Aluminum Bioaccumulation in Reed Canary Grass (Phalaris arundinacea L.) from Rivers in Southwestern Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Material
2.3. Aluminum Content Determination
2.4. Statistical Analysis of the Results
3. Results and Discussion
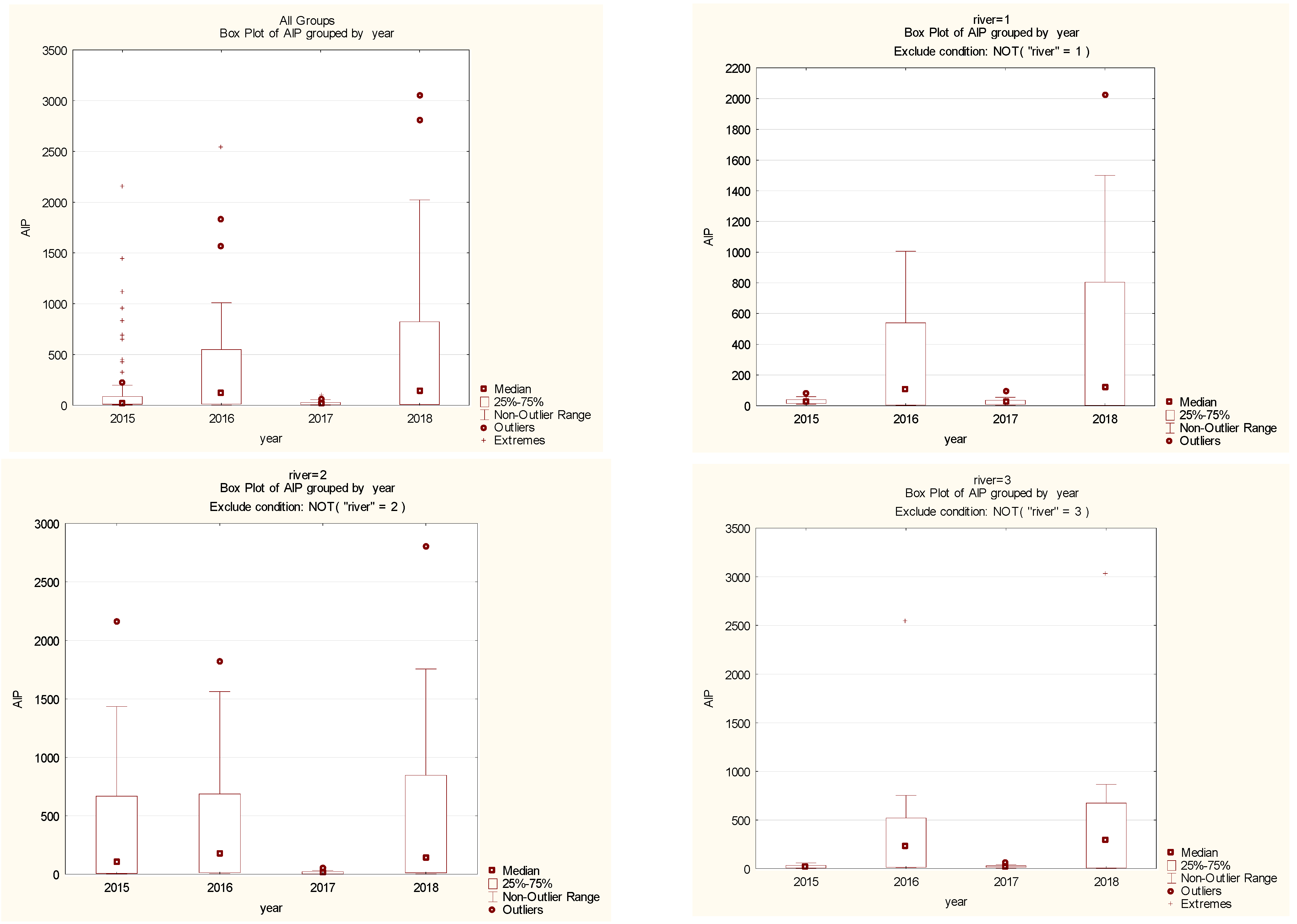
3.1. Aluminum in Aquatic Plants
3.2. Metal Pollution Index (MPI) of Aquatic Plants with Aluminum
3.3. Bioaccumulation of Aluminum in Aquatic Plants in Relation to Water (BCFW)
3.4. Bioaccumulation of Aluminum in Aquatic Plants in Relation to Bottom Sediments (BCFB)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kotowski, M.; Saczuk, M. Aluminium in water and soil environment. Ekoinżynieria 1997, 2, 22–29. [Google Scholar]
- Gensemer, R.W.; Playle, R.C. The Bioavailability and Toxicity of Aluminium in Aquatic Environments. Crit. Rev. Environ. Sci. Technol. 1999, 29, 315–450. [Google Scholar] [CrossRef]
- Barabasz, W.; Albińska, D.; Jaśkowska, M.; Lipiec, J. Ecotoxicology of Aluminium. Pol. J. Environ. Stud. 2002, 11, 199–203. [Google Scholar]
- Gworek, B. Aluminium in the natural environment and its toxicity. Ochr. Środ. Zas. Nat. 2006, 29, 27–38. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Kluczka, J.; Zołotajkin, M.; Ciba, J. Speciation of Aluminium in the water and bottom sediment of fish-breeding ponds. Arch. Environ. Prot. 2012, 38, 83–96. [Google Scholar] [CrossRef] [Green Version]
- Shetty, R.; Vidya, C.S.N.; Prakash, N.B.; Lux, A.; Vaculík, M. Aluminum toxicity in plants and its possible mitigation in acid soils by biochar: A review. Sci. Total Environ. 2021, 765, 142744. [Google Scholar] [CrossRef]
- Czamara, W.; Krężel, J.; Łomotowski, J. Influence of reservoir retention on surface water quality in the Nysa Szalona catchment area. Sci. J. Agric. Acad. Wrocław 1994, 2, 246. [Google Scholar]
- Samecka-Cymerman, A.; Kempers, A.J. Concentrations of heavy metals and plant nutrients in water, sediments and aquatic macrophytes of anthropogenic lakes (former open cut brown coal mines) differing in stage of acidification. Sci. Total Environ. 2001, 281, 87–98. [Google Scholar] [CrossRef]
- Bonanno, G. Arundo donax as a potential biomonitor of trace element contamination in water and sediment. Ecotoxicol. Environ. Saf. 2012, 80, 20–27. [Google Scholar] [CrossRef]
- Główny Inspektorat Ochrony Środowiska (GIOŚ). Chief Inspectorate of Environmental Protection. Report on the State of the Environment. Warsaw; Chief Inspectorate of Environmental Protection: Warsaw, Poland, 2018. [Google Scholar]
- Kowal, A. Treatment of water from the Dobromierz dam reservoir. Ochr. Środ. 1991, 1, 35–38. [Google Scholar]
- Opaliński, C.; Pasis, J. Water Reservoir on the Nysa Szalona River in Słup; ODGW: Wrocław, Poland, 1974. [Google Scholar]
- Broś, K. Operating Manual for the Słup Reservoir on the Nysa Szalona R; ODGW: Wrocław, Poland, 1995. [Google Scholar]
- Szoszkiewicz, K.; Jusik, S.; Zgoła, T. Key to the determination of macrophytes for the assessment of the ecological status of surface waters. In Environmental Protection Inspectorate; Library of Environmental Monitoring: Warsaw, Poland, 2010. [Google Scholar]
- Kopecký, M.; Mráz, P.; Kolář, L.; Váchalová, R.; Bernas, J.; Konvalina, P.; Perná, K.; Murindangabo, Y.; Menšík, L. Effect of Fertilization on the Energy Profit of Tall Wheatgrass and Reed Canary Grass. Agronomy 2021, 11, 445. [Google Scholar] [CrossRef]
- Souček, J.; Jasinskas, A.; Sillinger, F.; Szalay, K. Determination of Mechanical and Energetic Properties of Reed Canary Grass Pellets Production. Acta Univ. Agric. Silvic. Mendel. Brun. 2019, 67, 757–762. [Google Scholar] [CrossRef] [Green Version]
- Usťak, S.; Šinko, J.; Muňoz, J. Reed canary grass (Phalaris arundinacea L.) as a promising energy crop. J. Cent. Eur. Agric. 2019, 20, 1143–1168. [Google Scholar] [CrossRef]
- Guidelines for Quality Assurance in Environmental Water Sampling and Handling. PN-ISO 5667-14. 2004.
- Guidelines for Sampling of Rivers and Streams. Water Quality. PN-ISO 5667-6. 2003.
- Guidelines for the Fixation and Handling of Samples. PN-EN ISO 5667-3. 2005.
- Varian Analytical Methods. Heavy Metals Test Procedure. PB10/I. 1998.
- Water Quality—Determination of Aluminum by Atomic absorption Spectrometry. PN-EN ISO 12020. 2002.
- Senze, M.; Kowalska-Góralska, M.; Czyż, K. Availability of aluminum in river water supplying dam reservoirs in Lower Silesia considering the hydrochemical conditions. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100535. [Google Scholar] [CrossRef]
- Senze, M.; Kowalska-Góralska, M.; Czyż, K.; Wondołowska-Grabowska, A.; Łuczyńska, J. Aluminum in Bottom Sediments of the Lower Silesian Rivers Supplying Dam Reservoirs vs. Selected Chemical Parameters. Int. J. Environ. Res. Public Health 2021, 18, 13170. [Google Scholar] [CrossRef] [PubMed]
- Jezierska, B.; Witeska, M. Metal toxicity to fish. Monogr. Univ. Podlas. 2001, 42, 318. [Google Scholar]
- Usero, J.; Gonzales-Regalado, E.; Garcia, I. Trace metals in bivalve molluscs Ruditapes decussatus and Ruditapes philippinarum from the Atlantic Coast of southern Spain. Environ. Int. 1997, 23, 291–298. [Google Scholar] [CrossRef]
- Institute of Meteorology and Water Management (IMGW) Institute of Meteorology and Water Management National Research Institute. 2021. Available online: http://imgw.pl (accessed on 16 November 2021).
- Świerk, D.; Szpakowska, B. Occurrence of heavy metals in aquatic macrophytes colonizing small aquatic ecosystem. Ecol. Chem. Eng. S. 2011, 18, 369–384. [Google Scholar]
- Samecka-Cymerman, A.; Kolon, K.; Kempers, A.J. A comparison of native and transplanted Frontinalis antipyretica Hedw. as biomonitors of water polluted with heavy metals. Sci. Total Environ. 2005, 341, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Samecka-Cymerman, A.; Kempers, A.J. Toxic metals in aquatic plants surviving in surface water polluted by copper mining industry. Ecotoxicol. Environ. Saf. 2004, 59, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Samecka-Cymerman, A.; Kempers, A.J. Bioindication of heavy metals by Mimulus guttatus from the Czeska Struga Stream in the Karkonosze Mountains, Poland. Bull. Environ. Contam. Toxicol. 1999, 63, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Senze, M.; Kowalska-Góralska, M.; Białowąs, H. Evaluation of the aluminium load in the aquatic environment of two small rivers in the Baltic Sea catchment area. J. Elementol. 2015, 20, 987–998. [Google Scholar] [CrossRef]
- Senze, M.; Kowalska-Góralska, M. Bioaccumulation of aluminium in hydromacrophytes in Polish coastal lakes. Limnol. Rev. 2014, 14, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Senze, M.; Kowalska-Góralska, M.; Czyżowicz, I. Bioaccumulation of aluminium in the aquatic environment of the Dobra river in Wrocław. Ecol. Chem. Eng. A 2011, 18, 1545–1549. [Google Scholar]
- Sprenger, M.; McIntosh, A. Relationship between concentration of aluminium, cadmium, lead, and zinc in water, sediments, and aquatic macrophytes in six acidic lakes. Arch. Environ. Contam. Toxicol. 1989, 18, 225–231. [Google Scholar] [CrossRef]
- Engleman, C.J., Jr.; McDiffett, W.F. Accumulation of aluminium and iron by bryophytes in streams affected by acid-mine drainage. Environ. Pollut. 1996, 94, 67–74. [Google Scholar] [CrossRef]
- Vardanyan, L.G.; Ingole, B.S. Studies on heavy metal accumulation in aquatic macrophytes from Sevan (Armenia) and Carambolim (India) lake systems. Environ. Int. 2006, 32, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Wu, H.; Hao, B.; Liu, G. Metal accumulation by submerged macrophytes in eutrophic lakes at the watershed scale. Environ. Sci. Pollut. Res. 2013, 20, 6999–7008. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, A.; Bauer, K.; Fraz-Gerstein, C.; de Menezes, M. Variation of nutrient and metal concentrations in aquatic macrophytes along the Rio Cachoeira in Bahia (Brasil). Environ. Int. 2002, 28, 165–171. [Google Scholar] [CrossRef]
- Bonanno, G. Trace element accumulation and distribution in the organs of Phragmites australis (common reed) and biomonitoring applications. Ecotoxicol. Environ. Saf. 2011, 74, 1057–1064. [Google Scholar] [CrossRef]
- Obek, E.; Sasmaz, A. Bioaccumulation of Aluminum by Lemna gibba L. from Secondary Treated Municipal Wastewater Effluents. Bull. Environ. Contam Toxicol. 2011, 86, 217–220. [Google Scholar] [CrossRef]
- Bonanno, G. Comparative performance of trace element bioaccumulation and biomonitoring in the plant species Typha domingensis, Phragmites australis and Arundo donax. Ecotoxicol. Environ. Saf. 2013, 97, 124–130. [Google Scholar] [CrossRef] [PubMed]
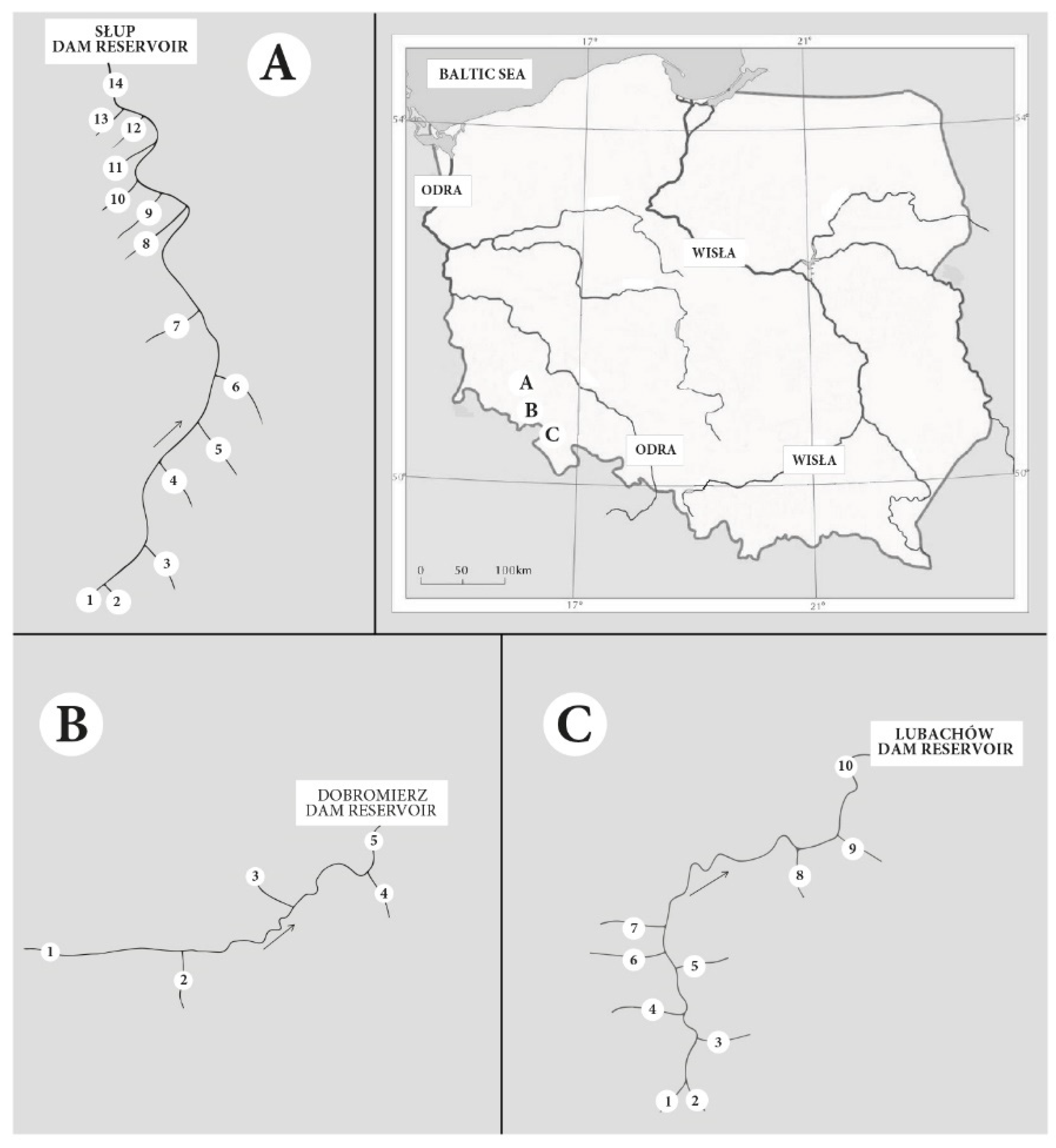



| Characteristics of the Rivers | River | ||
|---|---|---|---|
| Strzegomka | Bystrzyca | Nysa Szalona | |
| Length (km) | 74.70 | 95.20 | 51.00 |
| Catchment area (km2) | 555.00 | 1767.80 | 443.10 |
| Springs, altitude m above sea level | Trójgarb 692.00 | The Suche and Sowie Mountains 618.00 | Mount Pustelnik 628.00 |
| Reservoir location m.a.s.l./reservoir type | 300–423 lowland and upland | 400–500 upland | 165–257 lowland |
| Catchment area above the dam reservoir (km2) | 70.32 | 130.69 | 374.81 |
| Tributaries above the reservoir: left-bank | Sikorka | Otłuczyna, Złota Woda, Rybna | Męcinka, Rowiec, Starucha, Jawornik, Puszówka, Nysa Mała, Kamiennik |
| Tributaries above the reservoir: right-bank | Polska Woda, Czyżynka | Złoty Potok, Kłobia, Potok Marcowy Duży, Jaworzynik, Walimianka | Ochodnik, Sadówka, Czyściel, Parowa, Kocik |
| Site/Material | 2015 | 2016 | 2017 | 2018 | ||
|---|---|---|---|---|---|---|
| Min–Max ± SD | ||||||
| Nysa Szalona | Below the springs | P | 13.24–423.46 218.35 ± 205.10 | 10.25–325.10 172.39 ± 162.38 | 3.15–24.75 13.97 ± 10.72 | 6.41–562.78 284.40 ± 277.98 |
| 3.15–562.78 172.28 ± 215.39 | ||||||
| BCFW | 78.64–3846.13 1956.64 ± 1876.74 | 103.81–2131.36 1064.65 ± 960.28 | 14.63–142.02 78.34 ± 63.12 | 106.34–6045.50 3070.36 ± 2963.77 | ||
| 14.63–6045.50 1542.50 ± 2127.80 | ||||||
| BCFB | 0.0056–0.2028 0.1042 ± 0.0985 | 0.0002–25.96 12.36 ± 12.38 | 0.0413–0.2224 0.1255 ± 0.0843 | 0.0006–0.2923 0.1463 ± 0.1457 | ||
| 0.0002–25.96 3.1848 ± 8.1486 | ||||||
| Tributaries | P | 5.73–2159.75 400.69 ± 549.99 | 5.96–1825.44 414.58 ± 513.10 | 2.11–62.78 14.19 ± 13.26 | 4.74–2812.89 509.91 ± 688.27 | |
| 2.11–2812.89 334.84 ± 544.01 | ||||||
| BCFW | 22.78–17,473.74 2982.60 ± 4478.19 | 68.24–13,333.54 2748.24 ± 3565.82 | 10.83–358.97 61.8798 ± 71.51 | 62.74–30,565.43 5679.84 ± 7497.01 | ||
| 10.83–30,565.43 2868.14 ± 5118.29 | ||||||
| BCFB | 0.0010–0.6444 0.0499 ± 0.1402 | 0.0001–80.43 13.66 ± 22.09 | 0.0010–1.2981 0.1020 ± 0.2611 | 0.0002–1.1101 0.0949 ± 0.2294 | ||
| 0.0001–80.43 3.476 ± 12.51 | ||||||
| Mouth to the reservoir | P | 7.12–698.65 352.84 ± 345.72 | 32.48–654.36 343.37 ± 310.86 | 20.09–28.99 24.48 ± 4.17 | 26.01–875.95 451.01 ± 424.64 | |
| 7.12–875.95 292.93 ± 353.44 | ||||||
| BCFW | 32.83–3772.12 1891.48 ± 1858.31 | 271.56–3346.98 1804.33 ± 1525.24 | 68.34–89.27 78.68 ± 9.81 | 316.79–8925.21 4592.84 ± 4273.36 | ||
| 32.83–8925.21 2091.83 ± 2935.64 | ||||||
| BCFB | 0.0056–0.0110 0.0083 ± 0.0027 | 0.2034–35.69 17.6765 ± 17.4764 | 0.0013–0.0387 0.0197 ± 0.0184 | 0.0129–0.0517 0.0324 ± 0.0193 | ||
| 0.0013–35.69 4.4342 ± 11.6107 | ||||||
| The whole | P | 5.73–2159.75 384.25 ± 522.58 | 5.96–1825.44 392.19 ± 488.35 | 2.11–62.78 14.91 ± 12.94 | 4.74–2812.89 489.60 ± 654.14 | |
| 2.11–2812.89 320.24 ± 517.41 | ||||||
| BCFW | 22.78–17,473.74 2831.38 ± 4221.96 | 68.24–13,333.54 2560.56 ± 3370.69 | 10.83–358.97 64.26 ± 68.62 | 62.74–30,565.43 5415.81 ± 7113.98 | ||
| 10.83–30,565.43 1975.94 ± 6625.33 | ||||||
| BCFB | 0.0010–0.6444 0.0508 ± 0.13 | 0.0001–71.98 13.82 ± 21.27 | 0.0010–1.2981 0.0978 ± 0.2438 | 0.0002–1.1102 0.0941 ± 0.2171 | ||
| 0.0001–80.43 39.03 ± 42.09 | ||||||
| Strzegomka | Below the springs | P | 8.13–45.89 26.99 ± 18.64 | 10.01–521.63 265.84 ± 255.57 | 12.24–42.99 27.62 ± 15.06 | 7.74–675.65 341.59 ± 333.82 |
| 7.74–675.65 165.51 ± 253.28 | ||||||
| BCFW | 46.09–346.90 195.22 ± 147.68 | 83.53–4958.48 2510.25 ± 2421.31 | 65.68–301.49 183.75 ± 116.35 | 67.65–6734.25 3396.86 ± 3328.15 | ||
| 46.09–6734.25 1571.52 ± 2500.40 | ||||||
| BCFB | 0.0038–0.0258 0.0148 ± 0.0109 | 0.0004–0.0282 0.0143 ± 0.0139 | 0.0045–0.0131 0.0088 ± 0.0042 | 0.0001–0.0100 0.0051 ± 0.0049 | ||
| 0.0001–0.0282 0.0107 ± 0.0102 | ||||||
| Tributaries | P | 4.23–59.41 22.35 ± 18.68 | 10.06–2536.87 631.25 ± 896.22 | 5.59–56.79 24.16 ± 16.30 | 3.55–3044.54 753.60 ± 1076.80 | |
| 3.55–3044.54 357.84 ± 777.59 | ||||||
| BCFW | 25.07–415.16 138.72 ± 129.82 | 78.017–16,534.75 4295.60 ± 5753.44 | 35.97–385.58 141.07 ± 114.68 | 26.60–14,347.52 3845.02 ± 5066.66 | ||
| 25.07–16,534.75 2105.10 ± 4311.41 | ||||||
| BCFB | 0.0020–0.0217 0.0091 ± 0.0068 | 0.0005–0.1553 0.0367 ± 0.0550 | 0.0019–0.0232 0.0092 ± 0.0070 | 0.0001–0.0403 0.0104 ± 0.0144 | ||
| 0.0001–0.1553 0.0163 ± 0.0311 | ||||||
| Mouth to the reservoir | P | 19.44–33.56 26.50 ± 6.91 | 20.10–503.98 261.98 ± 241.47 | 25.11–29.54 27.38 ± 1.91 | 15.10–610.93 313.11 ± 297.48 | |
| 15.10–610.93 157.24 ± 232.42 | ||||||
| BCFW | 109.98–217.37 163.81 ± 52.14 | 126.63–4124.19 2112.67 ± 1980.60 | 142.80–180.8 161.59 ± 15.88 | 77.28–3372.21 1686.11 ± 1605.54 | ||
| 77.28–4124.19 1031.04 ± 1550.05 | ||||||
| BCFB | 0.0067–0.0092 0.0079 ± 0.0012 | 0.0010–0.0102 0.0056 ± 0.0050 | 0.0088–0.0125 0.0107 ± 0.0017 | 0.0003–0.0099 0.0051 ± 0.0050 | ||
| 0.0003–0.0125 0.0073 ± 0.0041 | ||||||
| The whole | P | 4.23–59.41 24.19 ± 17.12 | 10.01–2536.87 484.31 ± 734.19 | 5.59–56.79 25.50 ± 14.43 | 3.55–3044.54 583.10 ± 882.82 | |
| 3.55–3044.54 279.25 ± 629.04 | ||||||
| BCFW | 25.07–415.16 155.04 ± 124.57 | 78.02–16534.75 3501.94 ± 4772.74 | 35.969–385.58 153.71 ± 104.58 | 26.60–14347.52 3323.61 ± 4339.81 | ||
| 25.07–16,534.75 1783.57 ± 3614.98 | ||||||
| BCFB | 0.0020–0.0258 0.010 ± 0.01 | 0.0004–0.1553 0.0259 ± 0.045 | 0.0019–0.0232 0.0094 ± 0.0058 | 0.0001–0.0402 0.0083 ± 0.0119 | ||
| 0.0001–0.1553 0.0134 ± 0.02 | ||||||
| Bystrzyca | Below the springs | P | 12.02–86.53 49.27 ± 37.25 | 4.52–456.65 230.41 ± 225.88 | 11.06–96.98 53.78 ± 42.71 | 2.36–698.45 350.32 ± 347.95 |
| 2.36–698.45 170.94 ± 244.72 | ||||||
| BCFW | 64.89–529.23 297.01 ± 232.10 | 25.64–2759.24 1368.09 ± 1342.24 | 55.70–499.18 277.35 ± 221.03 | 17.29–7124.64 35.67 ± 3550.23 | ||
| 17.29–7124.64 1377.54 ± 2328.33 | ||||||
| BCFB | 0.0032–0.0386 0.0209 ± 0.0178 | 0.0029–0.0829 0.0429 ± 0.0400 | 0.0037–0.0083 0.0063 ± 0.0021 | 0.0007–0.1759 0.0882 ± 0.0876 | ||
| 0.0007–0.1759 0.0396 ± 0.0580 | ||||||
| Tributaries | P | 10.25–45.53 26.73 ± 11.54 | 2.56–1005.99 232.37 ± 348.95 | 3.01–43.79 20.75 ± 13.36 | 1.25–2022.55 565.10 ± 644.67 | |
| 1.25–2022.55 233.99 ± 431.20 | ||||||
| BCFW | 82.95–518.78 211.68 ± 111.36 | 15.25–8974.08 2571.14 ± 2884.50 | 21.76–294.87 139.58 ± 90.54 | 10.91–16,268.30 4961.98 ± 5542.95 | ||
| 10.91–16,268.30 1971.10 ± 3702.05 | ||||||
| BCFB | 0.0013–0.0278 0.0082 ± 0.0060 | 0.0002–0.7058 0.1489 ± 0.1907 | 0.0002–0.0289 0.0075 ± 0.0086 | 0.0004–0.8087 0.2460 ± 0.2892 | ||
| 0.0002–0.8087 0.1026 ± 0.2005 | ||||||
| Mouth to the reservoir | P | 35.41–60.15 47.78 ± 12.36 | 8.63–202.33 105.45 ± 96.82 | 33.56–55.45 44.47 ± 10.91 | 5.54–251.67 128.49 ± 122.94 | |
| 5.54–251.67 81.55 ± 86.67 | ||||||
| BCFW | 411.12–495.81 453.15 ± 41.74 | 62.39–1642.21 851.14 ± 787.53 | 231.16–300.73 265.92 ± 30.75 | 52.60–2279.64 1136.56 ± 1084.59 | ||
| 52.60–2279.64 676.70 ± 751.64 | ||||||
| BCFB | 0.0039–0.0586 0.0313 ± 0.0274 | 0.0032–0.0671 0.0351 ± 0.0319 | 0.0030–0.0139 0.0085 ± 0.0054 | 0.0019–0.1251 0.0635 ± 0.0615 | ||
| 0.0019–0.1252 0.0346 ± 0.0422 | ||||||
| The whole | P | 10.25–86.53 31.09 ± 18.35 | 2.56–1005.99 292.28 ± 328.78 | 3.01–96.98 26.43 ± 21.68 | 1.25–2022.55 499.96 ± 604.60 | |
| 1.25–2022.55 212.44 ± 397.17 | ||||||
| BCFW | 64.89–529.23 244.36 ±144.82 | 15.25–8974.08 2278.84 ± 2693.27 | 21.76–499.18 165.98 ± 119.73 | 10.91–16268.30 4440.01 ± 5229.03 | ||
| 10.91–16,268.30 1782.30 ± 3424.89 | ||||||
| BCFB | 0.0013–0.0586 0.0117 ± 0.0138 | 0.0002–0.7058 0.1266 ± 0.1769 | 0.0002–0.0289 0.0075 ± 0.0079 | 0.0004–0.8087 0.2119 ± 0.2696 | ||
| 0.0002–0.8087 0.0895 ± 0.1826 | ||||||
| Site | Nysa Szalona | Site | Strzegomka | Site | Bystrzyca | ||||
|---|---|---|---|---|---|---|---|---|---|
| Spring | Autumn | Spring | Autumn | Spring | Autumn | ||||
| P | 1 | 3.15–13.27 8.30 ± 3.79 | 24.66–562.78 336.26 ± 197.46 | 1 | 7.74–12.89 9.74 ± 1.89 | 42.45–675.65 321.28 ± 282.43 | 1 | 2.36–12.02 7.49 ± 4.14 | 86.52–698.45 334.39 ± 257.54 |
| BCFW | 14.63–107.77 76.94 ± 37.24 | 141.03–6045.50 3008.06 ± 2181.28 | 46.09–93.40 68.17 ± 14.85 | 298.55–6734.25 3074.86 ± 2825.53 | 17.29–64.92 41.09 ± 19.97 | 497.07–7124.64 2713.99 ± 2696.23 | |||
| BCFB | 0.0002–0.2224 0.0540 ± 0.09 | 0.4134–25.9640 6.3215 ± 10.64 | 0.0001–0.0050 0.0022 ± 0.01 | 0.0100–0.0282 0.0192 ± 0.0078 | 0.0007–0.0055 0.0027 ± 0.0014 | 0.0082–0.1759 0.0764 ± 0.0632 | |||
| P | 2 | 5.96–13.98 10.11 ± 2.85 | 15.07–456.72 289.67 ± 165.34 | 2 | 3.55–13.86 7.99 ± 4.09 | 5.59–589.65 266.75 ± 260.41 | 2 | 2.40–15.33 8.31 ± 5.59 | 43.09–852.63 372.50 ± 345.16 |
| BCFW | 45.56–123.96 74.59 ± 29.31 | 99.15–5367.66 2590.09 ± 1860.43 | 25.07–82.31 51.57 ± 23.97 | 35.97–5245.72 2313.77 ± 2283.04 | 17.56–107.58 52.73 ± 37.22 | 227.28–8685.02 3181.98 ± 3425.10 | |||
| BCFB | 0.0001–0.0426 0.0114 ± 0.02 | 0.0265–14.2383 3.6325 ± 6.07 | 0.0001–0.0046 0.0018 ± 0.01 | 0.0019–0.0235 0.0093 ± 0.01 | 0.0002–0.0058 0.0029 ± 0.01 | 0.0031–0.3284 0.1458 ± 0.14 | |||
| P | 3 | 4.89–12.48 8.62 ± 3.33 | 2.88–2812.89 1700.17 ± 1042.23 | 3 | 5.94–15.70 11.04 ± 4.43 | 27.57–3044.54 1410.21 ± 1391.81 | 3 | 1.25–42.64 21.99 ± 20.09 | 25.32–756.55 336.62 ± 317.63 |
| BCFW | 22.70–131.07 76.38 ± 50.32 | 19.39–30,565.43 15196.28 ± 10,796.41 | 26.60–94.89 57.91 ± 26.53 | 143.35–16,534.75 7726.89 ± 7604.01 | 10.91–289.33 148.53 ± 135.29 | 144.63–6295.96 2643.00 ± 2556.23 | |||
| BCFB | 0.0002–0.0135 0.0039 ± 0.01 | 0.0010–71.9811 18.43 ± 30.91 | 0.0001–0.0051 0.0023 ± 0.01 | 0.0108–0.1553 0.0569 ± 0.06 | 0.0002–0.0268 0.0086 ± 0.01 | 0.0023–0.2572 0.1048 ± 0.11 | |||
| P | 4 | 2.38–15.46 9.89 ± 5.03 | 62.26–907.59 651.79 ± 342.28 | 4 | 15.10–25.86 20.30 ± 3.52 | 29.00–610.93 294.18 ± 265.56 | 4 | 4.55–10.26 6.93 ± 2.10 | 6.95–1499.99 558.79 ± 613.82 |
| BCFW | 11.39–182.74 92.09 ± 69.78 | 354.21–9984.44 5735.40 ± 3471.46 | 49.31–141.27 94.90 ± 34.04 | 378.44–5303.83 2385.56 ± 2078.02 | 31.26–83.07 50.39 ± 19.78 | 42.65–13,636.27 4855.80 ± 5458.77 | |||
| BCFB | 0.0002–0.0087 0.0029 ± 0.01 | 0.0304–44.27 11.24 ± 19.06 | 0.0003–0.0098 0.0047 ± 0.01 | 0.0144–0.0392 0.0229 ± 0.01 | 0.0002–0.0050 0.0018 ± 0.01 | 0.0044–0.6044 0.2420 ± 0.25 | |||
| P | 5 | 9.52–24.89 15.40 ± 5.85 | 19.46–1146.79 776.85 ± 443.04 | 5 | 15.10–25.89 20.30 ± 3.52 | 29.00–610.93 294.181 ± 265.56 | 5 | 3.25–16.53 9.88 ± 6.09 | 25.34–701.65 281.30 ± 278.73 |
| BCFW | 32.63–185.02 118.72 ± 54.61 | 108.34–13,985.24 6954.68 ± 4870.60 | 77.28–150.24 117.74 ± 25.57 | 174.35–4124.19 1944.34 ± 1771.01 | 25.26–131.06 74.07 ± 47.85 | 163.81–6558.71 2467.29 ± 2669.07 | |||
| BCFB | 0.0024–1.1128 0.3085 ± 0.46 | 0.0018–80.43 34.62 ± 35.46 | 0.0003–0.0092 0.0048 ± 0.0040 | 0.0066–0.0125 0.0098 ± 0.0021 | 0.0004–0.0060 0.0030 ± 0.01 | 0.0011–0.2761 0.1022 ± 0.11 | |||
| P | 6 | 4.45–14.65 7.90 ± 4.06 | 21.34–652.88 360.19 ± 250.55 | 6 | 2.07–13.43 5.96 ± 4.47 | 38.22–1142.60 469.18 ± 462.21 | |||
| BCFW | 20.53–121.91 57.04 ± 38.55 | 127.22–7574.05 3135.21 ± 2782.74 | 15.22–93.84 40.85 ± 30.82 | 288.48–10,929.71 4306.13 ± 4325.73 | |||||
| BCFB | 0.0002–0.4849 0.1234 ± 0.21 | 0.0034–4.3189 1.0909 ± 1.86 | 0.0002–0.0061 0.0026 ± 0.01 | 0.0013–0.8087 0.2761 ± 0.32 | |||||
| P | 7 | 3.24–20.94 10.99 ± 6.80 | 6.12–1756.99 1190.62 ± 693.31 | 7 | 2.39–32.42 15.68 ± 12.99 | 5.22–1364.60 562.75 ± 579.67 | |||
| BCFW | 13.65–249.57 97.81 ± 92.54 | 23.59–19,048.06 10,771.61 ± 6871.59 | 18.93–170.23 95.41 ± 73.17 | 41.73–12,167.75 4761.89 ± 5023.88 | |||||
| BCFB | 0.0011–0.5429 0.1896 ± 0.22 | 0.0033–13.9559 3.5327 ± 6.02 | 0.0002–0.0148 0.0068 ± 0.01 | 0.0012–0.6069 0.2343 ± 0.25 | |||||
| P | 8 | 4.84–18.52 13.44 ± 5.1458 | 16.43–873.98 589.70 ± 337.54 | 8 | 3.57–15.64 7.56 ± 4.73 | 30.74–2022.55 773.12 ± 823.47 | |||
| BCFW | 19.93–172.80 121.37 ± 58.79 | 44.73–9543.77 5016.18 ± 3387.07 | 31.02–152.21 64.84 ± 50.50 | 223.81–16,268.30 6326.52 ± 6601.39 | |||||
| BCFB | 0.0009–0.5095 0.1659 ± 0.20 | 0.0082–3.9993 1.0164 ± 1.72 | 0.0005–0.0063 0.0027 ± 0.01 | 0.0023–0.7707 0.3725 ± 0.37 | |||||
| P | 9 | 2.91–13.78 9.07 ± 4.62 | 5.54–502.99 343.94 ± 198.14 | 9 | 2.33–25.63 11.59 ± 9.56 | 32.09–681.52 301.67 ± 278.90 | |||
| BCFW | 13.26–145.15 72.24 ± 50.54 | 14.77–6207.73 3028.52 ± 2181.75 | 22.08–182.32 83.26 ± 66.19 | 236.87–5394.65 2385.76 ± 2220.92 | |||||
| BCFB | 0.0016–0.8808 0.2416 ± 0.37 | 0.0029–2.5686 0.6507 ± 1.11 | 0.0006–0.0102 0.0053 ± 0.01 | 0.0058–0.2785 0.1295 ± 0.12 | |||||
| P | 10 | 5.85–14.26 10.66 ± 3.13 | 8.45–315.98 194.78 ± 115.28 | 10 | 5.54–60.15 32.43 ± 25.41 | 33.56–251.67 130.67 ± 97.74 | |||
| BCFW | 31.19–368.31 129.37 ± 127.35 | 24.51–2777.56 1519.65 ± 1005.70 | 52.60–495.81 227.08 ± 182.66 | 231.16–2279.64 1126.31 ± 832.02 | |||||
| BCFB | 0.0032–0.3712 0.1169 ± 0.15 | 0.0030–2.5596 0.6426 ± 1.11 | 0.0019–0.0586 0.0167 ± 0.0242 | 0.0039–0.1252 0.0525 ± 0.0483 | |||||
| P | 11 | 9.51–32.89 18.44 ± 8.88 | 21.33–1337.87 873.57 ± 505.78 | ||||||
| BCFW | 85.75–203.99 127.99 ± 47.12 | 74.77–14,697.91 7074.12 ± 5110.67 | |||||||
| BCFB | 0.0031–1.2981 0.3371 ± 0.55 | 0.0085–65.5874 16.1847 ± 27.95 | |||||||
| P | 12 | 8.49–14.75 12.06 ± 2.23 | 16.43–542.56 364.39 ± 204.36 | ||||||
| BCFW | 35.40–135.92 86.27 ± 38.53 | 48.38–5891.04 2701.08 ± 2098.58 | |||||||
| BCFB | 0.0033–0.0575 0.0235 ± 0.02 | 0.0061–14.8612 3.69 ± 6.37 | |||||||
| P | 13 | 2.11–32.60 17.38 ± 13.24 | 26.45–821.91 556.51 ± 311.61 | ||||||
| BCFW | 10.83–311.54 152.44 ± 122.77 | 75.89–9962.59 3906.23 ± 3666.38 | |||||||
| BCFB | 0.0034–0.0460 0.0179 ± 0.02 | 0.0103–1.0699 0.2803 ± 0.45 | |||||||
| P | 14 | 7.12–32.56 21.57 ± 9.39 | 28.44–875.95 564.28 ± 320.14 | ||||||
| BCFW | 32.83–322.10 175.21 ± 125.64 | 87.30–8925.21 4008.45 ± 3142.18 | |||||||
| BCFB | 0.0056–0.2041 0.0651 ± 0.08 | 0.0013–35.69 8.80 ± 15.21 | |||||||
| Year | Nysa Szalona | Bystrzyca | Strzegomka |
|---|---|---|---|
| 2015 | 72.17 | 26.46 | 18.07 |
| 2016 | 98.61 | 48.64 | 101.77 |
| 2017 | 10.21 | 18.62 | 21.33 |
| 2018 | 103.35 | 48.95 | 85.90 |
| Average | 71.08 | 35.67 | 56.76 |
| Nysa Szalona | Bystrzyca | Strzegomka | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Material | Plant | Sediment | Water | Plant | Sediment | Water | Plant | Sediment | Water |
| MPI | 71.08 | 277.26 | 0.0903 | 35.67 | 189.41 | 0.0396 | 56.76 | 50.46 | 0.0826 |
| Pollution degree | very high | highest | no effect | high | highest | no effect | very high | high | no effect |
| Nysa Szalona | Bystrzyca | Strzegomka | |
|---|---|---|---|
| Below the springs | 39.19 | 37.35 | 39.34 |
| Pollution degree | high | ||
| Mouth to the reservoir | 79.36 | 41.98 | 46.81 |
| Pollution degree | very high | high | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senze, M.; Kowalska-Góralska, M.; Czyż, K. Aluminum Bioaccumulation in Reed Canary Grass (Phalaris arundinacea L.) from Rivers in Southwestern Poland. Int. J. Environ. Res. Public Health 2022, 19, 2930. https://doi.org/10.3390/ijerph19052930
Senze M, Kowalska-Góralska M, Czyż K. Aluminum Bioaccumulation in Reed Canary Grass (Phalaris arundinacea L.) from Rivers in Southwestern Poland. International Journal of Environmental Research and Public Health. 2022; 19(5):2930. https://doi.org/10.3390/ijerph19052930
Chicago/Turabian StyleSenze, Magdalena, Monika Kowalska-Góralska, and Katarzyna Czyż. 2022. "Aluminum Bioaccumulation in Reed Canary Grass (Phalaris arundinacea L.) from Rivers in Southwestern Poland" International Journal of Environmental Research and Public Health 19, no. 5: 2930. https://doi.org/10.3390/ijerph19052930
APA StyleSenze, M., Kowalska-Góralska, M., & Czyż, K. (2022). Aluminum Bioaccumulation in Reed Canary Grass (Phalaris arundinacea L.) from Rivers in Southwestern Poland. International Journal of Environmental Research and Public Health, 19(5), 2930. https://doi.org/10.3390/ijerph19052930






