Is Population Density Associated with Non-Communicable Disease in Western Developed Countries? A Systematic Review
Abstract
:1. Introduction
- Does the mortality rate of residents in Western developed countries differ by degree of population density?
- Does the morbidity rate of residents in Western developed countries differ by degree of population density?
- To what degree do socioeconomic determinants explain any differences in morbidity or mortality rates?
2. Materials and Methods
2.1. Search Strategy
2.2. Quality Assessment
3. Results
3.1. Data Extraction
3.2. Study Characteristics
4. Quality Assessment
5. Associations between Population Density and Health Outcomes
5.1. Owing to Heterogeneity of Study Designs, We Used an Aggregative Approach to Provide a Narrative Overview of Existing Empirical Evidence. Studies Were Categorised into the Following Themes for Analysis
- All-cause mortality
- Cause-specific mortality:
- cancer (all cancer combined, all child cancer, and 15 different types of cancer)
- respiratory (lung cancer, COPD, broad category lung disease/respiratory)
- cardiovascular (stroke, heart disease)
- Morbidity
- cancer (all cancer combined, all child cancer, and 17 different types of cancer)
- diabetes (type 1 and type 2)
- respiratory (lung cancer, asthma, broad category lung disease/respiratory)
- cardiovascular
- neurological
- congenital
5.2. Key Findings
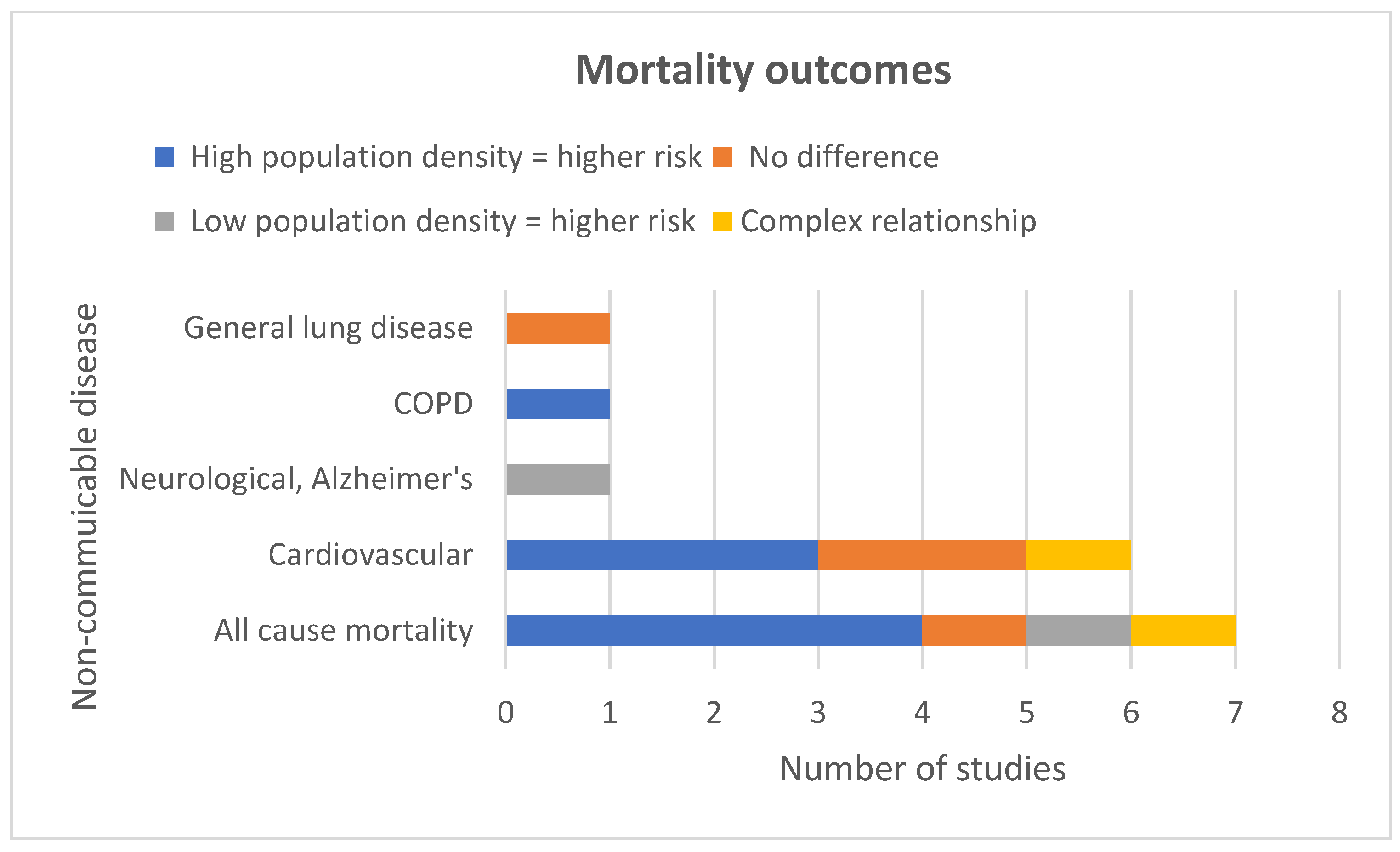
5.2.1. All-Cause Mortality
5.2.2. Cause Specific Mortality
5.2.3. Morbidity
Cancers
Asthma
Club foot
Diabetes
6. Discussion
6.1. Study Limitations
6.2. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organsation (WHO). Noncommuniable Diseases and Their Risk Factors. Available online: https://www.who.int/ncds/en/ (accessed on 27 October 2021).
- Pescheny, J.V.; Randhawa, G.; Pappas, Y. The impact of social prescribing services on service users: A systematic review of the evidence. Eur. J. Public Health 2020, 30, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Chawińska, E.; Tukiendorf, A.; Miszczyk, L. Interrelation between population density and cancer incidence in the province of Opole, Poland. Contemp. Oncol. 2014, 18, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Agovino, M.; Aprile, M.C.; Garofalo, A.; Mariani, A. Cancer mortality rates and spillover effects among different areas: A case study in Campania (southern Italy). Soc. Sci. Med. 2018, 204, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Conroy, S.M.; Shariff-Marco, S.; Yang, J.; Hertz, A.; Cockburn, M.; Shvetsov, Y.B.; Clarke, C.A.; Abright, C.L.; Haiman, C.A.; Le Marchand, L.; et al. Characterizing the neighborhood obesogenic environment in the Multiethnic Cohort: A multi-level infrastructure for cancer health disparities research. Cancer Causes Control 2018, 29, 167–183. [Google Scholar] [CrossRef]
- Gomez, S.L.; Glaser, S.L.; McClure, L.A.; Shema, S.J.; Kealey, M.; Keegan, T.H.M.; Satariano, W.A. The California Neighborhoods Data System: A new resource for examining the impact of neighborhood characteristics on cancer incidence and outcomes in populations. Cancer Causes Control 2011, 22, 631–647. [Google Scholar] [CrossRef] [Green Version]
- Institute, G.H.B. Urban Planning, Environment and Health Initiative. Available online: https://www.isglobal.org/en/urban-planning (accessed on 27 October 2021).
- Forsyth, A. Congested cities vs. sprawl makes you fat: Unpacking the health effects of planning density. TPR Town Plan. Rev. 2018, 89, 333–354. [Google Scholar]
- Cyril, S.; Oldroyd, J.C.; Renzaho, A. Urbanisation, urbanicity, and health: A systematic review of the reliability and validity of urbanicity scales. BMC Public Health 2013, 13, 513. [Google Scholar] [CrossRef] [Green Version]
- Galea, S.; Freudenberg, N.; Vlahov, D. Cities and population health. Soc. Sci. Med. 2005, 60, 1017–1033. [Google Scholar] [CrossRef]
- Gopinath, S.; Ortqvist, E.; Norgren, S.; Green, A.; Sanjeevi, C.B. Variations in incidence of type 1 diabetes in different municipalities of stockholm. Ann. N. Y. Acad. Sci. 2008, 1150, 200–207. [Google Scholar] [CrossRef]
- Dimitrovová, K.; Costa, C.; Santana, P.; Perelman, J. Evolution and financial cost of socioeconomic inequalities in ambulatory care sensitive conditions: An ecological study for Portugal, 2000–2014. Int. J. Equity Health 2017, 16, 145. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, T.L.; Lea, C.S.; Brinkley, J.; Zervos, E.E. Colorectal cancer outcome inequalities: Association between population density, race, and socioeconomic status. Rural Remote Health 2014, 14, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Requia, W.J.; Koutrakis, P.; Arain, A. Modeling spatial distribution of population for environmental epidemiological studies: Comparing the exposure estimates using choropleth versus dasymetric mapping. Environ. Int. 2018, 119, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, K.M.; LaGory, M. Unhealthy Cities Poverty, Race, and Place in America; Routledge: New York, NY, USA, 2011. [Google Scholar]
- Silveira, C.; Roebeling, P.; Lopes, M.; Ferreira, J.; Costa, S.; Teixeira, J.P.; Borrego, C.; Miranda, A.I. Assessment of health benefits related to air quality improvement strategies in urban areas: An Impact Pathway Approach. J. Environ. Manag. 2016, 183, 694–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martenies, S.E.; Milando, C.W.; Williams, G.O.; Batterman, S.A. Disease and health inequalities attributable to air pollutant exposure in Detroit, Michigan. Int. J. Environ. Res. Public Health 2017, 14, 1243. [Google Scholar] [CrossRef] [Green Version]
- Su, J.G.; Apte, J.S.; Lipsitt, J.; Garcia-Gonzales, D.A.; Beckerman, B.S.; de Nazelle, A.; Texcalac-Sangrador, J.L.; Jerrett, M. Populations potentially exposed to traffic-related air pollution in seven world cities. Environ. Int. 2015, 78, 82–89. [Google Scholar] [CrossRef]
- Villanueva, K.; Badland, H.; Kvalsvig, A.; O’Connor, M.; Christian, H.; Woolcock, G.; Giles-Corti, B.; Goldfeld, S. Can the Neighborhood Built Environment Make a Difference in Children’s Development? Building the Research Agenda to Create Evidence for Place-Based Children’s Policy. Acad. Pediatrics 2016, 16, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Vares, D.A.; St-Pierre, L.S.; Persinger, M.A. Correlations between U.S. county annual cancer incidence and population density. Am. J. Cancer Res. 2015, 5, 3467–3474. [Google Scholar]
- Nasca, P.C.; Burnett, W.S.; Greenwald, P.; Brennan, K.; Wolfgang, P.; Carlton, K. Population Density As An Indicator Of Urban-Rural Differences in Cancer Incidence, Upstate New York, 1968–19721. Am. J. Epidemiol. 1980, 112, 362–375. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Carnegie, E. Does Living in Areas of Relatively High Population Density, within Western Developed Countries, Increase One’s Risk of Developing Non-Communicable Disease? A Systematic Review of the Literature. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019119391 (accessed on 25 October 2021).
- Blakely, T.A.; Woodward, A.J. Ecological effects in multi-level studies. J. Epidemiol. Community Health 2000, 54, 367. [Google Scholar] [CrossRef] [Green Version]
- Javier, C.-R.; Suchithra, N.; Peter, D.S.; Paul, J. Mortality and morbidity in populations in the vicinity of coal mining: A systematic review. BMC Public Health 2018, 18, 721. [Google Scholar] [CrossRef] [Green Version]
- Cohen, B.L. Invited Commentary: In Defense of Ecologic Studies for Testing a Linear-No Threshold Theory. Am. J. Epidemiol. 1994, 139, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Dufault, B.; Klar, N. The Quality of Modern Cross-Sectional Ecologic Studies: A Bibliometric Review. Am. J. Epidemiol. 2011, 174, 1101–1107. [Google Scholar] [CrossRef]
- Altekruse, S.F.; Huang, L.; Cucinelli, J.E.; McNeel, T.S.; Wells, K.M.; Oliver, M.N. Spatial patterns of localized-stage prostate cancer incidence among white and black men in the southeastern United States, 1999–2001. Cancer Epidemiol. Prev. Biomark. 2010, 19, 1460–1467. [Google Scholar] [CrossRef] [Green Version]
- Balamurugan, A.; Delongchamp, R.; Bates, J.H.; Mehta, J.L. The neighborhood where you live is a risk factor for stroke. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 668–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ball, S.J.; Haynes, A.; Jacoby, P.; Pereira, G.; Miller, L.J.; Bower, C.; Davis, E.A. Spatial and temporal variation in type 1 diabetes incidence in Western Australia from 1991 to 2010: Increased risk at higher latitudes and over time. Health Place 2014, 28, 194–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, A.F.; Moncrief, T.; Huang, B.; Simmons, J.M.; Sauers, H.; Chen, C.; Kahn, R.S. Inequalities in neighborhood child asthma admission rates and underlying community characteristics in one US county. J. Pediatrics 2013, 163, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Beenackers, M.A.; Oude Groeniger, J.; Kamphuis, C.B.M.; Van Lenthe, F.J. Urban population density and mortality in a compact Dutch city: 23-year follow-up of the Dutch GLOBE study. Health Place 2018, 53, 79–85. [Google Scholar] [CrossRef]
- Canchola, A.J.; Shariff-Marco, S.; Yang, J.; Albright, C.; Hertz, A.; Park, S.-Y.; Shvetsov, Y.B.; Monroe, K.R.; Le Marchand, L.; Gomez, S.L.; et al. Association between the neighborhood obesogenic environment and colorectal cancer risk in the Multiethnic Cohort. Cancer Epidemiol. 2017, 50, 99–106. [Google Scholar] [CrossRef]
- Carsin, A.E.; Sharp, L.; Comber, H. Geographical, urban/rural and socioeconomic variations in nonmelanoma skin cancer incidence: A population-based study in Ireland. Br. J. Dermatol. 2011, 164, 822–829. [Google Scholar] [CrossRef]
- Chaix, B.; Rosvall, M.; Lynch, J.; Merlo, J. Disentangling contextual effects on cause-specific mortality in a longitudinal 23-year follow-up study: Impact of population density or socioeconomic environment? Int. J. Epidemiol. 2006, 35, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Chaix, B.; Rosvall, M.; Merlo, J. Assessment of the magnitude of geographical variations and socioeconomic contextual effects on ischaemic heart disease mortality: A multilevel survival analysis of a large Swedish cohort. J. Epidemiol. Community Health 2007, 61, 349–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrabose, M.; Owen, N.; Giles-Corti, B.; Turrell, G.; Carver, A.; Sugiyama, T. Urban Densification and 12-Year Changes in Cardiovascular Risk Markers. J. Am. Heart Assoc. 2019, 8, 1. [Google Scholar] [CrossRef]
- Chouaïd, C.; Debieuvre, D.; Durand-Zaleski, I.; Fernandes, J.; Scherpereel, A.; Westeel, V.; Blein, C.; Gaudin, A.-F.; Ozan, N.; Leblanc, S.; et al. Survival inequalities in patients with lung cancer in France: A nationwide cohort study (the TERRITOIRE Study). PLoS ONE 2017, 12, e0182798. [Google Scholar] [CrossRef] [PubMed]
- Colli, J.; Lee, B.R.; Thomas, R. Population densities in relation to bladder cancer mortality rates in America from 1950 to 1994. Int. Urol. Nephrol. 2012, 44, 443–449. [Google Scholar] [CrossRef]
- DeRouen, M.C.; Schupp, C.W.; Yang, J.; Koo, J.; Hertz, A.; Shariff-Marco, S.; Cockburn, M.; Nelson, D.O.; Ingles, S.A.; Cheng, I.; et al. Impact of individual and neighborhood factors on socioeconomic disparities in localized and advanced prostate cancer risk. Cancer Causes Control 2018, 29, 951–966. [Google Scholar] [CrossRef]
- Drewnowski, A.; Rehm, C.D.; Moudon, A.V.; Arterburn, D. The geography of diabetes by census tract in a large sample of insured adults in King County, Washington, 2005–2006. Prev. Chronic Dis. 2014, 11, E125. [Google Scholar] [CrossRef] [Green Version]
- du Prel, J.B.; Icks, A.; Grabert, M.; Holl, R.W.; Giani, G.; Rosenbauer, J. Socioeconomic conditions and type 1 diabetes in childhood in North Rhine-Westphalia, Germany. Diabetologia 2007, 50, 720–728. [Google Scholar] [CrossRef] [Green Version]
- Elliott, J.C.; Lucas, R.M.; Clements, M.S.; Bambrick, H.J. Population density determines the direction of the association between ambient ultraviolet radiation and type 1 diabetes incidence. Pediatric Diabetes 2010, 11, 394–402. [Google Scholar] [CrossRef]
- Erwin, P.C.; Fitzhugh, E.C.; Brown, K.C.; Looney, S.; Forde, T. Health Disparities in Rural Areas: The Interaction of Race, Socioeconomic Status, and Geography. J. Health Care Poor Underserved 2010, 21, 931–945. [Google Scholar] [CrossRef]
- Faka, A.; Chalkias, C.; Montano, D.; Georgousopoulou, E.N.; Tripitsidis, A.; Koloverou, E.; Tousoulis, D.; Pitsavos, C.; Panagiotakos, D.B. Association of Socio-Environmental Determinants with Diabetes Prevalence in the Athens Metropolitan Area, Greece: A Spatial Analysis. Rev. Diabet. Stud. RDS 2018, 14, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fecht, D.; Fortunato, L.; Morley, D.; Hansell, A.L.; Gulliver, J. Associations between urban metrics and mortality rates in England. Environ. Health 2016, 15 (Suppl. 1), 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallberg, Ö. Adverse health indicators correlating with sparsely populated areas in Sweden. Eur. J. Cancer Prev. 2007, 16, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Hipp, J.A.; Chalise, N. Spatial Analysis and Correlates of County-Level Diabetes Prevalence, 2009–2010. Prev. Chronic Dis. 2015, 12, E08. [Google Scholar] [CrossRef] [Green Version]
- Holmqvist, B.M.; Lofman, O.; Samuelsson, U. A low incidence of Type 1 diabetes between 1977 and 2001 in south-eastern Sweden in areas with high population density and which are more deprived. Diabet. Med. A J. Br. Diabet. Assoc. 2008, 25, 255–260. [Google Scholar] [CrossRef]
- Howe, H.L.; Keller, J.E.; Lehnherr, M. Relation between population density and cancer incidence, Illinois, 1986–1990. Am. J. Epidemiol. 1993, 138, 29–36. [Google Scholar] [CrossRef]
- Krogsgaard, M.R.; Jensen, P.K.; Kjaer, I.; Husted, H.; Lorentzen, J.; Hvass-Christensen, B.; Christensen, S.B.; Larsen, K.; Sonne-Holm, S. Increasing incidence of club foot with higher population density: Incidence and geographical variation in Denmark over a 16-year period--an epidemiological study of 936,525 births. Acta Orthop. 2006, 77, 839–846. [Google Scholar] [CrossRef] [Green Version]
- Liese, A.D.; Lamichhane, A.P.; Garzia, S.C.A.; Puett, R.C.; Porter, D.E.; Dabelea, D.; D’Agostino, R.B., Jr.; Standiford, D.; Liu, L. Neighborhood characteristics, food deserts, rurality, and type 2 diabetes in youth: Findings from a case-control study. Health Place 2018, 50, 81–88. [Google Scholar] [CrossRef]
- Lovasi, G.S.; Quinn, J.W.; Neckerman, K.M.; Perzanowski, M.S.; Rundle, A. Children living in areas with more street trees have lower prevalence of asthma. J. Epidemiol. Community Health 2008, 62, 647–649. [Google Scholar] [CrossRef] [Green Version]
- Mahoney, M.C.; LaBrie, D.S.; Nasca, P.C.; Wolfgang, P.E.; Burnett, W.S. Population density and cancer mortality differentials in New York State, 1978-1982. Int. J. Epidemiol. 1990, 19, 483–490. [Google Scholar] [CrossRef]
- Manda, S.O.M.; Feltbower, R.G.; Gilthorpe, M.S. Investigating spatio-temporal similarities in the epidemiology of childhood leukaemia and diabetes. Eur. J. Epidemiol. 2009, 24, 743–752. [Google Scholar] [CrossRef]
- McNally, R.J.Q.; Alston, R.D.; Cairns, D.P.; Eden, O.B.; Birch, J.M. Geographical and ecological analyses of childhood acute leukaemias and lymphomas in north-west England. Br. J. Haematol. 2003, 123, 60–65. [Google Scholar] [CrossRef] [PubMed]
- McNally, R.J.Q.; Alston, R.D.; Cairns, D.P.; Eden, O.B.; Kelsey, A.M.; Birch, J.M. Geographical and ecological analyses of childhood Wilms’ tumours and soft-tissue sarcomas in North West England. Eur. J. Cancer 2003, 39, 1586–1593. [Google Scholar] [CrossRef]
- McNally, R.J.Q.; Basta, N.O.; Errington, S.; James, P.W.; Norman, P.D.; Hale, J.P.; Pearce, M.S. Socioeconomic patterning in the incidence and survival of teenage and young adult men aged between 15 and 24 years diagnosed with non-seminoma testicular cancer in northern england. Urol. Oncol. 2015, 33, 506-e9. [Google Scholar] [CrossRef] [Green Version]
- Meijer, M.; Kejs, A.M.; Stock, C.; Bloomfield, K.; Ejstrud, B.; Schlattmann, P. Population density, socioeconomic environment and all-cause mortality: A multilevel survival analysis of 2.7 million individuals in Denmark. Health Place 2012, 18, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Meijer, M.; Bloomfield, K.; Engholm, G. Neighbourhoods matter too: The association between neighbourhood socioeconomic position, population density and breast, prostate and lung cancer incidence in Denmark between 2004 and 2008. J. Epidemiol. Community Health 2013, 67, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Muquit, S.; Parks, R.; Basu, S. Socio-economic characteristics of patients with glioblastoma multiforme. J. Neuro-Oncol. 2015, 125, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.C.; Khanna, S.; Dwivedi, P.; Huang, D.; Huang, Y.; Tasdizen, T.; Brunisholz, K.D.; Li, F.; Gorman, W.; Nguyen, T.T. Using Google Street View to examine associations between built environment characteristics and US health outcomes. Prev. Med. Rep. 2019, 14, 100859. [Google Scholar] [CrossRef]
- Phillips, D.I.W.; Osmond, C.; Williams, M.L.; Jones, A. Air pollution in early life and adult mortality from chronic rheumatic heart disease. Int. J. Epidemiol. 2017, 46, 1107–1114. [Google Scholar] [CrossRef] [Green Version]
- Puett, R.C.; Lamichhane, A.P.; Nichols, M.D.; Lawson, A.B.; Standiford, D.A.; Liu, L.; Dabelea, D.; Liese, A.D. Neighborhood context and incidence of type 1 diabetes: The SEARCH for Diabetes in Youth study. Health Place 2012, 18, 911–916. [Google Scholar] [CrossRef] [Green Version]
- Rogers, M.A.M. Onset of type 1 diabetes mellitus in rural areas of the USA. J. Epidemiol. Community Health 2019, 73, 1136–1138. [Google Scholar] [CrossRef] [PubMed]
- Rooney, J.P.; Tobin, K.; Crampsie, A.; Vajda, A.; Heverin, M.; McLaughlin, R.; Staines, A.; Hardiman, O. Social deprivation and population density are not associated with small area risk of amyotrophic lateral sclerosis. Env. Res. 2015, 142, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, U.; Westerberg, L.; Aakesson, K.; Birkebæk, N.H.; Bjarnason, R.; Drivvoll, A.K.; Skrivarhaug, T.; Svensson, J.; Thorsson, A.; Hanberger, L. Geographical variation in the incidence of type 1 diabetes in the Nordic countries: A study within NordicDiabKids. Pediatric Diabetes 2020, 21, 259–265. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Klug, M.G.; Rundquist, B.C. An exploration of colorectal cancer incidence rates in North Dakota, USA, via structural equation modeling. Int. J. Colorectal Dis. 2019, 34, 1571–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, K.M.; Abhinav, K.; Wijesekera, L.; Ganesalingam, J.; Goldstein, L.H.; Janssen, A.; Dougherty, A.; Willey, E.; Stanton, B.R.; Turner, M.R.; et al. The association between ALS and population density: A population based study. Amyotroph. Lateral Scler. 2010, 11, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Sharp, L.; Donnelly, D.; Hegarty, A.; Carsin, A.-E.; Deady, S.; McCluskey, N.; Gavin, A.; Comber, H. Risk of several cancers is higher in urban areas after adjusting for socioeconomic status. Results from a two-country population-based study of 18 common cancers. J. Urban Health 2014, 91, 510–525. [Google Scholar] [CrossRef] [Green Version]
- Sheehan, A.; Freni Sterrantino, A.; Fecht, D.; Elliott, P.; Hodgson, S. Childhood type 1 diabetes: An environment-wide association study across England. Diabetologia 2020, 63, 964–976. [Google Scholar] [CrossRef] [Green Version]
- Staines, A.; Bodansky, H.J.; McKinney, P.A.; Alexander, F.E.; McNally, R.J.; Law, G.R.; Lilley, H.E.; Stephenson, C.; Cartwright, R.A. Small area variation in the incidence of childhood insulin-dependent diabetes mellitus in Yorkshire, UK: Links with overcrowding and population density. Int. J. Epidemiol. 1997, 26, 1307–1313. [Google Scholar] [CrossRef] [Green Version]
- Tunstall, H.; Mitchell, R.; Gibbs, J.; Platt, S.; Dorling, D. Socio-demographic diversity and unexplained variation in death rates among the most deprived parliamentary constituencies in Britain. J. Public Health 2012, 34, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Van Cauwenberg, J.; Dunstan, D.; Cerin, E.; Koohsari, M.J.; Sugiyama, T.; Owen, N. Population density is beneficially associated with 12-year diabetes risk marker change among residents of lower socio-economic neighborhoods. Health Place 2019, 57, 74–81. [Google Scholar] [CrossRef] [Green Version]
- van der Aa, M.A.; de Vries, E.; Hoekstra, H.J.; Coebergh, J.W.W.; Siesling, S. Sociodemographic factors and incidence of melanoma in the Netherlands, 1994–2005. Eur. J. Cancer 2011, 47, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, V.; Garcia, A.M. Mortality and socioeconomic indicators in Spain 1962–1991. Eur. J. Public Health 2000, 10, 268–273. [Google Scholar] [CrossRef] [Green Version]
- Wickrama, K.A.T.; Wickrama, K.A.S.; Romas, J.A. The relationship of individual, family, and community characteristics with physical health: An adult study in 27 rural Minnesota counties. J. Rural Health 2005, 21, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Factor, R.; Waldron, I. Contemporary Population Densities and Human Health. Nature 1973, 243, 381–384. [Google Scholar] [CrossRef]
- Kirmeyer, S.L. Urban Density and Pathology: A Review of Research. Environ. Behav. 1978, 10, 247–269. [Google Scholar] [CrossRef]
- Dennis, M.; Cook, P.A.; James, P.; Wheater, C.P.; Lindley, S.J. Relationships between health outcomes in older populations and urban green infrastructure size, quality and proximity. BMC Public Health 2020, 20, 626. [Google Scholar] [CrossRef]
- McCullough, M.L.; Wan, N.; Pezzolesi, M.G.; Collins, T.W.; Grineski, S.E.; Dennis Wei, Y.; Lazaro-Guevara, J.; Frodsham, S.G.; Vanderslice, J.A.; Holmen, J.R.; et al. Type 1 Diabetes incidence among youth in Utah: A geographical analysis. Soc. Sci. Med. 2021, 278, 113952. [Google Scholar] [CrossRef]
- Gülden, E. Lifestyle factors affecting the gut microbiota’s relationship with Type 1 Diabetes. Curr. Diabetes Rep. 2018, 18, 111. [Google Scholar] [CrossRef]
- Bremberg, S. Rural-urban mortality inequalities in four Nordic welfare states. Scand. J. Public Health 2020, 48, 791–793. [Google Scholar] [CrossRef]
- Lin, L.; Li, Z.; Yan, L.; Liu, Y.; Yang, H.; Li, H. Global, regional, and national cancer incidence and death for 29 cancer groups in 2019 and trends analysis of the global cancer burden, 1990–2019. J. Hematol. Oncol. 2021, 14, 197. [Google Scholar] [CrossRef]
- Borck, R.; Schrauth, P. Population density and urban air quality. Reg. Sci. Urban Econ. 2021, 86, 103596. [Google Scholar] [CrossRef]
- Datzmann, T.; Markevych, I.; Trautmann, F.; Heinrich, J.; Schmitt, J.; Tesch, F. Outdoor air pollution, green space, and cancer incidence in Saxony: A semi-individual cohort study. BMC Public Health 2018, 18, 715. [Google Scholar] [CrossRef] [PubMed]
- Lanzinger, S.; Altug, H.; Schikowski, T.; Khodaverdi, S.; Rosenbauer, J.; Rathmann, W.; Praedicow, K.; Schönau, E.; Holl, R.W. Longitudinal relationship of particulate matter and metabolic control and severe hypoglycaemia in children and adolescents with type 1 diabetes. Environ. Res. 2022, 203, 111859. [Google Scholar] [CrossRef] [PubMed]
- Giles-Corti, B.; Vernez-Moudon, A.; Reis, R.; Turrell, G.; Dannenberg, A.L.; Badland, H.; Foster, S.; Lowe, M.; Sallis, J.F.; Stevenson, M.; et al. City planning and population health: A global challenge. Lancet 2016, 388, 2912–2924. [Google Scholar] [CrossRef]
- Prueitt, R.L.; Li, W.; Edwards, L.; Zhou, J.; Goodman, J.E. Systematic review of the association between long-term exposure to fine particulate matter and mortality. Int. J. Environ. Health Res. 2021, 1–39. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnegie, E.R.; Inglis, G.; Taylor, A.; Bak-Klimek, A.; Okoye, O. Is Population Density Associated with Non-Communicable Disease in Western Developed Countries? A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 2638. https://doi.org/10.3390/ijerph19052638
Carnegie ER, Inglis G, Taylor A, Bak-Klimek A, Okoye O. Is Population Density Associated with Non-Communicable Disease in Western Developed Countries? A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(5):2638. https://doi.org/10.3390/ijerph19052638
Chicago/Turabian StyleCarnegie, Elaine Ruth, Greig Inglis, Annie Taylor, Anna Bak-Klimek, and Ogochukwu Okoye. 2022. "Is Population Density Associated with Non-Communicable Disease in Western Developed Countries? A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 5: 2638. https://doi.org/10.3390/ijerph19052638
APA StyleCarnegie, E. R., Inglis, G., Taylor, A., Bak-Klimek, A., & Okoye, O. (2022). Is Population Density Associated with Non-Communicable Disease in Western Developed Countries? A Systematic Review. International Journal of Environmental Research and Public Health, 19(5), 2638. https://doi.org/10.3390/ijerph19052638





