The Inhaler Technique Questionnaire (InTeQ): Development and Validation of a Brief Patient-Reported Measure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Development of the Inhaler Technique Questionnaire (InTeQ)
2.2. Study Design and Participants
2.3. Study Variables
2.4. Analytical Strategy
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Iniciative for Asthma. Global Strategy for Asthma Management and Prevention. 2021. Available online: https://ginasthma.org/ (accessed on 25 June 2021).
- The Global Asthma Report 2018; Global Asthma Network: Auckland, New Zealand, 2018.
- Crompton, G. A brief history of inhaled asthma therapy over the last fifty years. Prim. Care Respir. J. 2006, 15, 326–331. [Google Scholar] [CrossRef] [Green Version]
- Dekhuijzen, P.N.R.; Vincken, W.; Virchow, J.C.; Roche, N.; Agusti, A.; Lavorini, F.; van Aalderen, W.M.; Price, D. Prescription of inhalers in asthma and COPD: Towards a rational, rapid and effective approach. Respir. Med. 2013, 107, 1817–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef]
- Sanchis, J.; Gich, I.; Pedersen, S. Systematic Review of Errors in Inhaler Use: Has Patient Technique Improved Over Time? Chest 2016, 150, 394–406. [Google Scholar] [CrossRef] [Green Version]
- Chrystyn, H.; Van Der Palen, J.; Sharma, R.; Barnes, N.; Delafont, B.; Mahajan, A.; Thomas, M. Device errors in asthma and COPD: Systematic literature review and meta-analysis. NPJ Prim. Care Respir. Med. 2017, 27, 22. [Google Scholar] [CrossRef]
- Van Boven, J.F.M.; Lavorini, F.; Dekhuijzen, P.N.R.; Blasi, F.; Price, D.B.; Viegi, G. Urging Europe to put non-adherence to inhaled respiratory medication higher on the policy agenda: A report from the First European Congress on Adherence to Therapy. Eur. Respir. J. 2017, 49, 1700076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocks, J.W.H.; Chrystyn, H.; van der Palen, J.; Thomas, M.; Yates, L.; Landis, S.H.; Driessen, M.T.; Gokhale, M.; Sharma, R.; Molimard, M. Systematic review of association between critical errors in inhalation and health outcomes in asthma and COPD. NPJ Prim. Care Respir. Med. 2018, 28, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usmani, O.S.; Lavorini, F.; Marshall, J.; Dunlop, W.C.N.; Heron, L.; Farrington, E.; Dekhuijzen, R. Critical inhaler errors in asthma and COPD: A systematic review of impact on health outcomes. Respir. Res. 2018, 19, 10. [Google Scholar] [CrossRef] [Green Version]
- Takemura, M.; Kobayashi, M.; Kimura, K.; Mitsui, K.; Masui, H.; Koyama, M.; Itotani, R.; Ishitoko, M.; Suzuki, S.; Aihara, K.; et al. Repeated instruction on inhalation technique improves adherence to the therapeutic regimen in asthma. J. Asthma 2010, 47, 202–208. [Google Scholar] [CrossRef]
- Azzi, E.; Srour, P.; Armour, C.L.; Rand, C.; Bosnic-Anticevich, S. Practice makes perfect: Self-reported adherence a positive marker of inhaler technique maintenance. Prim. Care Respir. J. 2017, 27, 29. [Google Scholar] [CrossRef] [Green Version]
- Bosnic-Anticevich, S.; Cvetkovski, B.; Azzi, E. Identifying Critical Errors: Addressing Inhaler Technique in the Context of Asthma Management. Pulm. Ther. 2018, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Price, D.; David-Wang, A.; Cho, S.H.; Ho, J.C.-M.; Jeong, J.-W.; Liam, C.-K.; Lin, J.; Muttalif, A.R.; Perng, D.-W.; Tan, T.-L.; et al. Asthma in Asia: Physician perspectives on control, inhaler use and patient communications. J. Asthma 2016, 53, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Bosnic-Anticevich, S.Z.; Sinha, H.; So, S.; Reddel, H.K. Metered-dose inhaler technique: The effect of two educational interventions delivered in community pharmacy over time. J. Asthma 2010, 47, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Basheti, I.A.; Bosnic-Anticevich, S.Z.; Armour, C.L.; Reddel, H.K. Checklists for powder inhaler technique: A review and recommendations. Respir. Care 2014, 59, 1140–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Martínez, C.E.; Sossa-Briceño, M.P.; Nino, G. A systematic review of instruments aimed at evaluating metered-dose inhaler administration technique in children. J. Asthma 2017, 54, 173–185. [Google Scholar] [CrossRef]
- Gleeson, P.K.; Feldman, S.; Apter, A.J. Controller Inhalers: Overview of Devices, Instructions for Use, Errors, and Interventions to Improve Technique. J. Allergy Clin. Immunol. Pract. 2020, 8, 2234–2242. [Google Scholar] [CrossRef]
- Erickson, S.R.; Horton, A.; Kirking, D.M. Assessing Metered-Dose Inhaler Technique: Comparison of Observation vs. Patient Self-Report. J. Asthma. 1998, 35, 575–583. [Google Scholar] [CrossRef]
- Ramadan, W.H.; Sarkis, A.; Aderian, S.S.; Milane, A. Asthma and COPD Patients’ Perception of Appropriate Metered-Dose Inhaler Technique. Dose-Response 2020, 18, 1559325820917832. [Google Scholar] [CrossRef] [PubMed]
- Kritikos, V.; Price, D.; Papi, A.; Infantino, A.; Ställberg, B.; Ryan, D.; Lavorini, F.; Chrystyn, H.; Haughney, J.; Lisspers, K.; et al. A multinational observational study identifying primary care patients at risk of overestimation of asthma control. NPJ Prim. Care Respir. Med. 2019, 29, 43. [Google Scholar] [CrossRef]
- Amin, A.N.; Ganapathy, V.; Roughley, A.; Small, M. Confidence in correct inhaler technique and its association with treatment adherence and health status among US patients with chronic obstructive pulmonary disease. Patient Prefer. Adherence 2017, 11, 1205–1212. [Google Scholar] [CrossRef] [Green Version]
- Kher, S.; Landau, H.; Hon, S.M.; Breeze, J.L.; Al-Naamani, N.; Paulus, J.K.; Martin, A.; Tsacoyianis, R. Inhaler use and education characteristics among English and non-English speaking patients: A pilot needs assessment survey. Patient Educ. Couns. 2019, 102, 932–936. [Google Scholar] [CrossRef]
- Chorão, P.; Pereira, A.M.; Fonseca, J.A. Inhaler devices in asthma and COPD—An assessment of inhaler technique and patient preferences. Respir. Med. 2014, 108, 968–975. [Google Scholar] [CrossRef] [Green Version]
- Barbara, S.A.; Kritikos, V.; Price, D.B.; Bosnic-Anticevich, S. Identifying patients at risk of poor asthma outcomes associated with making inhaler technique errors. J. Asthma 2020, 58, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Litt, H.K.; Press, V.G.; Hull, A.; Siros, M.; Luna, V.; Volerman, A. Association between inhaler technique and confidence among hospitalized children with asthma. Respir. Med. 2020, 174, 106191. [Google Scholar] [CrossRef]
- Sulaiman, I.; Seheult, J.; Machale, E.; Boland, F.; O’dwyer, S.M.; Rapcan, V.; D’Arcy, S.; Cushen, B.; Mokoka, M.; Killane, I.; et al. A Method to Calculate Adherence to Inhaled Therapy that Reflects the Changes in Clinical Features of Asthma. Ann. Am. Thorac. Soc. 2016, 13, 1894–1903. [Google Scholar] [CrossRef] [Green Version]
- Greene, G.; Costello, R.W.; Cushen, B.; Sulaiman, I.; Hale, E.M.; Conroy, R.M.; Doyle, F. A novel statistical method for assessing effective adherence to medication and calculating optimal drug dosages. PLoS ONE 2018, 13, e0195663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toomey, E.; Hardeman, W.; Hankonen, N.; Byrne, M.; McSharry, J.; Matvienko-Sikar, K.; Lorencatto, F. Focusing on fidelity: Recommendations for improving intervention fidelity within trials of health behavioral interventions. Ann. Behav. Med. 2020, 8, 132–151. [Google Scholar] [CrossRef]
- Van Ganse, E.; Texier, N.; Dima, A.L.; Laforest, L.; Ferrer, M.; Hernandez, G.; Schuck, S.; Herbage, S.; Vial, D.; de Bruin, M.; et al. Assessment of the safety of long-acting β2 -agonists in routine asthma care: The ASTRO-LAB protocol. NPJ Prim. Care Respir. Med. 2015, 25, 15040. [Google Scholar] [CrossRef]
- Dima, A.L.; van Ganse, E.; Stadler, G.; de Bruin, M. Does adherence to inhaled corticosteroids predict asthma-related outcomes over time? A cohort study. Eur. Respir. J. 2019, 54, 1900901. [Google Scholar] [CrossRef]
- Hernandez, G.; Dima, A.L.; Pont, À.; Garin, O.; Martí-Pastor, M.; Alonso, J.; van Ganse, E.; Lafores, L.; de Bruin, M.; Mayoral, K.; et al. Impact of asthma on women and men: Comparison with the general population using the eq-5d-5l questionnaire. PLoS ONE 2018, 13, e0202624. [Google Scholar] [CrossRef]
- Dima, A.L.; van Ganse, E.; Laforest, L.; Texier, N.; de Bruin, M. Measuring medication adherence in asthma: Development of a novel self-report tool. Psychol. Health 2017, 32, 1288–1307. [Google Scholar] [CrossRef] [PubMed]
- Rosseel, Y. lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Dima, A.L. Scale validation in applied health research: Tutorial for a 6-step R-based psychometrics protocol. Health Psychol. Behav. Med. 2018, 6, 136–161. [Google Scholar] [CrossRef] [Green Version]
- Hemker, B.T.; Sijtsma, K.; Molenaar, I.W. Selection of Unidimensional Scales from a Multidimensional Item Bank in the Polytomous Mokken I RT Model. Appl. Psychol. Meas. 2016, 19, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Champely, S. Basic Functions for Power Analysis [R Package pwr Version 1.3-0]. Available online: https://cran.r-project.org/package=pwr (accessed on 24 July 2021).
- Giraud, V.; Roche, N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur. Respir. J. 2002, 19, 246–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duerden, M.; Price, D. Training issues in the use of inhalers. Dis. Manag. Health Outcomes 2001, 9, 75–87. [Google Scholar] [CrossRef]
- Lavorini, F.; Magnan, A.; Christophe Dubus, J.; Voshaarg, T.; Corbettaa, L.; Broedersh, M.; Dekhuijzenh, R.; Sanchisi, J.; Viejo, J.L.; Barnes, P.; et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir. Med. 2008, 102, 593–604. [Google Scholar] [CrossRef] [Green Version]
- Price, D.B.; Román-Rodríguez, M.; McQueen, R.B.; Bosnic-Anticevich, S.; Carter, V.; Gruffydd-Jones, K.; Haughney, J.; Henrichsen, S.; Hutton, C.; Infantino, A.; et al. Inhaler Errors in the CRITIKAL Study: Type, Frequency, and Association with Asthma Outcomes. J. Allergy Clin. Immunol. Pract. 2017, 5, 1071–1081. [Google Scholar] [CrossRef]
- Williams, M.V.; Baker, D.W.; Honig, E.G.; Lee, T.M.; Nowlan, A. Inadequate literacy is a barrier to asthma knowledge and self-care. Chest 1998, 114, 1008–1015. [Google Scholar] [CrossRef]
- Jahedi, L.; Downie, S.R.; Saini, B.; Chan, H.K.; Bosnic-Anticevich, S. Inhaler Technique in Asthma: How Does It Relate to Patients’ Preferences and Attitudes Toward Their Inhalers? J. Aerosol. Med. Pulm. Drug Deliv. 2017, 30, 42–52. [Google Scholar] [CrossRef]
- Volerman, A.; Carpenter, D.; Press, V. What can be done to impact respiratory inhaler misuse: Exploring the problem, reasons, and solutions. Expert Rev. Respir. Med. 2020, 14, 791–805. [Google Scholar] [CrossRef]
- Takaku, Y.; Kurashima, K.; Ohta, C.; Ishiguro, T.; Kagiyama, N.; Yanagisawa, T.; Takayanagi, N. How many instructions are required to correct inhalation errors in patients with asthma and chronic obstructive pulmonary disease? Respir. Med. 2017, 123, 110–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basheti, I.A.; Obeidat, N.M.; Reddel, H.K. Effect of novel inhaler technique reminder labels on the retention of inhaler technique skills in asthma: A single-blind randomized controlled trial. NPJ Prim. Care Respir. Med. 2017, 27, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melani, A.S.; Bonavia, M.; Cilenti, V.; Cinti, C.; Lodi, M.; Martucci, P.; Serra, M.; Scichilone, N.; Sestini, P.; Aliani, M.; et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir. Med. 2011, 105, 930–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rootmensen, G.N.; Van Keimpema, A.R.; Jansen, H.M.; de Haan, R.J. Predictors of Incorrect Inhalation Technique in Patients with Asthma or COPD: A Study Using a Validated Videotaped Scoring Method. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Melani, A.S.; Zanchetta, D.; Barbato, N.; Sestini, P.; Cinti, C.; Canessa, P.A.; Aiolfi, S.; Neri, M. Inhalation technique and variables associated with misuse of conventional metered-dose inhalers and newer dry powder inhalers in experienced adults. Ann. Allergy Asthma Immunol. 2004, 93, 439–446. [Google Scholar] [CrossRef]
- Diggory, P.; Fernandez, C.; Humphrey, A.; Jones, V.; Murphy, M. Comparison of elderly people’s technique in using two dry powder inhalers to deliver zanamivir: Randomised controlled trial. BMJ 2001, 322, 577. [Google Scholar] [CrossRef] [Green Version]
- Paasche-Orlow, M.K.; Riekert, K.A.; Bilderback, A.; Chanmugam, A.; Hill, P.; Rand, C.S.; Brancati, F.L.; Krishnan, J.A. Tailored Education May Reduce Health Literacy Disparities in Asthma Self-Management. Am. J. Respir. Crit. Care Med. 2005, 172, 980–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartert, T.V.; Windom, H.H.; Peebles, R.S.; Freidhoff, L.R.; Togias, A. Inadequate outpatient medical therapy for patients with asthma admitted to two urban hospitals. Am. J. Med. 1996, 100, 386–394. [Google Scholar] [CrossRef]
- Lohr, K.N. Assessing health status and quality-of-life instruments: Attributes and review criteria. Qual. Life Res. 2002, 11, 193–205. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. A note on the use of the intraclass correlation coefficient in the evaluation of agreement between two methods of measurement. Comput. Biol. Med. 1990, 20, 337–340. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, M.J.; Martin, M.L.; Williams, P.V.; Gallet, C.L.; Miller, M.C.; Bennett, A.V.; May, R.W.; Lampl, K.L.; Ramachandran, S. Evaluation of inhaler device technique in caregivers of young children with asthma. Pediatr. Allergy Immunol. Pulmonol. 2010, 23, 113–120. [Google Scholar] [CrossRef]
- Boccuti, L.; Celano, M.; Geller, R.J.; Phillips, K.M. Development of a scale to measure children’s metered-dose inhaler and spacer technique. Ann. Allergy Asthma Immunol. 1996, 77, 217–221. [Google Scholar] [CrossRef]
- Sleath, B.; Ayala, G.X.; Gillette, C.; Williams, D.; Davis, S.; Tudor, G.; Yeatts, K.; Washington, D. Provider demonstration and assessment of child device technique during pediatric asthma visits. Pediatrics 2011, 127, 642–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.-H.; Yin, T.J.C.; Huang, J.-L. An exploration of the skills needed for inhalation therapy in schoolchildren with asthma in Taiwan. Ann. Allergy Asthma Immunol. 2002, 89, 311–315. [Google Scholar] [CrossRef]
- Chrystyn, H.; Audibert, R.; Keller, M.; Quaglia, B.; Vecellio, L.; Roche, N. Real-life inhaler adherence and technique: Time to get smarter! Respir. Med. 2019, 158, 24–32. [Google Scholar] [CrossRef]
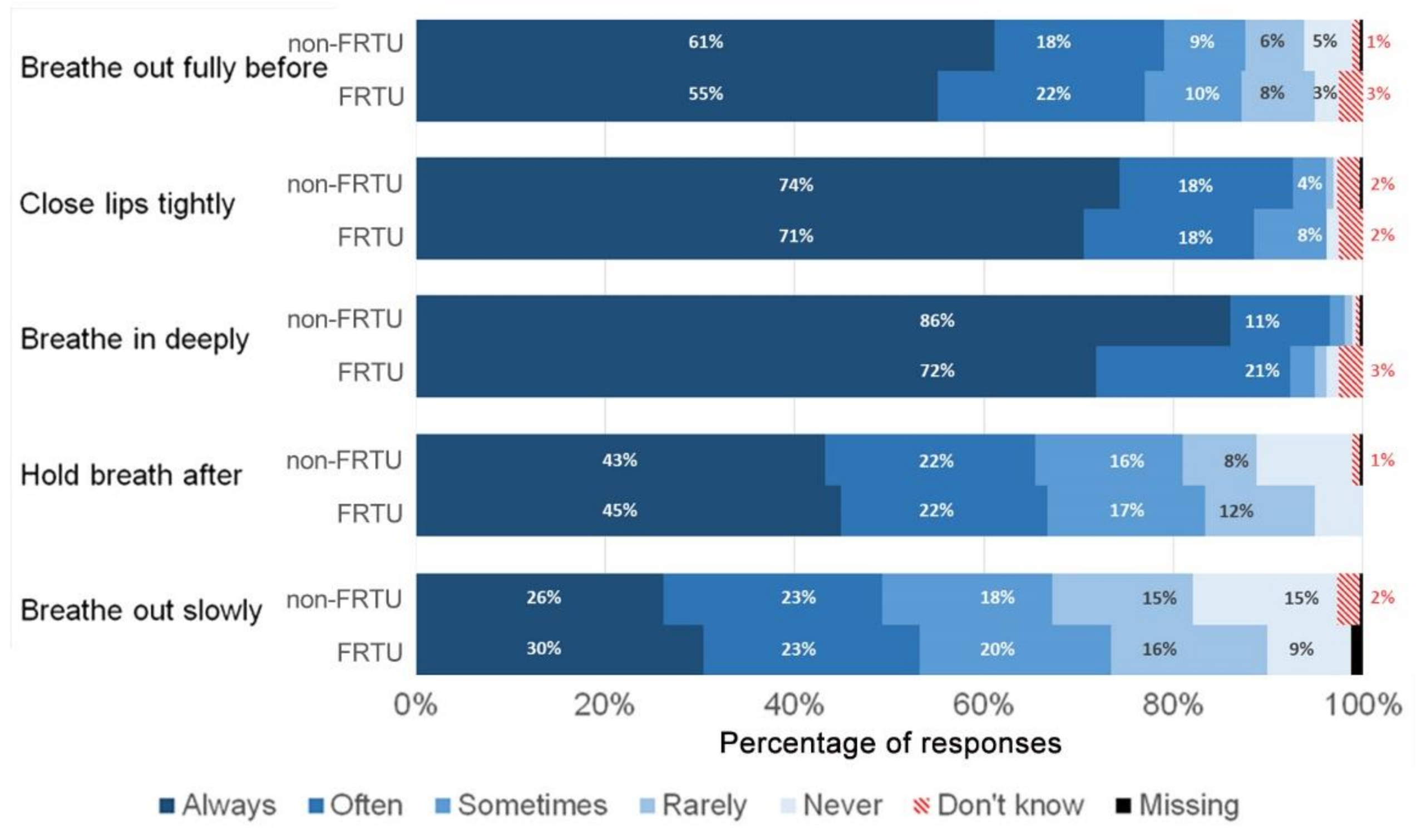
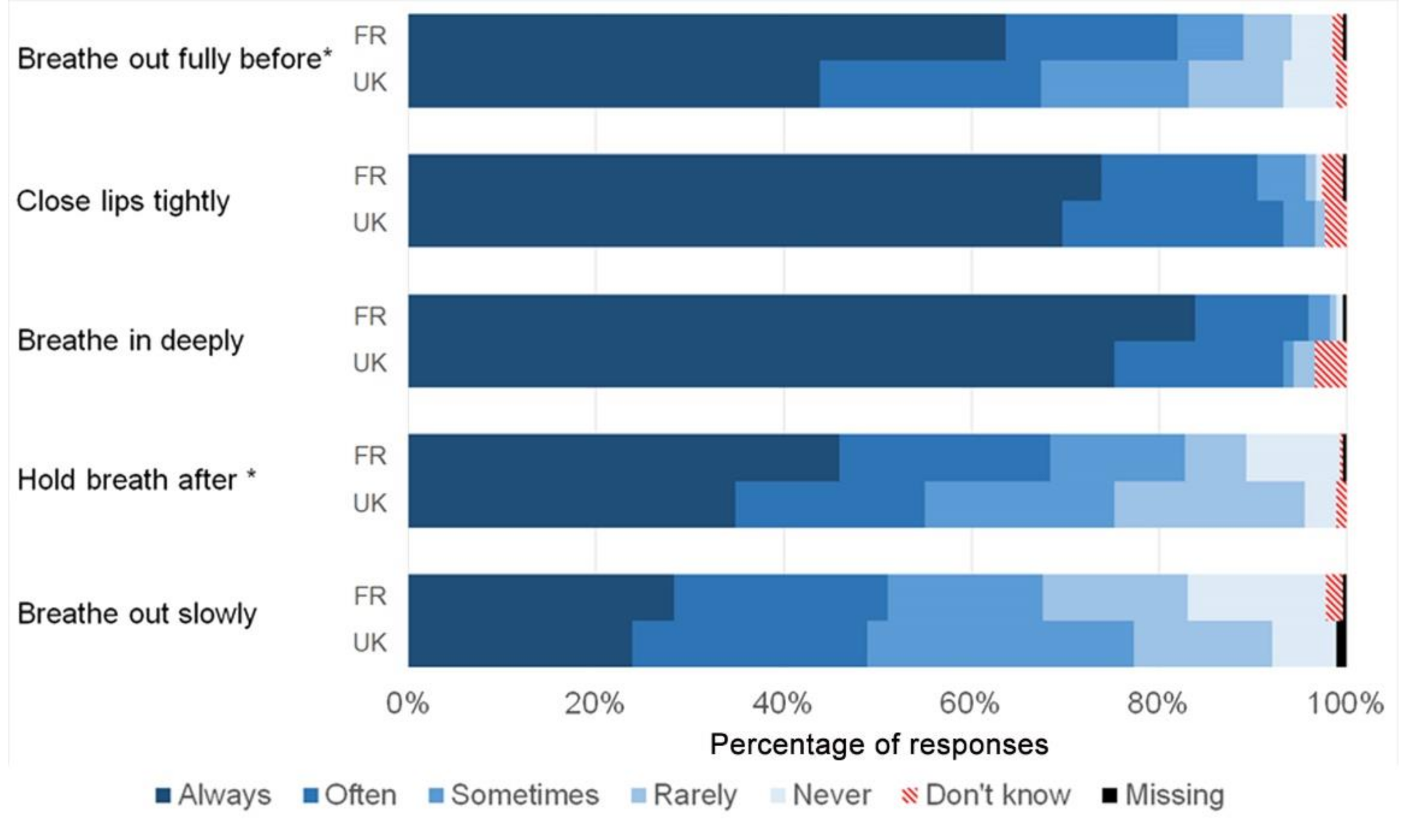


| Items | Response Options |
|---|---|
| Breathe out fully before use | Always |
| Close lips tightly around the mouthpiece | Often |
| Breathe in deeply through the mouthpiece | Sometimes |
| Hold my breath for at least 10 s after breathing in | Rarely |
| Breathe out very slowly after use | Never |
| Don’t Know | |
| Verbatim response regarding options, emerged during a cognitive interview | |
| “I think I know how to do it; the doctor asks me to do it in front of them every time I have an asthma clinic. Sometimes I do feel like it is not doing anything, so I do wonder. I take the blue inhaler with me and take a couple of puffs before exercising, and this really worked […] because of actually paying attention on […] how I use it […]. [But] for the morning and evening one, I take it rather quickly, and sometimes I wonder if I shouldn’t actually focus more on how I do it, because sometimes I just take a tiny breath in, and sometimes I am tired or I just woke up and I don’t have any breath capacity, so I do wonder if I do it properly. It’s not because I don’t know, it’s just that it becomes more usual that I use it in a really quick way.” | |
| Primary Care Records | ||
| Demographic variables | ||
| Age, mean (SD) | 28.0 (8.6) | |
| Sex | Male | 151 (41.8%) |
| Female | 210 (58.2%) | |
| Country | France | 272 (75.3%) |
| United Kingdom | 89 (24.7%) | |
| Severity markers 12 months before enrolment | ||
| Asthma-related comorbidities | 0 | 81 (41.1%) |
| 1 | 84 (42.6%) | |
| ≥2 | 32 (16.2%) | |
| Missing | 164 (45.2%) | |
| Oral corticosteroids courses | 0 | 251 (70.7%) |
| ≥1 | 104 (29.3%) | |
| Missing | 6 (1.7%) | |
| Hospitalizations | No | 86 (96.6%) |
| Yes | 3 (3.4%) | |
| Missing | 272 (75.3%) | |
| Telephone interviews at baseline | ||
| Inhaled controller treatment | Corticosteroids | 92 (25.5%) |
| Long-acting beta-agonists | 14 (3.9%) | |
| Corticosteroids and long-acting beta-agonists | 255 (70.6%) | |
| Inhaler device for controller treatment | Dry powder (DPI) | 208 (63.8%) |
| Metered-dose (MDI) | 61 (18.7%) | |
| Breath-actuated metered-dose (BA-MDI) | 15 (4.6%) | |
| More than one type of device | 42 (12.9%) | |
| Missing | 35 (10.7%) | |
| Reliever treatment use | Every day | 38 (11.4%) |
| Almost every day | 40 (12.0%) | |
| Once or twice every week | 87 (26.0%) | |
| Less than once a week | 119 (35.6%) | |
| Never | 50 (15.0%) | |
| Missing | 27 (7.5%) | |
| Adherence (Medication Intake Survey–Asthma) | Low (≤50%) | 81 (27.3%) |
| Intermediate (>50–<100%) | 87 (29.3%) | |
| Complete (100%) | 129 (43.4%) | |
| Missing | 64 (17.7%) | |
| Online survey at baseline | ||
| Use of spacer during the last 4 months | Always | 26 (7.2%) |
| Often | 13 (3.6%) | |
| Sometimes | 9 (2.5%) | |
| Rarely | 18 (5.0%) | |
| Never | 293 (81.6%) | |
| Missing | 2 (0.6%) | |
| Make an inhaler use plan together | 1 (Not at all) | 86 (24.2%) |
| with healthcare practitioners | 2 | 38 (10.7%) |
| 3 | 24 (6.7%) | |
| 4 (In general) | 88 (24.7%) | |
| 5 | 36 (10.1%) | |
| 6 | 34 (9.6%) | |
| 7 (In a lot of detail) | 50 (14.0%) | |
| Missing | 32 (8.2%) | |
| Healthcare practitioners taught how to use inhalers | 1 (Not at all) | 85 (23.9%) |
| 2 | 24 (6.7%) | |
| 3 | 20 (5.6%) | |
| 4 (In general) | 65 (18.3%) | |
| 5 | 31 (8.7%) | |
| 6 | 50 (14.0%) | |
| 7 (In a lot of detail) | 81 (22.8%) | |
| Missing | 32 (8.2%) | |
| Text messages and telephone interviews | ||
| Number of exacerbations during the year of follow-up | 0 | 223 (71.9%) |
| 1 | 58 (18.7%) | |
| 2 | 19 (6.1%) | |
| 3–5 | 10 (3.2%) | |
| Missing | 51 (14.1%) |
| Item | Mean (SD a) | Skew | Inter-Item Correlations | |||||
|---|---|---|---|---|---|---|---|---|
| Breathe Out Fully Before | Close Lips Tightly | Breathe In Deeply | Hold Breath After | Breathe Out Slowly | Hi b (SE c) | |||
| Breathe out fully before | 0.73 (1.12) | 1.51 | 1 | 0.497 (0.053) | ||||
| Close lips tightly | 0.34 (0.67) | 2.42 | 0.33 | 1 | 0.566 (0.053) | |||
| Breathe in deeply | 0.23 (0.59) | 3.45 | 0.35 | 0.55 | 1 | 0.708 (0.059) | ||
| Hold breath after | 1.16 (1.30) | 0.85 | 0.32 | 0.20 | 0.29 | 1 | 0.593 (0.047) | |
| Breathe out slowly | 1.61 (1.36) | 0.37 | 0.24 | 0.30 | 0.24 | 0.58 | 1 | 0.738 (0.045) |
| Scale | H d (SE) | |||||||
| InTeQ scale | 0.607 (0.040) | |||||||
| Make an Inhaler Use Plan Together a | Teach How to Use the Inhaler | Use of Spacer b | ||||
|---|---|---|---|---|---|---|
| 1–5: Not Discussed/ Only in General | 6–7: Discussed in Detail | 1–5: Not Discussed/Only in General | 6–7: Discussed in Detail | Always–Sometimes | Rarely–Never | |
| Subjects | 272 | 84 | 225 | 131 | 30 | 68 |
| Breathe out fully before | ||||||
| Always | 151 (55.5%) | 57 (67.9%) | 183 (81.3%) | 108 (82.4%) | 11 (37.9%) | 40 (61.5%) |
| Often–Sometimes | 81 (29.8%) | 22 (26.2%) | 35 (15.6%) | 18 (13.7%) | 12 (41.4%) | 15 (27.7%) |
| Rarely–Never | 36 (13.2%) | 4 (4.8%) | 6 (2.7%) | 2 (1.5%) | 6 (20.7%) | 7 (10.8%) |
| Don’t know | 3 (1.1%) | 1 (1.2%) | 1 (0.4%) | 2 (1.5%) | 0 (0.0%) | 0 (0.0%) |
| p-value | 0.049 | 0.246 | 0.097 | |||
| Close lips tightly | ||||||
| Always | 127 (56.4%) | 81 (61.8%) | 110 (40.4%) | 45 (53.6%) | 21 (70.0%) | 49 (72.1%) |
| Often–Sometimes | 65 (28.9%) | 38 (29.0%) | 102 (37.5%) | 27 (32.1%) | 9 (30.0%) | 18 (26.5%) |
| Rarely–Never | 30 (13.3%) | 10 (7.6%) | 52 (19.1%) | 12 (14.3%) | 0 (0.0%) | 1 (1.5%) |
| Don’t know | 3 (1.3%) | 1 (0.8%) | 3 (1.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| p-value | 0.108 | 0.585 | 0.760 | |||
| Breathe in deeply | ||||||
| Always | 191 (70.2%) | 68 (81.0%) | 92 (40.9%) | 63 (48.1%) | 19 (63.3%) | 56 (82.4%) |
| Often–Sometimes | 66 (24.3%) | 16 (19.0%) | 84 (37.3%) | 45 (34.4%) | 8 (26.7%) | 11 (16.2%) |
| Rarely–Never | 6 (2.2%) | 0 (0.0%) | 44 (19.6%) | 20 (15.3%) | 2 (6.7%) | 0 (0.0%) |
| Don’t know | 8 (2.9%) | 0 (0.0%) | 1 (0.4%) | 2 (1.5%) | 1 (3.3%) | 1 (1.5%) |
| p-value | 0.227 | 0.507 | 0.070 | |||
| Hold breath after | ||||||
| Always | 162 (72.0%) | 97 (74.0%) | 69 (25.4%) | 28 (33.3%) | 8 (26.7%) | 37 (54.4%) |
| Often–Sometimes | 54 (24.0%) | 28 (21.4%) | 114 (41.9%) | 36 (42.9%) | 13 (43.3%) | 22 (32.4%) |
| Rarely–Never | 5 (2.2%) | 1 (0.8%) | 77 (28.3%) | 18 (21.4%) | 9 (30.0%) | 8 (11.8%) |
| Don’t know | 4 (1.8%) | 4 (3.1%) | 3 (1.1%) | 0 (0.0%) | 0 (0.0%) | 1 (1.5%) |
| p-value | 0.176 | 0.381 | 0.035 | |||
| Breathe out slowly | ||||||
| Always | 216 (79.4%) | 75 (89.3%) | 55 (24.4%) | 42 (32.1%) | 5 (16.7%) | 23 (34.4%) |
| Often–Sometimes | 45 (16.5%) | 8 (9.5%) | 96 (42.7%) | 54 (41.2%) | 23 (50.0%) | 31 (46.3%) |
| Rarely–Never | 7 (2.6%) | 1 (1.2%) | 66 (29.3%) | 29 (22.1%) | 10 (33.3%) | 12 (17.9%) |
| Don’t know | 3 (1.1%) | 0 (0.0%) | 1 (0.4%) | 2 (1.5%) | 0 (0.0%) | 1 (1.5%) |
| p-value | 0.333 | 0.355 | 0.178 | |||
| Quality of inhalation technique according to InTeQ global score | ||||||
| Poor (0–2 “Always”) | 114 (41.9%) | 22 (26.2%) | 91 (40.1%) | 45 (34.4%) | 17 (56.7%) | 24 (35.3%) |
| Fair (3 “Always”) | 71 (26.1%) | 24 (28.6%) | 62 (27.6%) | 33 (25.2%) | 6 (20.0%) | 16 (23.5%) |
| Good (4–5 “Always”) | 87 (32.0%) | 38 (45.2%) | 72 (32.0%) | 53 (40.5%) | 7 (23.3%) | 28 (41.2%) |
| p-value | 0.023 | 0.264 | 0.120 | |||
| 12 Months | % of Agreement (95%CI a) | Kappa (SE b) | |||
|---|---|---|---|---|---|
| InTeQ Item | Baseline | Always | Often–Never | ||
| Breathe out fully before | Always | 36 | 6 | 73.8 (68.8–78.7) | 0.468 (0.097) |
| Often–Never | 15 | 23 | |||
| Close lips tightly | Always | 51 | 9 | 78.8 (74.2–83.3) | 0.443 (0.114) |
| Often–Never | 8 | 12 | |||
| Breathe in deeply | Always | 60 | 11 | 80.0 (75.5–84.5) | 0.224 (0.135) |
| Often–Never | 5 | 4 | |||
| Hold breath after | Always | 27 | 10 | 78.8 (74.2–83.3) | 0.570 (0.092) |
| Often–Never | 7 | 36 | |||
| Breathe out slowly | Always | 15 | 5 | 81.3 (76.8–85.8) | 0.551 (0.105) |
| Often–Never | 9 | 46 | |||
| InTeQ global score | Mean (SD c) baseline | Mean (SD c) 12 m | Mean (SD c) change | p-value | ICC d |
| 2.8 (1.5) | 2.9 (1.7) | 0.06 (1.34) | 0.769 | 0.775 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizano-Barrantes, C.; Garin, O.; Dima, A.L.; van Ganse, E.; de Bruin, M.; Belhassen, M.; Mayoral, K.; Pont, À.; Ferrer, M. The Inhaler Technique Questionnaire (InTeQ): Development and Validation of a Brief Patient-Reported Measure. Int. J. Environ. Res. Public Health 2022, 19, 2591. https://doi.org/10.3390/ijerph19052591
Lizano-Barrantes C, Garin O, Dima AL, van Ganse E, de Bruin M, Belhassen M, Mayoral K, Pont À, Ferrer M. The Inhaler Technique Questionnaire (InTeQ): Development and Validation of a Brief Patient-Reported Measure. International Journal of Environmental Research and Public Health. 2022; 19(5):2591. https://doi.org/10.3390/ijerph19052591
Chicago/Turabian StyleLizano-Barrantes, Catalina, Olatz Garin, Alexandra L. Dima, Eric van Ganse, Marijn de Bruin, Manon Belhassen, Karina Mayoral, Àngels Pont, and Montse Ferrer. 2022. "The Inhaler Technique Questionnaire (InTeQ): Development and Validation of a Brief Patient-Reported Measure" International Journal of Environmental Research and Public Health 19, no. 5: 2591. https://doi.org/10.3390/ijerph19052591
APA StyleLizano-Barrantes, C., Garin, O., Dima, A. L., van Ganse, E., de Bruin, M., Belhassen, M., Mayoral, K., Pont, À., & Ferrer, M. (2022). The Inhaler Technique Questionnaire (InTeQ): Development and Validation of a Brief Patient-Reported Measure. International Journal of Environmental Research and Public Health, 19(5), 2591. https://doi.org/10.3390/ijerph19052591







