Potential Effects of Romanian Propolis Extracts against Pathogen Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Propolis Sampling
2.2. Chemical Characterization of Raw Propolis
2.2.1. Qualitative Identification of Flavones’ Presence
2.2.2. Identification of Aromatic Acids
2.2.3. Quantification of the Phenolic Compounds
2.2.4. Determination of Flavonoid Content
2.3. The Antioxidant Activity of Propolis
2.4. Preparation of the Propolis Extracts
2.4.1. Preparation of the Aqueous Propolis Extracts
2.4.2. Preparation of the Ethanolic Extracts of Propolis
2.5. Identification of Quercetin and Rutin in Aqueous and Ethanolic Propolis Extracts
2.6. Molecular Descriptors for Rutin and Quercetin
2.7. Antimicrobial Activity of Aqueous Propolis Extracts
2.7.1. Micro-Organisms and Culture Conditions
2.7.2. Determination of the Antibacterial Properties of the Aqueous Propolis Extracts—Agar Disk Diffusion Method
2.7.3. Minimum Inhibitory Concentrations (MICs) of the Aqueous Propolis Extracts
2.8. Statistical Analysis
3. Results
3.1. Chemical Characterization of the Propolis Samples
3.2. Identification of Quercetin and Rutin in Aqueous and Ethanol Propolis Extracts
| Sample | Propolis Extract | Quercetin (mg/mL); RSD% | Rutin (mg/mL); RSD% | ||
|---|---|---|---|---|---|
| S4 | Aqueous | 0.74; 2.94 | 0.0143; 1.47 | ||
| Ethanolic 25% | 0.69; 2.83 | 0.0120; 1.38 | |||
| Ethanolic 50% | 0.83; 1.91 | 0.0057; 2.67 | |||
| Ethanolic 99% | 1.12; 1.64 | 0.0048; 2.81 | |||
| Ethanolic (99%) | Aqueous | Ethanolic (99%) | Aqueous | ||
| S1 | 1.04 | 0.57 | 0.0030 | 0.0153 | |
| S2 | 1.02 | 0.62 | 0.0127 | 0.0168 | |
| S3 | 1.20 | 0.62 | 0.0094 | 0.0080 | |
| S5 | 0.92 | 0.67 | 0.0027 | 0.0148 | |
| S6 | 1.10 | 0.83 | 0.0071 | 0.0196 | |
| S7 | 0.86 | 0.64 | 0.0046 | 0.0128 | |
| S8 | 0.83 | 0.82 | 0.0018 | 0.0171 | |
3.3. Molecular Descriptors for Rutin and Quercetin
3.4. Antimicrobial Activity of Aqueous Propolis Extracts
Minimum Inhibitory Concentration (MIC)
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rebolledo Ranz, R.E.R. Actuality and Trends of Beekeeping Worldwide. In Beekeeping-New Challenges; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/chapters/67687 (accessed on 12 January 2022).
- Hung, K.-L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172140. [Google Scholar] [CrossRef]
- Winfree, R.; Aguilar, R.; Vázquez, D.P.; LeBuhn, G.; Aizen, M.A. A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 2009, 9, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Hristov, P.; Shumkova, R.; Palova, N.; Neov, B. Factors Associated with Honey Bee Colony Losses: A Mini-Review. Vet. Sci. 2020, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Vercelli, M.; Croce, L.; Mancuso, T. An Economic Approach to Assess the Annual Stock in Beekeeping Farms: The Honey Bee Colony Inventory Tool. Sustainability 2020, 12, 9258. [Google Scholar] [CrossRef]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and modern uses of natural honey in human diseases: A review. Iran. J. Basic Med. Sci. 2013, 16, 731–742. [Google Scholar] [PubMed]
- Rocha, B.; Bueno, P.C.P.; Vaz, M.M.D.O.L.L.; Nascimento, A.P.; Ferreira, N.U.; Moreno, G.D.P.; Rodrigues, M.R.; Costa-Machado, A.R.D.M.; Barizon, E.A.; Campos, J.C.L.; et al. Evaluation of a Propolis Water Extract Using a Reliable RP-HPLC Methodology and In Vitro and In Vivo Efficacy and Safety Characterisation. Evid.-Based Complement. Altern. Med. 2013, 2013, 670451. [Google Scholar] [CrossRef]
- Krause, J.; Tobin, G. Discovery, Development, and Regulation of Natural Products. In Using Old Solutions to New Problems-Natural Drug Discovery in the 21st Century; Kulka, M., Ed.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Calixto, J.B. Twenty-five years of research on medicinal plants in Latin America: A personal view. J. Ethnopharmacol. 2005, 100, 131–134. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Nell, R.A.; Buckendorf, J.E.; Kúsz, N.; Mwangi, P.W.; Berkecz, R.; Rédei, D.; Vasas, A.; Spivak, A.M.; Hohmann, J. Bioactive Compounds from Euphorbia usambarica Pax. with HIV-1 Latency Reversal Activity. Pharmaceuticals 2021, 14, 653. [Google Scholar] [CrossRef]
- Parate, S.; Kumar, V.; Lee, G.; Rampogu, S.; Hong, J.C.; Lee, K.W. Marine-Derived Natural Products as ATP-Competitive mTOR Kinase Inhibitors for Cancer Therapeutics. Pharmaceuticals 2021, 14, 282. [Google Scholar] [CrossRef]
- Vica, M.L.; Glevitzky, M.; Dumitrel, G.A.; Junie, L.M.; Popa, M. Antibacterial activity of different natural honeys from Transylvania, Romania. J. Environ. Sci. Health B 2014, 49, 176–181. [Google Scholar] [CrossRef]
- De Castro, S.L. Propolis: Biological and pharmacological activities. Therapeutic uses of this bee-product. Annu. Rev. Biomed. Sci. 2001, 3, 49–83. [Google Scholar] [CrossRef]
- Lavigne, J.-P.; Ranfaing, J.; Dunyach-Remy, C.; Sotto, A. Synergistic Effect of Propolis and Antibiotics on Uropathogenic Escherichia coli. Antibiotics 2020, 9, 739. [Google Scholar] [CrossRef]
- Junie, L.M.; Vica, M.L.; Glevitzky, M.; Matei, H.V. Physico-chemical characterization and antibacterial activity of different types of honey tested on strains isolated from hospitalized patients. J. Apic. Sci. 2016, 60, 5–18. [Google Scholar] [CrossRef]
- Al-Waili, N.; Al-Ghamdi, A.; Ansari, M.J.; Al-Attal, Y.; Salom, K. Synergistic effects of honey and propolis toward drug multiresistant Staphylococcus aureus, Escherichia coli and Candida albicans isolates in single and polymicrobial cultures. Int. J. Med. Sci. 2012, 9, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jin, X.; Chen, Y.; Song, Z.; Jiang, X.; Hu, F.; Conlon, M.; Topping, D.L. Polyphenol-Rich Propolis Extracts Strengthen Intestinal Barrier Function by Activating AMPK and ERK Signaling. Nutrients 2016, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, A. Evidence on the Health Benefits of Supplemental Propolis. Nutrients 2019, 11, 2705. [Google Scholar] [CrossRef]
- Batista, L.L.V.; Campesatto, E.A.; Assis, M.L.B.; Barbosa, A.P.F.; Grillo, L.A.M.; Dornelas, C.B. Comparative study of topical green and red propolis in the repair of wounds induced in rats. Rev. Col. Bras. Cir. 2012, 39, 515–520. [Google Scholar] [CrossRef]
- Farooqui, T.; Farooqui, A.A. Beneficial effects of propolis on human health and neurological diseases. Front. Biosci. 2012, 4, 779–793. [Google Scholar] [CrossRef]
- Hochheim, S.; Guedes, A.; Faccin-Galhardi, L.; Rechenchoski, D.Z.; Nozawa, C.; Linhares, R.E.; da Filho, H.H.S.; Rau, M.; Siebert, D.A.; Micke, G.; et al. Determination of phenolic profile by HPLC–ESI-MS/MS, antioxidant activity, in vitro cytotoxicity and anti-herpetic activity of propolis from the Brazilian native bee Melipona quadrifasciata. Rev. Bras. Farmacogn. 2019, 29, 339–350. [Google Scholar] [CrossRef]
- Shashikala, A.; Harini, B.; Reddy, M.S. HPLC analysis of flavonoids from propolis of different honeybee species in selected locations of bangalore. Int. J. Pharm. Sci. Res. 2019, 10, 5423–5429. [Google Scholar]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic properties of bioactive compounds from different honeybee products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Markham, K.R.; Mitchell, K.A.; Wilkins, A.L.; Daldy, J.A.; Lu, Y. HPLC and GC-MS identification of the major organic constituents in New Zeland propolis. Phytochemistry 1996, 42, 205–211. [Google Scholar] [CrossRef]
- Bonvehí, J.S.; Coll, F.V. The composition, active components and bacteriostatic activity of propolis in dietetics. J. Am. Oil Chem. Soc. 1994, 71, 529–532. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Park, D.H.; Shin, E.J.; Kwon, M.S.; Ko, K.H.; Kim, W.K.; Jhoo, J.H.; Jhoo, W.K.; Wie, M.B.; Jung, B.D.; et al. Antioxidant propolis attenuates kainate-induced neurotoxicity via adenosine A1 receptor modulation in the rat. Neurosci. Lett. 2004, 355, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Osés, S.M.; Pascual-Maté, A.; Fernández-Muiño, M.A.; López-Díaz, T.M.; Sancho, M.T. Bioactive properties of honey with propolis. Food Chem. 2016, 196, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Pujirahayu, N.; Bhattacharjya, D.K.; Suzuki, T.; Katayama, T. α-Glucosidase Inhibitory Activity of Cycloartane-Type Triterpenes Isolated from Indonesian Stingless Bee Propolis and Their Structure-Activity Relationship. Pharmaceuticals 2019, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Adebooye, O.C.; Vijayalakshmi, R.; Singh, V. Peroxidase activity, chlorophylls and antioxidant profile of two leaf vegetables (Solanum nigrum L. and Amaranthus cruentus L.) under six pretreatment methods before cooking. Int. J. Food Sci. Technol. 2008, 43, 173–178. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Bendary, E.; Francis, R.R.; Ali, H.M.G.; Sarwat, M.I.; El Hady, S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef]
- Wang, K.; Ping, S.; Huang, S.; Hu, L.; Xuan, H.; Zhang, C.; Hu, F. Molecular mechanisms underlying the in vitro anti-inflammatory effects of a flavonoid-rich ethanol extract from chinese propolis (poplar type). Evid. Based Complement. Altern. Med. 2013, 2013, 127672. [Google Scholar] [CrossRef]
- Miyataka, H.; Nishiki, H.; Matsumoto, H.; Fijimoto, T.; Matsuka, M.; Satoh, T. Evaluation of Propolis. I. Evaluation of Brazilian and Chinese Propolis by Enzymatic and Physico-Chemical Methods. Biol. Pharm. Bull. 1997, 20, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Menniti-Ippolito, F.; Mazzanti, G.; Vitalone, A.; Firenzuoli, F.; Santuccio, C. Surveillance of suspected adverse reactions to natural health products: The case of propolis. Drug Saf. 2008, 31, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Mărghitaș, L.A. Albinele și Produsele Lor, 2nd ed.; Ceres Publishing: Bucharest, Romania, 2008; pp. 374–375. [Google Scholar]
- Vica, M.L.; Glevitzky, I.; Glevitzky, M.; Siserman, C.V.; Matei, H.V.; Teodoru, C.A. Antibacterial Activity of Propolis Extracts from the Central Region of Romania against Neisseria gonorrhoeae. Antibiotics 2021, 10, 689. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.K. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Marcucci, M.C.; Ferreres, F.; Garcıa-Viguera, C.; Bankova, V.S.; De Castro, S.L.; Dantas, A.P.; Valente, P.H.; Paulino, N. Phenolic compounds from Brazilian propolis with pharmacological activities. J. Ethnopharmacol. 2001, 74, 105–112. [Google Scholar] [CrossRef]
- Mărghitaş, L.A.; Dezmirean, D.; Moise, A.; Mihai, C.; Laslo, L. DPPH method for evaluation of propolis antioxidant activity. Bull. Univ. Agric. Sci. 2009, 66, 253–258. [Google Scholar] [CrossRef]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Nikolova-Damyanova, B.; Sabatini, A.G.; Marcazzan, G.L.; Bogdanov, S. Validated methods for the quantification of biologically active constituents of poplar type propolis. Phytochem. Anal. 2004, 15, 235–240. [Google Scholar] [CrossRef]
- HyperChem™, Release 7.01, Molecular Modeling System. Demo Version. Hypercub Inc. Available online: https://hiper.com (accessed on 15 December 2021).
- Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing. 2020. Available online: https://clsi.org/media/3481/m100ed30_sample.pdf (accessed on 29 July 2021).
- Box, G.E.P.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters: Design, Innovation, and Discovery, 2nd ed.; John Wiley & Sons Press: Hoboken, NJ, USA, 2005; Volume 2, p. 214. [Google Scholar]
- Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Propolis, Bee Honey, and Their Components Protect against Coronavirus Disease 2019 (COVID-19): A Review of In Silico, In Vitro, and Clinical Studies. Molecules 2021, 26, 1232. [Google Scholar] [CrossRef] [PubMed]
- Guler, H.I.; Tatar, G.; Yildiz, O.; Belduz, A.O.; Kolayli, S. Investigation of potential inhibitor properties of ethanolic propolis extracts against ACE-II receptors for COVID-19 treatment by molecular docking study. Arch. Microbiol. 2021, 203, 3557–3564. [Google Scholar] [CrossRef] [PubMed]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Rutin: A Potential Antiviral for Repurposing as a SARS-CoV-2 Main Protease (Mpro) Inhibitor. Nat. Prod. Commun. 2021, 16, 1934578X21991723. [Google Scholar] [CrossRef]
- Inokuchi, Y.; Shimazawa, M.; Nakajima, Y.; Suemori, S.; Mishima, S.; Hara, H. Brazilian Green Propolis Protects against Retinal Damage In Vitro and In Vivo. Evid.-Based Complement. Altern. Med. 2006, 3, 71–77. [Google Scholar] [CrossRef]
- Bazmandegan, G.; Boroushaki, M.T.; Shamsizadeh, A.; Ayoobi, F.; Hakimizadeh, E.; Allahtavakoli, M. Brown propolis attenuates cerebral ischemia-induced oxidative damage via affecting antioxidant enzyme system in mice. Biomed. Pharmacother. 2017, 85, 503–510. [Google Scholar] [CrossRef]
- Farooqui, T.; Farooqui, A.A. Molecular mechanism underlying the therapeutic activities of propolis: A critical review. Curr. Nutr. Food Sci. 2010, 6, 186–199. [Google Scholar] [CrossRef]
- Filipič, B.; Gradišnik, L.; Exel Gregorič, D.; Pereyra, A.; Ružić-Sabljić, E.; Mazija, H. The Polyphenol content and antimicrobial activity of selected Propolis’ extracts. J. Agric. Sci. Technol. 2020, 10, 350–364. [Google Scholar] [CrossRef]
- Luo, C.; Zou, X.; Li, Y.; Sun, C.; Jiang, Y.; Wu, Z. Determination of flavonoids in propolis-rich functional foods by reversed phase high performance liquid chromatography with diode array detection. Food Chem. 2011, 127, 314–320. [Google Scholar] [CrossRef]
- Ruoff, K.; Iglesias, M.T.; Luginbühl, W.; Bosset, J.O.; Bogdanov, S.; Amadò, R. Quantitative analysis of physical and chemical measurands in honey by mid-infrared spectrometry. Eur. Food. Res. Technol. 2006, 223, 22–29. [Google Scholar] [CrossRef]
- Cao, W.; Liu, H.; Cheng, N.; Gao, H.; Wang, B.; Zheng, J. LC with electrochemical detection for analysis of caffeic acid and caffeic acid phenyl ester in propolis. Chromatographia 2011, 73, 1864–1867. [Google Scholar] [CrossRef]
- Pellati, F.; Orlandini, G.; Pinetti, D.; Benvenuti, S. HPLC-DAD and HPLC-ESI-MS/MS methods for metabolite profiling of propolis extracts. J. Pharm. Biomed. Anal. 2011, 55, 934–948. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yan, Q.H.; Ma, J.Y.; Wang, Q.; Zhang, J.W.; Xi, G.X. High Performance Liquid Chromatographic Determination of Phenolic Compounds in Propolis. Trop. J. Pharm. Res. 2013, 12, 771–776. [Google Scholar] [CrossRef][Green Version]
- García-Viguera, C.; Ferreres, F.; Tomás-Barberán, F.A. Study of Canadian Propolis by GC-MS and HPLC. Z. Naturforsch. 1993, 48, 731–735. [Google Scholar] [CrossRef]
- Saad, M.A.; Salam, R.M.A.; Kenawy, S.A.; Attia, A.S. Pinocembrin attenuates hippocampal inflammation, oxidative perturbations and apoptosis in a rat model of global cerebral ischemia reperfusion. Pharmacol. Rep. 2015, 67, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Yesiltas, B.; Capanoglu, E.; Firatligil-Durmus, E.; Sunay, A.E.; Samanci, T.; Boyacioglu, D. Investigating the in-vitro bioaccessibility of propolis and pollen using a simulated gastrointestinal digestion System. J. Apic. Res. 2014, 53, 101–108. [Google Scholar] [CrossRef]
- Bonvehí, J.S.; Coll, F.V. Phenolic composition of propolis from China and from South America. Z. Naturforsch. 1994, 49, 712–718. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Miao, Y.; Cui, Q.; Zhao, W.; Zhang, J.; Wang, H. Pinocembrin protects SH-SY5Y cells against MPP+-induced neurotoxicity through the mitochondrial apoptotic pathway. J. Mol. Neurosci. 2014, 53, 537–545. [Google Scholar] [CrossRef]
- Mateescu, C.; Dumitru, I.F. Propolisul şi Extractele de Propolis; Ilex Publishing House: Bucharest, Romania, 2001; pp. 87–94. [Google Scholar]
- Freires, I.A.; de Alencar, S.M.; Rosalen, P.L. A pharmacological perspective on the use of Brazilian Red Propolis and its isolated compounds against human diseases. Eur. J. Med. Chem. 2016, 110, 267–279. [Google Scholar] [CrossRef]
- Mihai, C.M.; Marghitas, L.A.; Dezmirean, D.S.; Chirila, F.; Moritz, R.F.; Schlüns, H. Interactions among flavonoids of propolis affect antibacterial activity against the honeybee pathogen Paenibacillus larvae. J. Invertebr. Pathol. 2012, 110, 68–72. [Google Scholar] [CrossRef]
- Haenen, G.R.M.M.; Arts, M.J.T.J.; Bast, A.; Coleman, M.D. Structure and activity in assessing antioxidant activity in vitro and in vivo: A critical appraisal illustrated with the flavonoids. Environ. Toxicol. Pharmacol. 2006, 21, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Nair, M.G.; Strasburg, G.M. Structure–Activity Relationships for Antioxidant Activities of a Series of Flavonoids in a Liposomal System. Free Radic. Biol. Med. 1998, 24, 1355–1363. [Google Scholar] [CrossRef]
- Da Silva, J.F.M.; de Souza, M.C.; Matta, S.R.; de Andrade, M.R.; Vidal, F.V.N. Correlation analysis between phenolic levels of Brazilian propolis extracts and their antimicrobial and antioxidant activities. Food Chem. 2006, 99, 431–435. [Google Scholar] [CrossRef]
- Ahn, M.R.; Kumazawa, S.; Usui, Y.; Nakamura, J.; Matsuka, M.; Zhu, F.; Nakayama, T. Antioxidant activity and constituents of propolis collected in various areas of China. Food Chem. 2007, 101, 1383–1392. [Google Scholar] [CrossRef]
- Lan, X.; Wang, W.; Li, Q.; Wang, J. The Natural Flavonoid Pinocembrin: Molecular Targets and Potential Therapeutic Applications. Mol. Neurobiol. 2016, 53, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Haddadin, M.S.Y.; Nazer, I.; Raddad, S.J.A.; Robinson, R.K. Effect of propolis on two bacterial species with probiotic potential. Pak. J. Nutr. 2008, 7, 391–394. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, propolis, and royal jelly: A comprehensive review of their biological actions and health benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Vica, M.L.; Glevitzky, M.; Tit, D.M.; Behl, T.; Heghedus-Mîndru, R.C.; Zaha, D.C.; Ursu, F.; Popa, M.; Glevitzky, I.; Bungau, S. The antimicrobial activity of honey and propolis extracts from the central region of Romania. Food Biosci. 2021, 41, 101014. [Google Scholar] [CrossRef]
- Cardinault, N.; Cayeux, M.-O.; Du Sert, P.P. La propolis: Origine, composition et propriétes. Phytothérapie 2012, 10, 298–304. [Google Scholar] [CrossRef]
- Daher, S.; Gülaçar, F.O. Analysis of phenolic and other aromatic compounds in honeys by solid-phase micro extraction followed by gas chromatographymass spectrometry. J. Agric. Food Chem. 2008, 56, 5775–5780. [Google Scholar] [CrossRef]
- Lopez, B.G.-C.; de Lourenco, C.C.; Alves, D.A.; Machado, D.; Lancellotti, M.; Sawaya, A.C.H.F. Antimicrobial and cytotoxic activity of red propolis: An alert for its safe use. J. Appl. Microbiol. 2015, 119, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Feás, X.; Iglesias, A.; Rodrigues, S.; Estevinho, L.M. Effect of Erica sp. Honey against Microorganisms of Clinical Importance: Study of the Factors Underlying this Biological Activity. Molecules 2013, 18, 4233–4246. [Google Scholar] [CrossRef] [PubMed]
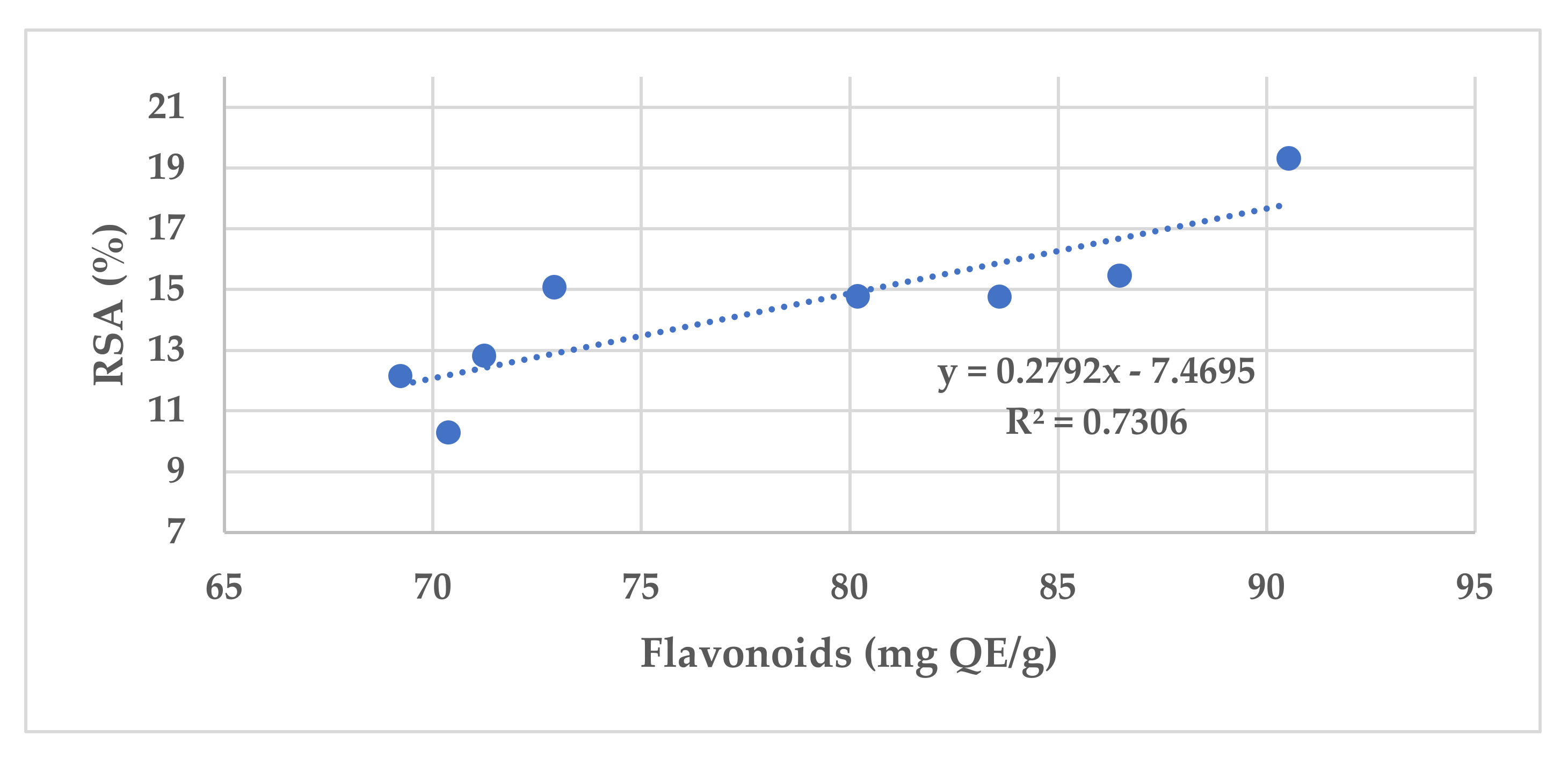
| Sample | Phenolic Compounds (mg GAE/g) | Flavonoids (mg QE/g) | Flavones | Aromatic Acids | RSA (%) |
|---|---|---|---|---|---|
| S1 | 187.9 ± 6.25 | 83.60 ± 0.05 | + | + | 14.75 |
| S2 | 172.2 ± 6.14 | 70.37 ± 0.03 | + | + | 10.29 |
| S3 | 158.8 ± 5.27 | 86.48 ± 0.02 | + | − | 15.46 |
| S4 | 203.3 ± 7.28 | 90.54 ± 0.06 | + | + | 19.31 |
| S5 | 181.5 ± 6.10 | 72.92 ± 0.07 | + | + | 16.07 |
| S6 | 134.7 ± 4.09 | 71.24 ± 0.02 | + | + | 13.82 |
| S7 | 190.6 ± 5.26 | 80.19 ± 0.01 | + | + | 14.78 |
| S8 | 169.1 ± 8.39 | 69.23 ± 0.04 | + | + | 11.15 |
| Molecular Descriptor | Rutin | Quercetin |
|---|---|---|
| A [Å2] | 783.58 | 443.05 |
| V [Å3] | 1320.04 | 704.27 |
| Log P | 11.21 | 2.52 |
| R [Å3] | 97.01 | 77.12 |
| α [Å3] | 44.18 | 23.99 |
| Hformation [kcal/mol] | −598.683 | 150.6105 |
| Ehidr [kcal/mol] | −22.54 | −10 |
| μt [D] | 2.162 | 2.352 |
| No. of –OH phenolic groups | 4 | 5 |
| Antioxidant activity [%] * | 90.9 * | 89.9 * |
| Strain | Inhibition Diameter Area (mm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample No. (0.1 g/mL) | Total ∑xj | Average xj | Ciprofloxacin (5 µg) | ||||||||
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | ||||
| S. aureus | 16 | 19 | 31 | 30 | 19 | 25 | 32 | 18 | 190 | 23.75 | 30 |
| B. cereus | 28 | 29 | 27 | 25 | 26 | 27 | 28 | 29 | 219 | 27.37 | 30 |
| B. subtilis | 29 | 23 | 27 | 28 | 24 | 29 | 27 | 29 | 216 | 27 | 30 |
| P. aeruginosa | 32 | 31 | 31 | 27 | 32 | 30 | 29 | 30 | 242 | 30.25 | 25 |
| E. coli | 32 | 26 | 19 | 32 | 18 | 30 | 27 | 22 | 206 | 25.75 | 29 |
| L. monocytogenes | 30 | 30 | 31 | 29 | 30 | 31 | 30 | 31 | 242 | 30.25 | 24 |
| S. typhimurium | 30 | 21 | 18 | 31 | 20 | 29 | 26 | 20 | 195 | 24.37 | 29 |
| Total ∑xi | 197 | 179 | 184 | 202 | 169 | 201 | 199 | 179 | ∑xij = 1510 | - | |
| Average xi | 28.14 | 25.57 | 26.28 | 28.85 | 24.14 | 28.71 | 28.42 | 25.57 | |||
| Sample No. | MIC (mg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| S. aureus | B. cereus | B. subtilis | P. aeruginosa | E. coli | L. monocytogenes | S.typhimurium | |
| S1 | 12.5 | 6.25 | 6.25 | 3.12 | 6.25 | 3.12 | 6.25 |
| S2 | 6.25 | 12.5 | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 |
| S3 | 6.25 | 3.12 | 6.25 | 3.12 | 6.25 | 3.12 | 12.5 |
| S4 | 3.12 | 1.56 | 3.12 | 1.56 | 3.12 | 1.56 | 3.12 |
| S5 | 25.0 | 6.25 | 12.5 | 12.5 | 25.0 | 6.25 | 12.5 |
| S6 | 6.25 | 3.12 | 3.12 | 1.56 | 3.12 | 1.56 | 3.12 |
| S7 | 6.25 | 3.12 | 3.12 | 3.12 | 6.25 | 3.12 | 3.12 |
| S8 | 12.5 | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 | 12.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vică, M.L.; Glevitzky, M.; Heghedűş-Mîndru, R.C.; Glevitzky, I.; Matei, H.V.; Balici, S.; Popa, M.; Teodoru, C.A. Potential Effects of Romanian Propolis Extracts against Pathogen Strains. Int. J. Environ. Res. Public Health 2022, 19, 2640. https://doi.org/10.3390/ijerph19052640
Vică ML, Glevitzky M, Heghedűş-Mîndru RC, Glevitzky I, Matei HV, Balici S, Popa M, Teodoru CA. Potential Effects of Romanian Propolis Extracts against Pathogen Strains. International Journal of Environmental Research and Public Health. 2022; 19(5):2640. https://doi.org/10.3390/ijerph19052640
Chicago/Turabian StyleVică, Mihaela Laura, Mirel Glevitzky, Ramona Cristina Heghedűş-Mîndru, Ioana Glevitzky, Horea Vladi Matei, Stefana Balici, Maria Popa, and Cosmin Adrian Teodoru. 2022. "Potential Effects of Romanian Propolis Extracts against Pathogen Strains" International Journal of Environmental Research and Public Health 19, no. 5: 2640. https://doi.org/10.3390/ijerph19052640
APA StyleVică, M. L., Glevitzky, M., Heghedűş-Mîndru, R. C., Glevitzky, I., Matei, H. V., Balici, S., Popa, M., & Teodoru, C. A. (2022). Potential Effects of Romanian Propolis Extracts against Pathogen Strains. International Journal of Environmental Research and Public Health, 19(5), 2640. https://doi.org/10.3390/ijerph19052640






