µg/mL (Day 5) Cmin: 13.9 µg/mL (Day 8) As a result of combination therapy, Cmin was in the optimal range of 3.7 to 7.2 mg/mL. | nd | [31] | |
| TZD | Population: n = 3 patients | Development of an assay system for simultaneous quantification of plasma concentrations of LZD, DAP, and TZD and its clinical application | CLcr 48.3–64.5 mL/min | 200 mg once daily | UPLC-MS/MS | -TZD showed good linearity over wide ranges of 5–5000 ng/mL. -The lower limited of quantification and three quality controls (QCs: low, medium and high) were less than 15% for both accu-racy and precision. -Recovery rate of TZD: more than 84.8% | Cpeak and Cmin of TZD ranged from 1.87 to 4.92 μg/mL and from 0.09 to 0.78 μg/mL | nd | [32] |
| Population: n = 3 patients | Development of an assay for simultaneous quantification of 12 antimicrobial agents commonly used in ICU and its clinical application | CLcr 51.7–60.4 mL/min | 200 mg once daily | UHPLC-MS/MS | -The concentration ranges of calibration curves for TZD was 0.01–5 μg/mL. -The measured concentrations in blanks were less than 20% of the peak response of the lower limited of quantification and less than 5% for internal standard | The ranges of Cmin and Cpeak in patients with CLcr of 51.7–60.4 mL/min were 0.06–0.12 and 2.67–4.01 μg/mL | nd | [33] | |
| LZD | Age: 78 Sex: male weight: 48.2 kg | Treatment of mediastinitis with TDM of serum and wound exudate concentrations of linezolid in renal function impaired patients. | Scr: 5.6 mg/dL glomerular filtration rate: 8.6 mL/min/1.73 m2 | 600 mg every 24 h After that, 300 mg every 24 h | HPLC | -Lower limit: 0.1 μg/mL -Intra/interday precision below 5.0% | Cmin: 11.5 μg/mL (Day 21) Cmin: 5.5 μg/mL (Day 55) | nd | [29] |
| Age: 77 Sex: female weight: 55 kg | TDM was effective in preventing thrombocytopenia with linezolid: a case report | CLcr 29.9 mL/min | 600 mg twice a day After that, 600 mg every 24 h | HPLC | -Lower limit: 0.25 μg/mL -Intra/interday precision below 5.0% | 39.4 µg/mL (Day 9) | nd | [30] | |
| Age: 79 Sex: female weight: 58.5 kg | Successful combination therapy with linezolid and rifampicin with appropriate management of linezolid TDM in MRSA osteomyelitis: a case report | Scr 0.4 mg/dL | 600 mg twice a day Thereafter, 300 mg twice a day At the time rifampicin is combined, 600 mg twice a day | HPLC | -Lower limit: 0.1 μg/mL -Intra/interday precision below 5.0% | Cmin: 15.1 µg/mL (Day 5) Cmin: 13.9 µg/mL (Day 8) As a result of combination therapy, Cmin was in the optimal range of 3.7 to 7.2 mg/mL. | nd | [31] | |
| TZD | Population: n = 3 patients | Development of an assay system for simultaneous quantification of plasma concentrations of LZD, DAP, and TZD and its clinical application | CLcr 48.3–64.5 mL/min | 200 mg once daily | UPLC-MS/MS | -TZD showed good linearity over wide ranges of 5–5000 ng/mL. -The lower limited of quantification and three quality controls (QCs: low, medium and high) were less than 15% for both accu-racy and precision. -Recovery rate of TZD: more than 84.8% | Cpeak and Cmin of TZD ranged from 1.87 to 4.92 μg/mL and from 0.09 to 0.78 μg/mL | nd | [32] |
| Population: n = 3 patients | Development of an assay for simultaneous quantification of 12 antimicrobial agents commonly used in ICU and its clinical application | CLcr 51.7–60.4 mL/min | 200 mg once daily | UHPLC-MS/MS | -The concentration ranges of calibration curves for TZD was 0.01–5 μg/mL. -The measured concentrations in blanks were less than 20% of the peak response of the lower limited of quantification and less than 5% for internal standard | The ranges of Cmin and Cpeak in patients with CLcr of 51.7–60.4 mL/min were 0.06–0.12 and 2.67–4.01 μg/mL | nd | [33] |
| Skin and Soft Tissue | Intrapulmonary | ||
|---|---|---|---|
| Adipose Tissue | Muscle | ELT | |
| AUC0–12 | 5.3 | 5.9 | NR |
| AUC0–24 | NR | NR | 106.0 |
| AUCtissue/AUCplasma | 1.1 | 1.2 | 39.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebihara, F.; Hamada, Y.; Kato, H.; Maruyama, T.; Kimura, T. Importance and Reality of TDM for Antibiotics Not Covered by Insurance in Japan. Int. J. Environ. Res. Public Health 2022, 19, 2516. https://doi.org/10.3390/ijerph19052516
Ebihara F, Hamada Y, Kato H, Maruyama T, Kimura T. Importance and Reality of TDM for Antibiotics Not Covered by Insurance in Japan. International Journal of Environmental Research and Public Health. 2022; 19(5):2516. https://doi.org/10.3390/ijerph19052516
Chicago/Turabian StyleEbihara, Fumiya, Yukihiro Hamada, Hideo Kato, Takumi Maruyama, and Toshimi Kimura. 2022. "Importance and Reality of TDM for Antibiotics Not Covered by Insurance in Japan" International Journal of Environmental Research and Public Health 19, no. 5: 2516. https://doi.org/10.3390/ijerph19052516
APA StyleEbihara, F., Hamada, Y., Kato, H., Maruyama, T., & Kimura, T. (2022). Importance and Reality of TDM for Antibiotics Not Covered by Insurance in Japan. International Journal of Environmental Research and Public Health, 19(5), 2516. https://doi.org/10.3390/ijerph19052516









