Changes in Ammonia-Oxidizing Archaea and Bacterial Communities and Soil Nitrogen Dynamics in Response to Long-Term Nitrogen Fertilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Sampling and Physicochemical Analyses
2.3. DNA Extraction and qPCR
2.4. Illumina Miseq Sequencing and Bioinformatic Analysis
2.5. Statistical Analysis
3. Results
3.1. Soil Physicochemical Parameters, PNA, Yield of Wheats, and NUE
3.2. Community Abundances of AOA and AOB
3.3. Richness and Diversity of AOA and AOB
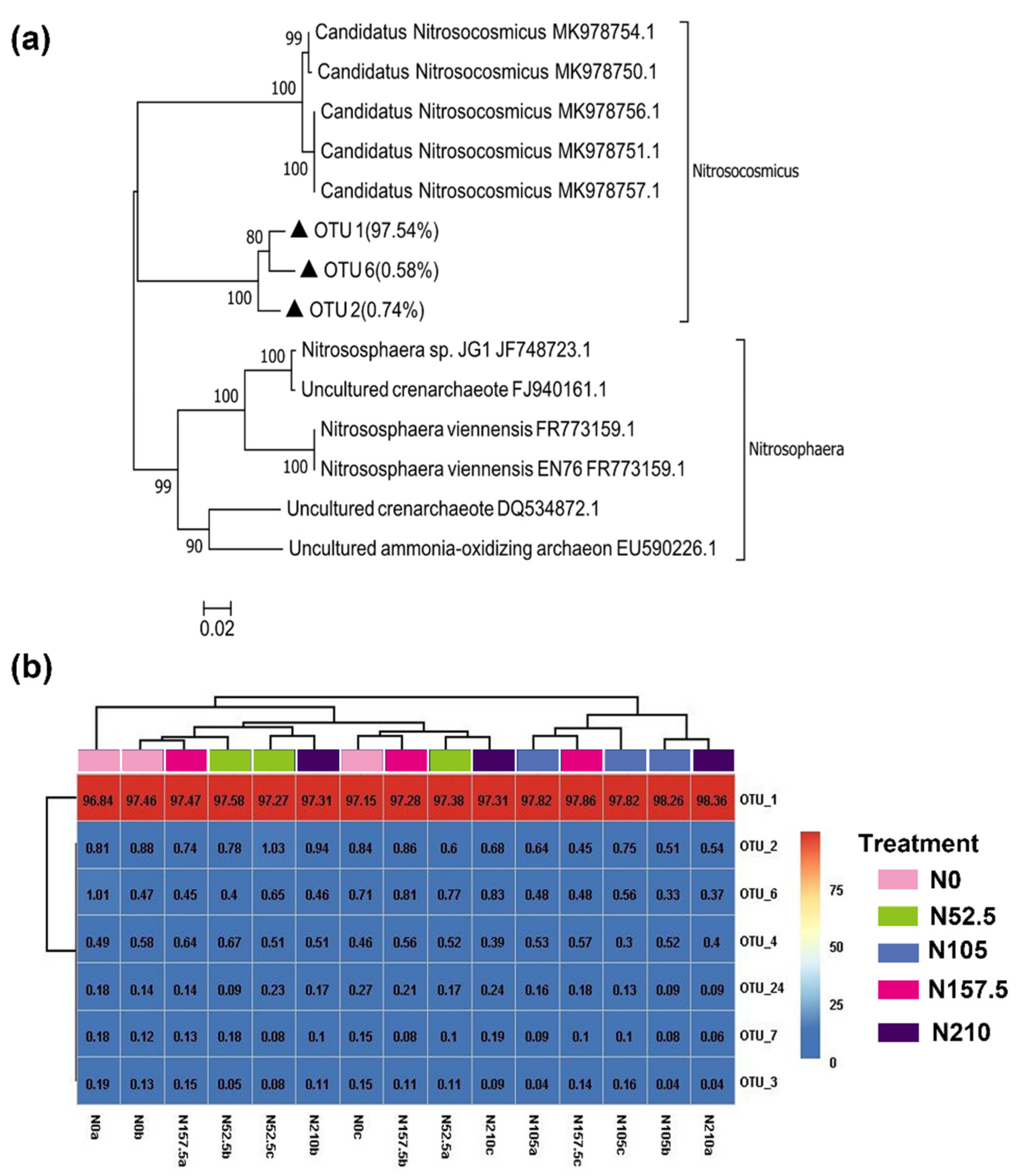
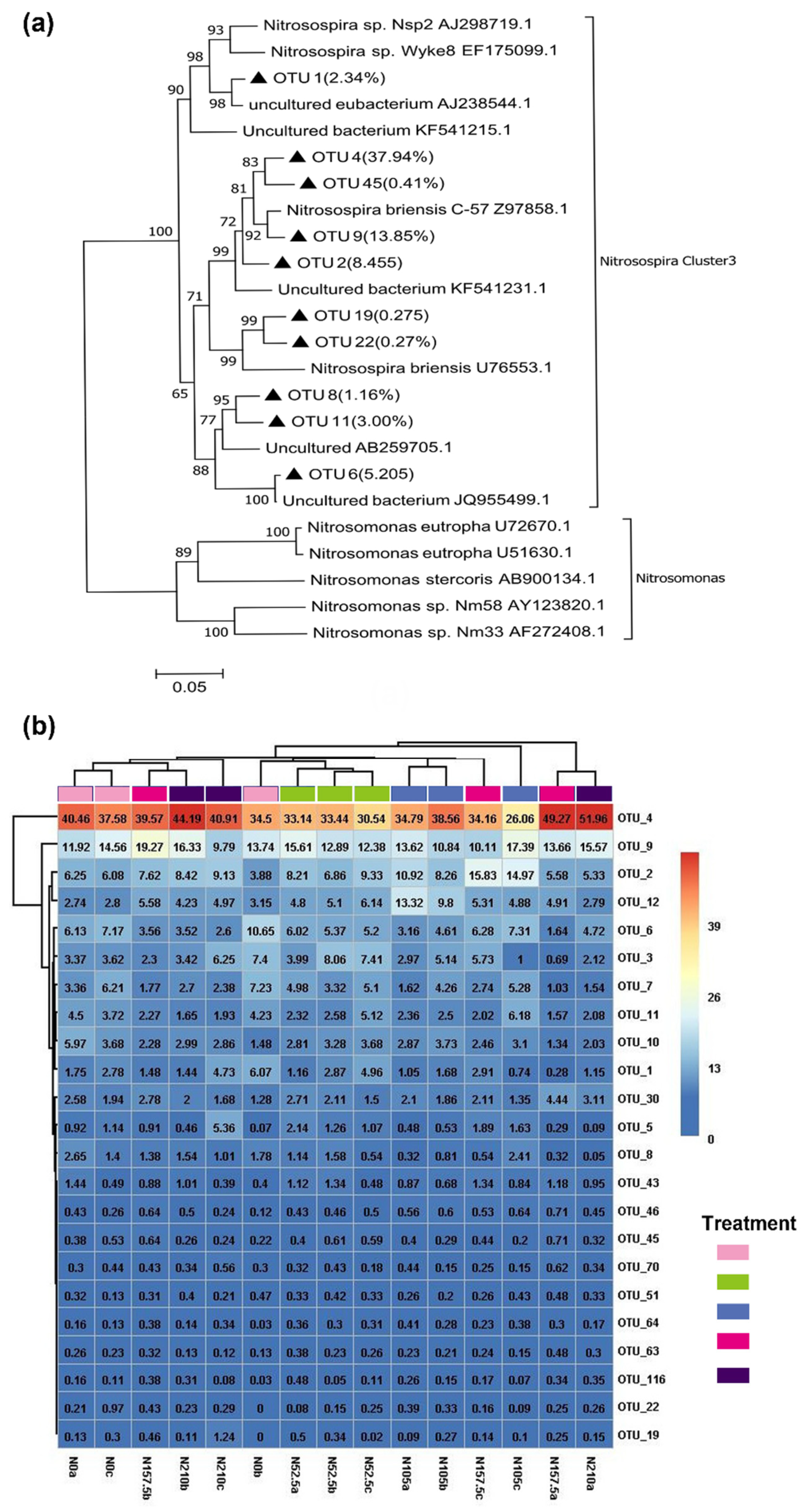
3.4. AOA and AOB Community
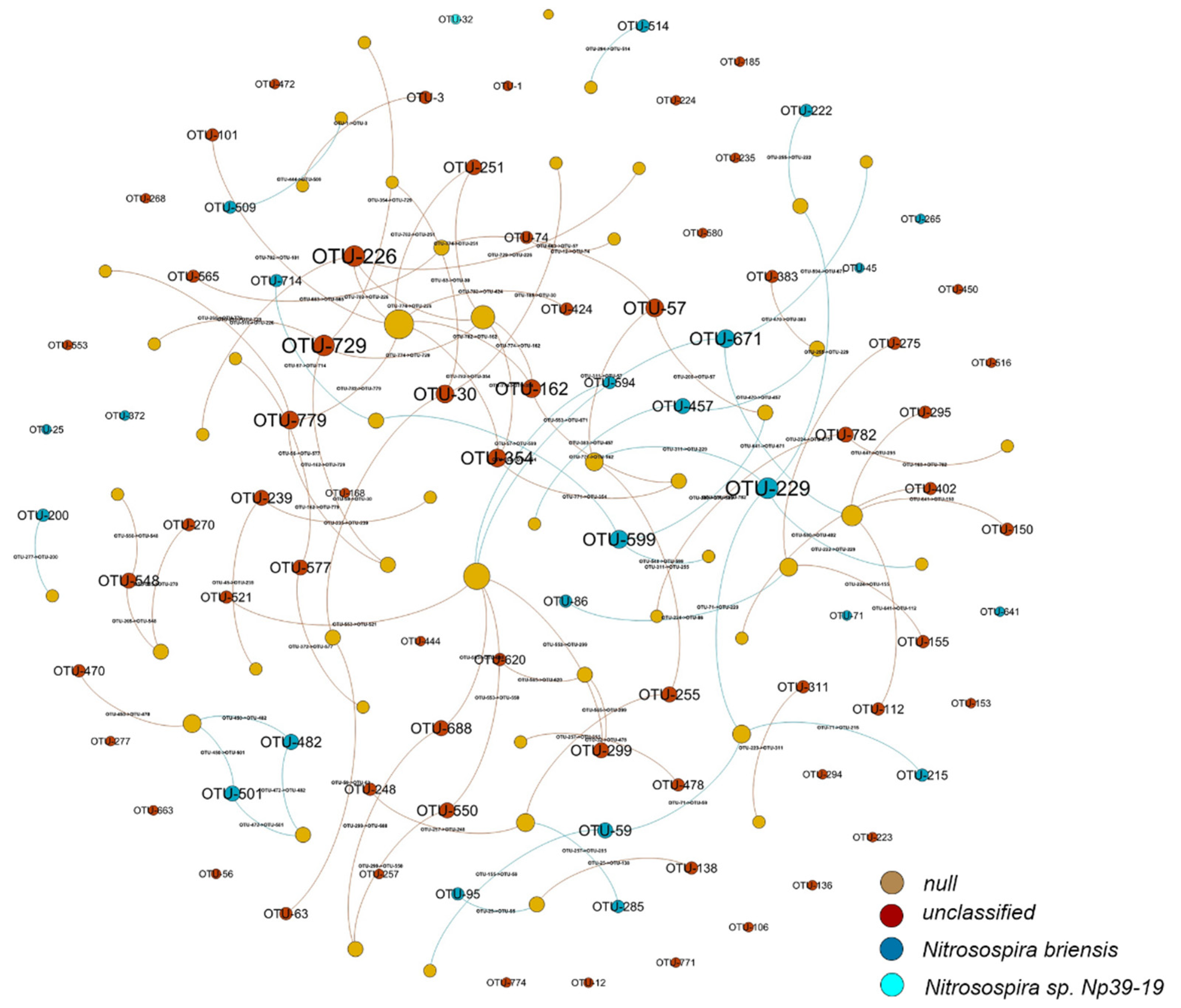
3.5. Network Associations among OTUs of AOB Species
3.6. Correlation of Ammonia-Oxidizing Communities with the Soil Properties
4. Discussion
4.1. Effect of N Fertilization on AOA and AOB Abundances
4.2. Composition of AOA and AOB
4.3. Relationships between Soil Properties and AOA and AOB Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hatzenpichler, R. Diversity, physiology, and niche differentiation of ammonia-oxidizing archaea. Appl. Environ. Microbiol. 2012, 78, 7501–7510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.; Bi, X.; Xu, C. Ammonia-Oxidizing Archaea (AOA) Play with Ammonia-Oxidizing Bacteria (AOB) in Nitrogen Removal from Wastewater. Archaea 2018, 2018, 8429145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhtar, H.; Lin, Y.-P.; Anthony, J. Ammonia Oxidizing Archaea and Bacteria in East Asian Paddy Soils—A Mini Review. Environments 2017, 4, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Kool, D.M.; Dolfing, J.; Wrage, N.; Van Groenigen, J.W. Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil. Soil Biol. Biochem. 2011, 43, 174–178. [Google Scholar] [CrossRef]
- Zhu, X.; Burger, M.; Doane, T.A.; Horwath, W.R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2 and NO under low oxygen availability. Proc. Natl. Acad. Sci. USA 2013, 110, 6328–6333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adair, K.L.; Schwarta, F. Evidence that ammonia-oxidizing archaea are more abundant than ammonia-oxidizing bacteria in semiarid soils of northern Arizona, USA. Microb. Ecol. 2008, 56, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Kowalchuk, G.A.; Stephen, J.R. Ammonia-oxidizing bacteria: A model for molecular microbial ecology. Annu. Rev. Microbiol. 2001, 55, 485–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prosser, J.I. Soil Nitrifiers and Nitrification; ASM Press: Washington, DC, USA, 2011; pp. 347–381. [Google Scholar]
- Li, Y.; Ding, K.; Wen, X.; Zhang, B.; Shen, B.; Yang, Y. A novel ammonia-oxidizing archaeon from wastewater treatment plant: Its enrichment, physiological and genomic characteristics. Sci. Rep. 2016, 6, 23747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzanakakis, V.A.; Paranychianakis, N.V. Divergent response of ammonia oxidizers to various amino acids. Appl. Soil Ecol. 2017, 114, 45–51. [Google Scholar] [CrossRef]
- Ouyang, Y.; Norton, J.M.; Stark, J.M.; Reeve, J.R.; Habteselassie, M.Y. Ammonia-oxidizing bacteria are more responsive than archaea to nitrogen source in an agricultural soil. Soil Biol. Biochem. 2016, 96, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Zeng, D.; Jiang, F.; Zhao, Q. Responses of biomass to the addition of water, nitrogen and phosphorus in Keerqin sandy grassland, Inner Mongolia, China. For. Res. 2009, 20, 23–26. [Google Scholar] [CrossRef]
- Wang, Z.; Zong, H.; Zheng, H.; Liu, G.; Chen, L.; Xing, B. Reduced nitrification and abundance of ammonia-oxidizing bacteria in acidic soil amended with biochar. Chemosphere. 2015, 138, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.K.; Hina, M.; Tahir, M.M. Effect of Azadirachta indica (neem), sodium thiosulphate and calcium chloride on changes in nitrogen transformations and inhibition of nitrification in soil incubated under laboratory conditions. Chemosphere 2011, 82, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- He, J.Z.; Shen, J.P.; Zhang, L.M.; Zhu, Y.G.; Zheng, Y.M.; Xu, M.G.; Di, H. Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ. Microbiol. 2007, 9, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, C.; Zhang, J.; Huang, Q.; Deng, H.; Deng, Y.; Zhong, W. Long-term fertilization effects on active ammonia oxidizers in an acidic upland soil in China. Soil Biol. Biochem. 2015, 84, 28–37. [Google Scholar] [CrossRef]
- Segal, L.M.; Miller, D.N.; McGhee, R.P.; Loecke, T.D.; Cook, K.L.; Shapiro, C.A.; Drijber, R.A. Bacterial and archaeal ammonia oxidizers respond differently to long-term tillage and fertilizer management at a continuous maize site. Soil Tillage Res. 2017, 168, 110–117. [Google Scholar] [CrossRef]
- Chu, H.; Fujii, T.; Morimoto, S.; Lin, X.; Yagi, K.; Hu, J.; Zhang, J. Community structure of ammonia-oxidizing bacteria under long-term application of mineral fertilizer and organic manure in a sandy loam soil. Appl. Environ. Microbiol. 2007, 73, 485–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Lu, L.; Wang, B.; Lin, X.; Zhu, J.; Cai, Z.; Yan, X.; Jia, Z. Long-term field fertilization significantly alters community structure of ammonia-oxidizing bacteria rather than archaea in a paddy soil. Soil Sci. Soc. Am. J. 2011, 75, 1431–1439. [Google Scholar] [CrossRef]
- Ai, C.; Liang, G.; Sun, J.; Wang, X.; He, P.; Zhou, W. Different roles of rhizosphere effect and long-term fertilization in the activity and community structure of ammonia oxidizers in a calcareous fluvo-aquic soil. Soil Biol. Biochem. 2013, 57, 30–42. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.Y.; Deng, X.; Herbert, S.; Xing, B.S. Impacts of adding biochar on nitrogen retention and bioavailability in agricultural soil. Geoderma 2013, 206, 32–39. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Shi, Y.; He, N.; Wen, X.; Yu, Q.; Zheng, C.; Sun, X.; Qiu, W. Responses of soil hydrolytic enzymes, ammonia-oxidizing bacteria and archaea to nitrogen applications in a temperate grassland in Inner Mongolia. Sci. Rep. 2016, 6, 32791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levicnik-Hofferle, S.; Nicol, G.W.; Ausec, L.; Mandic-Mulec, I.; Prosser, J.I. Stimulation of thaumarchaeal ammonia oxidation by ammonia derived from organic nitrogen but not added inorganic nitrogen. FEMS Microbiol. Ecol. 2012, 80, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Di, H.J.; Cameron, K.C. Nitrate leaching in temperate agroecosystems: Sources, factors and mitigating strategies. Nutr. Cycl. Agroecosyst. 2002, 64, 237–256. [Google Scholar] [CrossRef]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Guo, Y.J.; Di, H.J.; Cameron, K.C.; Li, B. Effect of application rate of a nitrification inhibitor, dicyandiamide (DCD), on nitrification rate, and ammonia-oxidizing bacteria and archaea growth in a grazed pasture soil: An incubation study. J. Soils Sediments 2014, 14, 897–903. [Google Scholar] [CrossRef]
- Kleineidam, K.; Košmrlj, K.; Kublik, S.; Palmer, I.; Pfab, H.; Ruser, R.; Schloter, M. Influence of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on ammonia-oxidizing bacteria and archaea in rhizosphere and bulk soil. Chemosphere 2011, 84, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Wang, J.G.; Almøy, T.; Bakken, L.R. Excessive use of nitrogen in Chinese agriculture results in high N2O/(N2O + N2) product ratio of denitrification, primarily due to acidification of the soils. Glob. Chang. Biol 2014, 20, 1685–1698. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Lu, C.; Ciais, P.; Michalak, A.M.; Canadell, J.G.; Saikawa, E.; Huntzinger, D.N.; Gurney, K.R.; Sitch, S.; Zhang, B. The terrestrial biosphere as a net source of greenhouse gases to the atmosphere. Nature 2016, 531, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Hangs, R.D.; Schoenau, J.J.; Lafond, G.; Breme, E. The effect of nitrogen fertilization and no-Till duration on soil nitrogen supply power and post-spring thaw greenhouse-Gas emissions. J. Plant Nutr. Soil Sci. 2013, 176, 227–237. [Google Scholar] [CrossRef]
- Zhu, Z.L.; Chen, D.L. Nitrogen fertilizer use in China–Contributions to food production, impacts on the environment and best management strategies. Nutr. Cycl. Agroecosyst. 2002, 63, 117–127. [Google Scholar] [CrossRef]
- Yang, S.M.; Malhi, S.S.; Song, J.R.; Xiong, Y.C.; Yue, W.Y.; Lu, L.L.; Wan, J.G.; Guo, T.W. Crop yield, nitrogen uptake and nitrate-Nitrogen accumulation in soil as affected by 23 annual applications of fertilizer and manure in the rainfed region of Northwestern China. Nutr. Cycl. Agroecosyst. 2006, 76, 81–94. [Google Scholar] [CrossRef]
- Directive, C. Concerning the protection of waters against pollution caused by nitrates from agricultural sources. Off. J. 1991, 375, 1–8. [Google Scholar]
- Schröder, J.J.; Scholefield, D.; Cabralet, F.; Hofmanal, G. The effects of nutrient losses from agriculture on ground and surface water quality: The position of science in developing indicators for regulation. Environ. Sci. Policy 2004, 7, 15–23. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, J.; Clark, C.; Naeem, S.; Pan, Q.; Huang, J.; Zhang, L.; Han, X. Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: Evidence from inner Mongolia Grasslands. Glob. Chang. Biol. 2010, 16, 358–372. [Google Scholar] [CrossRef]
- Prosser, J.I.; Nicol, G.W. Archaeal and bacterial ammonia- oxidisers in soil: The quest for niche specialisation and differentiation. Trends Microbiol. 2012, 20, 523–531. [Google Scholar] [CrossRef] [Green Version]
- Azziz, G.; Trasante, T.; Monza, J.; Irisarri, P. The effect of soil type, rice cultivar and water management on ammonia-oxidizing archaea and bacteria populations. Appl. Soil Ecol. 2016, 100, 8–17. [Google Scholar] [CrossRef]
- Zhang, L.M.; Hu, H.W.; Shen, J.P.; He, J.Z. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. Multidiscip. J. Microb. Ecol. 2012, 6, 1032–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsiknia, M.; Paranychianakis, N.V.; Varouchakis, E.A.; Nikolaidis, N.P. Environmental drivers of the distribution of nitrogen functional genes at a watershed scale. FEMS Microbiol. Ecol. 2015, 91, fiv052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.C.; Schleper, C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 2006, 442, 806–809. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.A.; Wakelin, S.A.; Fillery, I.R.P.; Roper, M.M. Factors affecting ammonia-oxidising microorganisms and potential nitrification rates in southern Australian agricultural soils. Soil Res. 2013, 51, 240–252. [Google Scholar] [CrossRef]
- Shen, J.P.; Zhang, L.M.; Zhu, Y.G.; Zhang, J.B.; He, J.Z. Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environ. Microbiol. 2008, 10, 1601–1611. [Google Scholar] [CrossRef]
- Jia, Z.; Conrad, R. Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ. Microbiol. 2009, 11, 1658–1671. [Google Scholar] [CrossRef] [PubMed]
- Tourna, M.; Freitag, T.E.; Nicol, G.W.; Prosser, J.I. Growth, activity and temperature responses of ammonia-oxidizing archaea and bacteria in soil N microcosms. Environ. Microbiol. 2008, 10, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhu, G.; Liu, R. How nitrification related N2O is associated with soil ammonia oxidizers in two contrasting soils in China. Sci. Total Environ. 2020, 770, 143212. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Wakelin, S.A.; Liang, Y.; Chu, G. Response of ammonia-oxidizing archaea and bacteria in calcareous soil to mineral and organic fertilizer application and their relative contribution to nitrification. Soil Biol. Biochem. 2017, 114, 20–30. [Google Scholar] [CrossRef]
- Yin, C.; Fan, F.L.; Song, A.L.; Fan, X.P.; Ding, H.; Ran, W.; Qiu, H.Z.; Liang, Y.C. The response patterns of community traits of N2O emission-related functional guilds to temperature across different arable soils under inorganic fertilization. Soil Biol. Biochem. 2017, 108, 65–77. [Google Scholar] [CrossRef]
- Xu, A.; Li, L.; Xie, J.; Wang, X.; Coulter, J.A.; Liu, C.; Wang, L. Effect of Long-Term Nitrogen Addition on Wheat Yield, Nitrogen Use Efficiency, and Residual Soil Nitrate in a Semiarid Area of the Loess Plateau of China. Sustainability 2020, 12, 1735. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization. The State of Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 1990. [Google Scholar]
- Chinese Soil Taxonomy Cooperative Research Group. Chinese Soil Taxonomy (Revised Proposal); Institute of Soil Science, Chinese Agricultural Science and Technology Press: Beijing, China, 1995. [Google Scholar]
- Bao, S.D. Analysis of Soil Agro-Chemistry; Agriculture Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- National Agriculture Technology Extension and Service Center (NATESC). Soil Analysis Technology Specification; China Agriculture Press: Beijing, China, 2006; pp. 47–49. (In Chinese) [Google Scholar]
- Li, X.; Deng, Y.; Li, Q.; Lu, C.; Wang, J.; Zhang, H.; Zhu, J.; Zhou, J.; He, Z. Shifts of functional gene representation in wheat rhizosphere microbial communities under elevated ozone. ISME J. 2013, 7, 660–671. [Google Scholar] [CrossRef] [Green Version]
- O’Kelly, B.C. Accurate Determination of Moisture Content of Organic Soils Using the Oven Drying Method. Dry. Technol. 2004, 22, 1767–1776. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, S.; Feng, X.; Fan, G.; Jetten, M.; Yin, C. Anammox bacterial abundance, biodiversity and activity in a constructed wetland. Environ. Sci. Technol. 2011, 45, 9951–9958. [Google Scholar] [CrossRef]
- Park, S.J.; Park, B.J.; Rhee, S.K. Comparative analysis of archaeal 16S rRNA and amoA genes to estimate the abundance and diversity of ammonia-oxidizing archaea in marine sediments. Extremophiles 2008, 12, 605–615. [Google Scholar] [CrossRef]
- Meinhardt, K.A.; Bertagnolli, A.; Pannu, M.W.; Strand, S.E.; Stahl, D.A. Evaluation of revised polymerase chain reaction primers for more inclusive quantification of ammonia-oxidizing archaea and bacteria: Revised primers for AOA and AOB quantification. Environ. Microbiol. Rep. 2015, 7, 1758–2229. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Chen, B.; Liu, Y.; Wang, C.; Gu, J.; Feng, H.; Du, G.; Ma, X. Higher Abundance of Ammonia Oxidizing Archaea than Ammonia Oxidizing Bacteria and Their Communities in Tibetan Alpine Meadow Soils under Long-term Nitrogen Fertilization. Geomicrobiol. J. 2014, 31, 597–604. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Lu, Q.; Raza, W.; Huang, Q.; Shen, Q. Ammonia oxidizer abundance in paddy soil profile with different fertilizer regimes. Appl. Soil Ecol. 2014, 84, 38–44. [Google Scholar] [CrossRef]
- Meyer, A.; Focks, A.; Radl, V.; Welzl, G.; Ingo, S.; Schloter, M. Influence of land use intensity on the diversity of ammonia oxidizing bacteria and archaea in soils from grassland ecosystems. Microb. Ecol. 2014, 67, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Okano, Y.; Hristova, K.R.; Leutenegger, C.M.; Jackson, L.E.; Denison, R.F.; Gebreyesus, B.; Lebauer, D.; Scow, K.M. Application of real-time PCR to study effects ofammonium on population size of ammonia-oxidizing bacteria in soil. Appl. Environ. Microbiol. 2004, 70, 1008–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermansson, A.; Lindgren, P.E. Quantification of ammonia oxidizing bacteria in arable soil by real-time PCR. Appl. Environ. Microbiol. 2001, 67, 972–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schauss, K.; Focks, A.; Leininger, S.; Kotzerke, A.; Heuer, H.; Thiele-Bruhn, W.; Sharma, B.; Matthies, M.; Smalla, K.; Munch, J.C.; et al. Dynamics and functional relevance of ammonia-oxidizing archaea in two agricultural soils. Environ. Microbiol. 2009, 11, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.F.; Shi, X.J.; Zheng, Y.; Qin, Z.X.; Xie, D.T.; Li, Z.L.; Guo, T. Abundance and community composition of ammonia-oxidizing bacteria and archaea in purple soil under long-term fertilization. Eur. J. Soil Biol. 2014, 60, 24–33. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C.; Shen, J.P.; Winefield, C.S.; Callaghan, M.O.; Bowatte, S.; He, J.Z. Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol. Ecol. 2010, 72, 386–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicol, G.W.; Leininger, S.; Schleper, C.; Prosser, J.I. The influence of soil pH on the diversity abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 2008, 10, 2966–2978. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Hu, Y.; Zeng, Z. Irrigation frequency alters the abundance and community structure of ammonia-oxidizing archaea and bacteria in a northern Chinese upland soil. Eur. J. Soil Biol. 2017, 83, 34–42. [Google Scholar] [CrossRef]
- Wessén, E.; Nyberg, K.; Jansson, J.K.; Hallin, S. Responses of bacterial and archaeal ammonia oxidizers to soil organic and fertilizer amendments under long-term management. Appl. Soil Ecol. 2010, 45, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, H.; Morimoto, S.; Hayatsu, M.; Hayakawa, A.; Sudo, S.; Yagi, K. Nitrification, ammonia-oxidizing communities, and N2O and CH4 fluxes in an imperfectly drained agricultural field fertilized with coated urea with and without dicyandiamide. Biol. Fertil. Soils 2013, 49, 213–223. [Google Scholar] [CrossRef]
- Xia, W.; Zhang, C.; Zeng, X.; Feng, Y.; Weng, J.; Lin, X.; Zhu, J.; Xiong, Z.; Xu, J.; Cai, Z. Autotrophic growth of nitrifying community in an agricultural soil. ISME J. 2011, 5, 1226–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auguet, J.C.; Barberán, A.; Casamayor, E.O. Global ecological patterns in uncultured Archaea. ISME J. 2011, 4, 182–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, S.T.; Berg-Lyons, D.; Caporaso, J.G.; Walters, W.A.; Knight, R.; Fierer, N. Examining the global distribution of dominant archaeal populations in soil. ISME J. 2011, 5, 908–917. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Wang, F.; Jia, X.; Chen, J. Distinct responses of ammonia-oxidizing bacteria and archaea to green manure combined with reduced chemical fertilizer in a paddy soil. J. Soils Sediments 2018, 19, 1613–1623. [Google Scholar] [CrossRef]
- Duan, P.; Wu, Z.; Zhang, Q.; Fan, C.; Xiong, Z. Thermodynamic responses of ammonia-oxidizing archaea and bacteria explain N2O production from greenhouse vegetable soils. Soil Biol. Biochem. 2018, 120, 37–47. [Google Scholar] [CrossRef]
- Xue, C.; Zhang, X.; Zhu, C.; Zhao, J.; Zhu, P.; Peng, C.; Ling, N.; Shen, Q.R. Quantitative and compositional responses of ammonia-oxidizing archaea and bacteria to long-term field fertilization. Sci. Rep. 2016, 6, 28981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Che, Z.; Cao, W.; Huang, T.; Wang, J.; Dong, Z. Changing roles of ammonia-oxidizing bacteria and archaea in a continuously acidifying soil caused by over-fertilization with nitrogen. Environ. Sci. Pollut. Res. 2016, 23, 11964–11974. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Xu, Z.W.; Hu, H.W.; Hu, Y.J.; Hao, Z.P.; Jiang, Y.; Chen, B.D. Responses of ammonia-oxidizing bacteria and archaea to nitrogen fertilization and precipitation increment in a typical temperate steppe in Inner Mongolia. Appl. Soil Ecol. 2013, 68, 36–45. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.M.; Shen, J.P.; Wei, W.X.; He, J.Z. Abundance and community structure of ammonia-oxidizing archaea and bacteria in an acid paddy soil. Biol. Fertil. Soils 2011, 47, 323–331. [Google Scholar] [CrossRef]
- Ke, X.; Angel, R.; Lu, Y.; Conrad, R. Niche differentiation of ammonia oxidizers and nitrite oxidizers in rice paddy soil. Environ. Microbiol. 2013, 15, 2275–2292. [Google Scholar] [CrossRef] [PubMed]
- Lentendu, G.; Wubet, T.; Chatzinotas, A.; Wilhelm, C.; Buscot, F.; Schlegel, M. Effects of long-term differential fertilization on eukaryotic microbial communities in an arable soil: A multiple barcoding approach. Mol. Ecol. 2014, 23, 3341–3355. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Ferguson, R.B.; Shapiro, C.A.; Drijber, R.A.; Walters, D.T. Does inorganic nitrogen fertilization improve soil aggregation? Insights from two long-term tillage experiments. J. Environ. Qual. 2014, 43, 995–1003. [Google Scholar] [CrossRef] [PubMed]




| Treatment | pH | TN (g kg−1) | NH4-N (mg kg−1) | NO3-N (mg kg−1) | TP (g kg−1) | AP (mg kg−1) | SM (%) | PNA (mg NO3-N kg−1 ha−1) | GY (kg ha−1) | NUE (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| N0 | 8.99 ± 0.04a | 0.91 ± 0.04a | 10.96 ± 0.13b | 12.92 ± 0.10e | 0.74 ± 0.03a | 18.16 ± 1.19b | 12.13 ± 0.75a | 1.22 ± 0.04c | 2696.65 ± 107.12c | - |
| N52.5 | 8.81 ± 0.02b | 0.95 ± 0.11a | 11.06 ± 0.45b | 16.23 ± 0.45d | 0.82 ± 0.07a | 19.99 ± 0.45b | 12.07 ± 0.38a | 1.69 ± 0.12b | 3270.2 ± 144.94b | 19.64 ± 2.4a |
| N105 | 8.72 ± 0.02bc | 1.05 ± 0.02a | 11.62 ± 1.32b | 21.42 ± 0.52c | 0.88 ± 0.05a | 22.39 ± 0.17a | 11.98 ± 0.22a | 2.32 ± 0.15a | 4007.88 ± 158.98a | 19.07 ± 0.56a |
| N157.5 | 8.67 ± 0.01c | 1.01 ± 0.03a | 16.47 ± 0.21a | 30.07 ± 0.56b | 0.88 ± 0.07a | 19.84 ± 0.57b | 11.4 ± 0.41a | 1.75 ± 0.12b | 4675.52 ± 33.67a | 16.3 ± 0.73ab |
| N210 | 8.68 ± 0.04c | 0.96 ± 0.01a | 17.01 ± 0.32a | 35.64 ± 0.35a | 0.87 ± 0.06a | 20.15 ± 0.69b | 11.23 ± 0.65a | 1.79 ± 0.14b | 4209.77 ± 156.71a | 13.61 ± 0.68b |
| ANOVA p-value | 0.002 | 0.410 | <0.001 | <0.001 | 0.417 | 0.032 | 0.647 | 0.001 | <0.001 | 0.042 |
| pH | TN | NH4-N | NO3-N | TP | AP | SM | GY | NUE | PNA | |
|---|---|---|---|---|---|---|---|---|---|---|
| AOA | 0.900 ** | −0.348 | −0.728 ** | −0.863 ** | −0.490 | −0.470 | 0.320 | −0.893 ** | 0.895 ** | −0.580 * |
| AOB | −0.802 ** | 0.563 * | 0.443 | 0.605 * | 0.515 * | 0.661 ** | −0.225 | 0.860 ** | −0.661 ** | 0.787 ** |
| AOA/AOB | 0.888 ** | −0.412 | −0.596 * | −0.763 ** | −0.513 | −0.573 * | 0.273 | −0.868 ** | 0.872 ** | −0.685 ** |
| PNA | −0.480 | 0.660 ** | 0.328 | 0.674 ** | 0.320 | 0.135 | −0.015 | 0.758 ** | −0.479 | - |
| Item | Alpha Index | pH | TN | NH4-N | NO3-N | TP | AP | SM | PNA |
|---|---|---|---|---|---|---|---|---|---|
| AOA | Chao1 | −0.554 * | 0.128 | 0.450 | 0.589 * | −0.112 | 0.284 | −0.463 | 0.080 |
| Simpson | 0.524 * | −0.323 | −0.254 | −0.330 | −0.328 | −0.665 ** | 0.088 | −0.700 ** | |
| Shannon | 0.510 | −0.297 | −0.236 | −0.309 | −0.387 | −0.688 ** | 0.031 | −0.713 ** | |
| Pielou_e | 0.474 | −0.222 | −0.262 | −0.335 | −0.219 | −0.639 * | 0.122 | −0.623 * | |
| Observed species | −0.545 * | 0.157 | 0.715 ** | 0.696 ** | 0.165 | 0.162 | −0.386 | 0.023 | |
| AOB | Chao1 | −0.304 | 0.327 | −0.189 | −0.095 | 0.396 | 0.715 ** | 0.166 | 0.703 ** |
| Simpson | 0.412 | 0.087 | −0.711 ** | −0.616 * | 0.188 | 0.058 | 0.144 | 0.357 | |
| Shannon | 0.062 | 0.422 | −0.529 * | −0.448 | 0.157 | 0.282 | 0.111 | 0.535 * | |
| Pielou_e | 0.530 * | −0.186 | −0.578 * | −0.497 | 0.299 | −0.182 | 0.206 | 0.241 | |
| Observed species | −0.420 | 0.618 * | −0.149 | −0.095 | 0.036 | 0.547 * | 0.067 | 0.488 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, A.; Li, L.; Xie, J.; Gopalakrishnan, S.; Zhang, R.; Luo, Z.; Cai, L.; Liu, C.; Wang, L.; Anwar, S.; et al. Changes in Ammonia-Oxidizing Archaea and Bacterial Communities and Soil Nitrogen Dynamics in Response to Long-Term Nitrogen Fertilization. Int. J. Environ. Res. Public Health 2022, 19, 2732. https://doi.org/10.3390/ijerph19052732
Xu A, Li L, Xie J, Gopalakrishnan S, Zhang R, Luo Z, Cai L, Liu C, Wang L, Anwar S, et al. Changes in Ammonia-Oxidizing Archaea and Bacterial Communities and Soil Nitrogen Dynamics in Response to Long-Term Nitrogen Fertilization. International Journal of Environmental Research and Public Health. 2022; 19(5):2732. https://doi.org/10.3390/ijerph19052732
Chicago/Turabian StyleXu, Aixia, Lingling Li, Junhong Xie, Subramaniam Gopalakrishnan, Renzhi Zhang, Zhuzhu Luo, Liqun Cai, Chang Liu, Linlin Wang, Sumera Anwar, and et al. 2022. "Changes in Ammonia-Oxidizing Archaea and Bacterial Communities and Soil Nitrogen Dynamics in Response to Long-Term Nitrogen Fertilization" International Journal of Environmental Research and Public Health 19, no. 5: 2732. https://doi.org/10.3390/ijerph19052732







