Exposure to High Precariousness Prevalence Negatively Impacts Drug Prescriptions of General Practitioners to Precarious and Non-Precarious Populations: A Retrospective Pharmaco-Epidemiological Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Precariousness Criteria in the French Healthcare System
2.2. Data Source
2.3. Data Selection
2.3.1. Data Selection Regarding Prescribers, Populations of Patients, Year of Prescription, and French Regions
- − BR: 3,341,188 insured people, including 149,115 precarious individuals (4.46% of the regional population). We selected BR because it has the lowest UMC population compared to other French regions.
- − CR: 2,635,080 insured people, including 172,758 precarious individuals (6.56% of the regional population).
- − OCR: 5,771,360 insured people, including 515,259 precarious individuals (8.82% of the regional population).
- − OSR: 1,890,901 insured people, including 613,453 precarious individuals (32.44% of the regional population). OSR has the highest UMC prevalence in France.
2.3.2. Data Selection Relative to Medications and Criteria of Principal Evaluation
2.4. Statistical Analyses
2.4.1. Linear Regression Analyses
2.4.2. Required Sample Size and Achieved Power Computations
3. Results
3.1. Effect of Populations on GPs Drug Prescriptions
3.2. Effect of the Interaction between Precariousness Prevalence and Population on GPs’ Drug Prescriptions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
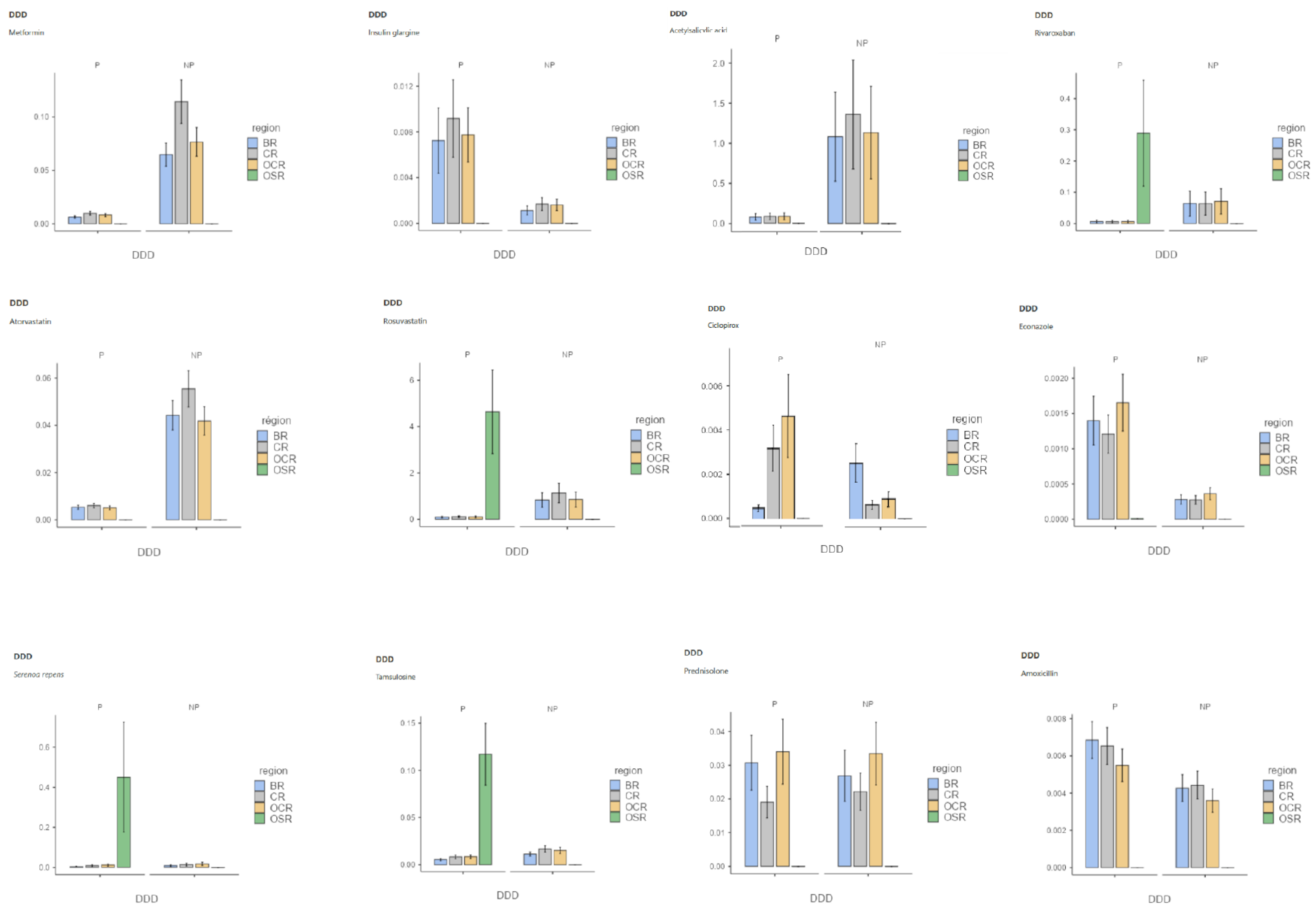
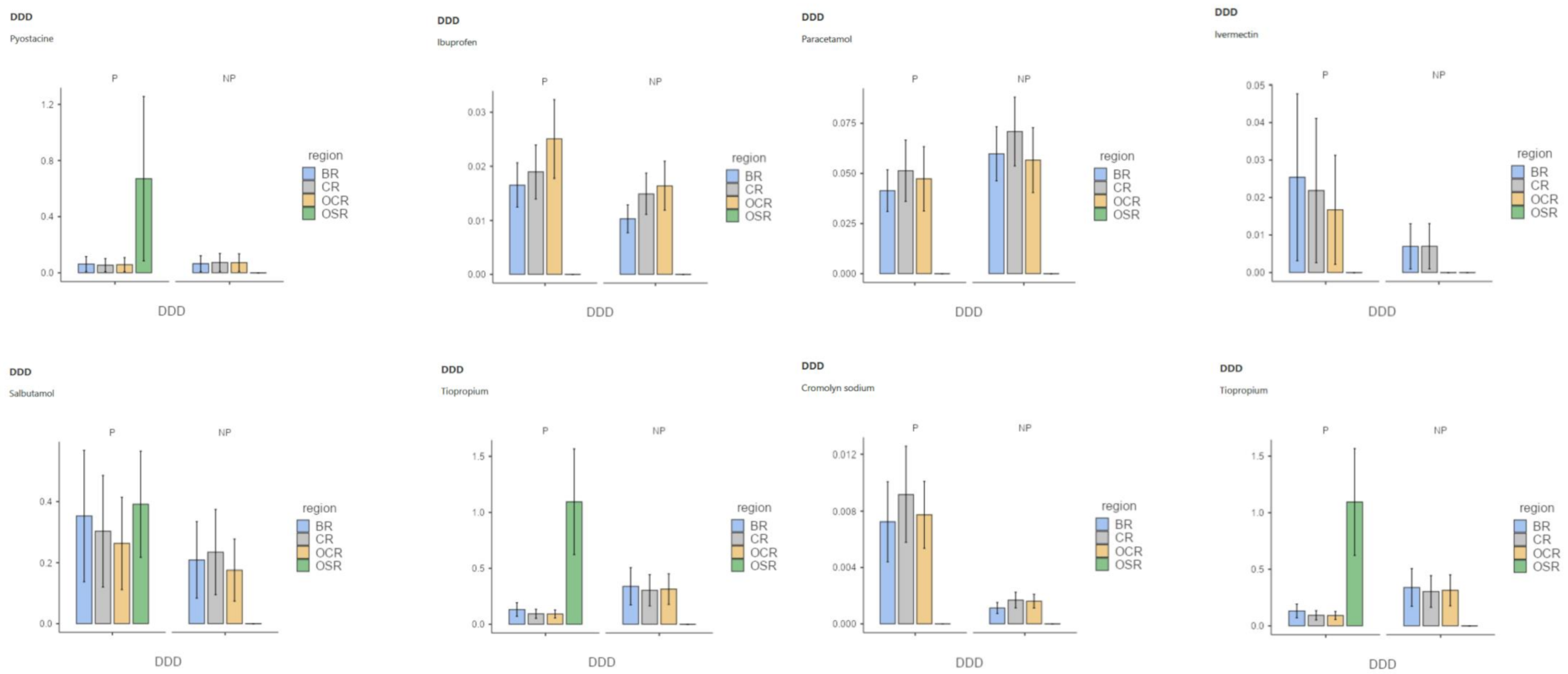
Appendix B
| Normality Test Shapiro–Wilk p-Value | Heteroskedasticity Test Goldfeld–Quandt p-Value | Durbin–Watson for Autocorrelation p-Value | Collinearity Statistics VIF | |||
|---|---|---|---|---|---|---|
| Prev. | Pop. | Prev. * Pop. | ||||
| Metformin | <0.001 | 1.000 | <0.001 | 2.00 | 2.36 | 3.36 |
| Insulin glargine | <0.001 | 1.000 | 0.468 | 2.13 | 2.34 | 3.56 |
| Acetysalicylic acid | <0.001 | 0.999 | 0.272 | 2.00 | 2.36 | 3.36 |
| Rivaroxaban | <0.001 | <0.001 | 0.052 | 2.00 | 2.36 | 3.36 |
| Atorvastatin | <0.001 | 1.000 | <0.001 | 2.00 | 2.36 | 3.36 |
| Rosuvastatin | <0.001 | 0.021 | 0.008 | 2.00 | 2.42 | 3.42 |
| Econazole | <0.001 | 0.918 | <0.001 | 2.00 | 2.36 | 3.36 |
| Ciclopirox | <0.001 | <0.001 | 0.018 | 2.00 | 2.36 | 3.36 |
| Serenoa repens | 0.018 | 0.104 | <0.001 | 2.25 | 2.62 | 3.37 |
| Tamsulosin | <0.001 | <0.001 | 0.044 | 2.00 | 2.36 | 3.36 |
| Prednisolone | <0.001 | 0.452 | 0.604 | 2.00 | 2.36 | 3.36 |
| Amoxicillin | <0.001 | 1.000 | <0.001 | 2.02 | 2.36 | 3.34 |
| Pyostacine | <0.001 | <0.001 | 0.088 | 2.00 | 2.36 | 3.36 |
| Ibuprofen | <0.001 | 0.042 | 0.134 | 2.00 | 2.36 | 3.36 |
| Paracetamol | <0.001 | 1.000 | 0.016 | 2.00 | 2.36 | 3.36 |
| Ivermectin | <0.001 | 0.992 | 0.146 | 2.00 | 2.36 | 3.36 |
| Salbutamol | <0.001 | 1.000 | 0.480 | 2.00 | 2.36 | 3.36 |
| Tiotropium | <0.001 | <0.001 | 0.848 | 2.00 | 2.36 | 3.36 |
| Cromolyn Sodium | <0.001 | 0.940 | <0.001 | 2.17 | 2.37 | 3.63 |
| Timolol | <0.001 | <0.001 | 0.002 | 2.00 | 2.36 | 3.36 |
References
- Lopez, A.M.; Comfort, M.; Powers, C.; Kral, A.H.; Lorvick, J. Structural vulnerability and supplemental security income: Subtle modes of punitive governance within federal social welfare. Hum. Organ. 2018, 77, 302–311. [Google Scholar] [CrossRef]
- Whittle, H.J.; Leddy, A.M.; Shieh, J.; Tien, P.C.; Ofotokum, I.; Adimora, A.A.; Turan, J.M.; Frongillo, E.A.; Turan, B.; Weiser, S.D. Precarity and health: Theorizing the intersection of multiple material-need insecurities, stigma, and illness among women in the United States. Soc. Sci. Med. 2020, 245, 112683. [Google Scholar] [CrossRef] [PubMed]
- Proctor, B.D.; Semega, J.L.; Kollar, M.A.; U.S. Census Bureau. Current Population Reports, P60-256(RV). Income and Poverty in the United States; U.S. Government Printing Office: Washington, DC, USA, 2016. [Google Scholar]
- Webber, D.; Callum, C. Persistent Poverty in the UK and EU: 2015 Rates of Persistent Relative Income Poverty for the UK Are Compared with Other EU Countries; Eurostat, Office for National Statistics: London, UK, 2017. [Google Scholar]
- La Situation Démographique en 2015—Insee Résultats. Available online: https://www.insee.fr/fr/statistiques/2851587 (accessed on 18 May 2021).
- Sumner, A.; Ortiz-Juarez, E.; Hoy, C. Precarity and the Pandemic. COVID-19 and Poverty Incidence, Intensity, and Severity in Developing Countries; WIDER Working Paper 77/UNU-WIDER; UNU-WIDER: Helsinki, Finland, 2020. [Google Scholar] [CrossRef]
- Marmot, M. Fair Society, Healthy Lives: The Marmot Review: Strategic Review of Health Inequalities in England Post-2010. 2010. Available online: https://www.parliament.uk/globalassets/documents/fair-society-healthy-lives-full-report.pdf (accessed on 18 May 2021).
- Tuppin, P.; Blotière, P.O.; Weill, A.; Ricordeau, P.; Allemand, H. Mortality and hospital admissions rates and diagnosis among individuals with low income and full health insurance coverage in France. Presse Med. 2011, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, S.F.; Rehkopf, D.; Tuljapurkar, S.; Horvitz, C.C. Poverty dynamics, poverty thresholds, and mortality. PLoS ONE 2018, 13, e0195734. [Google Scholar] [CrossRef]
- Allonier, C.; Boisguerin, B.; Le Fur, P. UMC Beneficiaries Report More Pathologies Than the Rest of the Population. 2006–2008 ESPS Surveys Results. Available online: http://www.irdes.fr/Publications/2012/Qes173.pdf (accessed on 2 September 2021).
- Lound, C.; Breen, A.; Flisher, A.J.; Kakuma, R.; Corrigal, J.; Joska, J.A.; Swartz, L.; Patel, V. Poverty and common mental disorders in low and middle income countries: A systematic review. Soc. Sci. Med. 2010, 71, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Zukiewicz-Sobczak, W.; Wroblewska, P.; Zwolinski, J.; Chmielewska-Badora, J.; Adamczuk, P.; Krasowska, E.; Zagorski, J.; Oniszczuk, A.; Piatek, J.; Silny, W. Obesity and poverty paradox in developed countries. Ann. Agric. Environ. Med. 2014, 21, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Moscrop, A.; MacPherson, P. Should doctors record their patients’ income? Br. J. Gen. Pract. 2014, 64, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Birault, F.; Mignot, S.; Caunes, N.; Boutin, P.; Bouquet, E.; Pérault-Pochat, M.C.; Thirioux, B. The characteristics of care provided to population(s) in precarious situations in 2015. A preliminary study on the Universal Health Cover in France. Int. J. Environ. Res. Public Health 2020, 17, 3305. [Google Scholar] [CrossRef] [PubMed]
- Bihan, H.; Laurent, S.; Sass, C.; Nguyen, G.; Huot, C.; Moulin, J.J.; Guegen, R.; Le Toumelin, P.; Le Clésiau, H.; La Rosa, E.; et al. Association among individual deprivation, glycemic control and diabetes complications: The EPICES scores. Diabetes Care 2005, 28, 2680–2685. [Google Scholar] [CrossRef] [PubMed]
- Van doorslaer, E.; Masseria, C.; Koolman, X.; OECD Health Equity Research Group. Inequalities in access to medical care by income in developed countries. CMAJ 2006, 174, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Tudrej, B.V.; Etonno, R.; Martinière, A.; Hervé, C.; Birault, F. Clinical public health, a model for identifying medico-social determinants of health? Ethics reflections and feedback on precariousness. Ethics Med. Public Health 2018, 6, 15–25. [Google Scholar] [CrossRef]
- De Oliveira, A.; Chavannes, B.; Steinecker, M.; Denantes, M.; Chastang, J.; Ibanez, G. How French general practitioners adapt their care to patients with social difficulties? Fam. Med. Community Health 2019, 7, e000044. [Google Scholar] [CrossRef] [PubMed]
- Barlow, P.; Mohan, G.; Nolan, A.; Lyons, S. Area-level deprivation and geographic factors influencing utilization of General Practitioner services. SSM Popul. Health 2021, 15, 100870. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, E.; Kohrt, B.A.; Norris, S.A.; Ndetei, D.; Prabhakaran, D. Non-communicable disease syndemics: Poverty, depression, and diabetes among low-income populations. Lancet 2017, 389, 951–963. [Google Scholar] [CrossRef]
- Hurst, J.R.; Agarwal, G.; van Boven, J.F.M.; Daivadanam, M.; Gould, G.S.; Wan-Chun Huang, E.; Maulik, P.K.; Miranda, J.J.; Owolabi, M.O.; Premji, S.S.; et al. GACD Multi-Morbidity Working Group. Critical review of multimorbidity outcome measures suitable for low-income and middle-income country settings: Perspectives from the Global Alliance for Chronic Diseases (GACD) researchers. BMJ Open 2020, 10, e037079. [Google Scholar] [CrossRef]
- WHO. The Anatomical Therapeutic Chemical Classification System with Defined Daily Doses (ATC/DDD); World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.has-sante.fr/upload/docs/application/pdf/hbp_2003_recommandations.pdf (accessed on 18 May 2021).
- Arber, S.; McKinlay, J.; Adams, A.; Marceau, L.; Link, C.; D’Donnell, A. Patient characteristics and inequalities in doctors’ diagnostic and management strategies relating to CHD: A video-simulation experiment. Soc. Sci. Med. 2006, 62, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Irving, M.; Delpierre, C.; Schieber, A.C.; Lepage, B.; Rolland, C.; Afrité, A.; Pascal, J.; Cases, C.; Lombrail, P.; Lang, T. Do general practitioners overestimate the health of their patients with lower education? Soc. Sci. Med. 2011, 73, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Bensing, J.M.; Tromp, E.; van Dulmen, S.; van den Brink-Muinen, A.; Verheul, W.; Schellevis, F. Shifts in doctor-patient communication between 1986 and 2002: A study of videotaped general practice consultations with hypertension patients. BMC Fam. Pract. 2006, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Fox, S.A.; Escarce, J.J. Socioeconomic and racial/ethnic differenes in the discussion of cancer screening: “Between-”versus “Within-” physician differences. Health Serv. Res. 2007, 42, 950–970. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.; Buckingham, C.D.; Lindenmeyer, A.; McKinlay, J.B.; Link, C.; Marceau, L.; Arber, S. The influence of patient and doctor gender on diagnosis coronary heart disease. Sociol. Health Illn. 2008, 30, 1–18. [Google Scholar] [CrossRef]
- Street, R.L.; Gordon, H.; Haidet, P. Physicians’ communication and perceptions of patients: Is it how they look, how they talk, or is it just the doctor? Soc. Sci. Med. 2007, 65, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Willems, S.; De Maesschalck, S.; Deveugele, M.; Derese, A.; De Maeseneer, J. Socio-economic status of the patient and doctor-patient communication: Does it make a difference? Patient Educ. Couns. 2005, 56, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Tuppin, P.; Rudant, P.; Constantinou, C.; Gastaldi-Ménager, A.; Rachas, L.; de Roquefeuil, G.; Maura, G.; Caillol, H.; Tajahmady, A.; Coste, J.; et al. Value of a national admistrative database to guide public decision: From the système national d’information interrégimes de l’Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev. Epidémiol. Santé Publique 2017, 65, 149–167. [Google Scholar] [CrossRef]
- Kim, E.H.; Larson, J.A.; Andriole, G.L. Management of Benign Prostatic Hyperplasia. Annu. Rev. Med. 2016, 67, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Conlon, R.; Saheb, H.; Ahmed, I.I.K. Glaucoma treatment trends: A review. Can. J. Ophtalmol. 2017, 52, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Lokeshwar, S.D.; Harper, B.T.; Webb, E.; Jordan, A.; Dykes, T.A.; Neal, D.E.; Terris, M.K.; Klaassen, Z. Epidemiology and treatment modalities for the management of benign prostatic hyperplasia. Transl. Androl. Urol. 2019, 8, 529–539. [Google Scholar] [CrossRef]
- Fouda, P.J.; Mpay, E.M.; Mekeme, J.M.; Angwafor, F.; Sow, M. Symptoms of men’s lower urinary tract in Yaounde’s Central Hospital: Report of 329. Health Sci. Dis. 2013, 14, 1–4. [Google Scholar]
- Liang, Y.; Du, F.; Thompson, I.M.; Turner, B.J. Limited PSA testing in indigent men in South Texas: An appropriate care or missing a prevention opportunity? Cancer Epidemiol. Biomark. Prev. 2012, 21, 1489–1496. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kupelian, V.; Weit, J.T.; O’Leary, M.P.; Kusek, J.W.; Litman, H.J.; Link, C.L.; McKinlay, J.B.; BACH Survey Investigators. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: The Boston Area Community Health (BACH) Survey. Arch. Intern. Med. 2006, 166, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Hoke, G.P.; MacWilliams, G.W. Epidemiology of benign prostatic hyperplasia and comorbidities in racial and ethnic minority populations. Am. J. Med. 2008, 121, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C. Prevalence of lower urinay tract symptoms in male aborigines and non-aborigines in eastern Taiwan. J. Formos. Med. Assoc. 2008, 107, 72–735. [Google Scholar] [CrossRef]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- Maple, P.A.; Hamilton-Miller, J.M.; Brumfitt, W. World-wide antibotic resistance in methicillin-resistant Staphylococcus aureus. Lancet 1989, 1, 537–540. [Google Scholar] [CrossRef]
- O’Brien, K.H. Social determinants of health: The how, who, and where screenings are occurring: A systematic review. Soc. Work Health Care 2019, 58, 719–745. [Google Scholar] [CrossRef] [PubMed]
- Chimelli, L.; Mahler-Araujo, M.B. Fungal infections. Brain Pathol. 1997, 7, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Pujalte, G.G.A.; Reese, S.T. Superficial fungal infections. Prim. Care 2015, 42, 501–516. [Google Scholar] [CrossRef] [PubMed]
- Thirioux, B.; Birault, F.; Jaafari, N. Empathy is a protective factor of burnout in physicians: New neuro-phenomenological hypotheses regarding empathy and sympathy in care relationship. Front. Psychol. 2016, 7, 763. [Google Scholar] [CrossRef]
- Hojat, M.; Louis, D.Z.; Maio, V.; Gonnella, J.S. Empathy and health care quality. Am. J. Med. Qual. 2013, 28, 6–7. [Google Scholar] [CrossRef]
- Maslach, C.; Jackson, E. The measurement of experienced burnout. J. Organ. Behav. 1981, 2, 99–113. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Bradley, K.A.; Wipf, J.E.; Black, A.L. Burnout and self-reported patient care in an internal medicine residency program. Ann. Intern. Med. 2002, 136, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Reader, T.W.; Gillepsie, A. Patient neglect in healthcare institutions: A systematic review and conceptual model. BMC Health Serv. Res. 2013, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Yugero, O.; Marsal, J.R.; Esquerda, M.; Galvan, L.; Soler-Gonzalez, J. Cross-sectional study of the association between empathy and burnout and drug prescribing in primary care. Prim. Health Care Res. Dev. 2019, 20, e145. [Google Scholar] [CrossRef] [PubMed]
- Yugero, O.; Esquerda, M.; Marsal, J.R.; Soler-Gonzales, J. Association between sick leave prescribing practices and physician burnout and empathy. PLoS ONE 2015, 10, e0133379. [Google Scholar] [CrossRef]
- Ortiz-Hernandez, L.; Pérez-Salgado, D.; Tamez-Gonzalez, S. Socioeconomic inequality and health in Mexico. Rev. Med. Inst. Mex. Seguro 2015, 53, 336–347. [Google Scholar]
- Saurel-Cubizolles, M.J.; Chastang, J.F.; Menvielle, G.; Leclerc, A.; Luce, D.; EDISC Group. Social inequalities in mortality by cause among men and women in France. J. Epidemiol. Community Health 2009, 63, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.S.; Khan, S.S.; Lloyd-Jones, D.M.; Sidney, S. Changes in mortality in top 10 causes of death from 2011 to J. Gen. Intern. Med. 2021, 36, 2517–2518. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.H. Measuring individual differences in empathy: Evidence for a multidimensional approach. J. Pers. Soc. Psychol. 1983, 44, 113–125. [Google Scholar] [CrossRef]

| Class | Medication | DDD NP (€) Mean ± SD | DDD P (€) Mean ± SD | Region | DDD NP (€) Mean ± SD | DDD P (€) Mean ± SD | DDD NP vs. DDD P (Pairwise Comparisons—One-Way ANOVA Kruskal–Wallis) p-Value |
|---|---|---|---|---|---|---|---|
| A | Metformin | 6.38−3 ± 2.51−1 | 6.09−3 ± 2.35−2 | BR | 6.38−3 ± 2.01−2 | 6.50−2 ± 2.02−1 | <0.001 |
| CR | 9.85−3 ± 3.23−2 | 1.15−1 ± 3.81−1 | <0.001 | ||||
| OCR | 9.96−3 ± 2.87−2 | 9.30−2 ± 2.71−1 | <0.001 | ||||
| OSR | 4.79−6 ± 3.02−7 | 8.59−7 ± 6.78−8 | <0.001 | ||||
| Insulin glargine | 2.82−2 ± 7.66−2 | 1.75−3 ± 4.68−3 | BR | 2.59−2 ± 4.78−2 | 2.01−3 ± 4.78−3 | 0.857 | |
| CR | 4.65−2 ± 1.11−1 | 2.66−3 ± 6.40−3 | 0.698 | ||||
| OCR | 3.47−2 ± 8.30−2 | 1.98−3 ± 4.73−3 | 0.848 | ||||
| OSR | 7.44−7 ± 2.62−7 | 4.25−6 ± 1.49−6 | 0.001 | ||||
| B | Acetylsalicylic acid | 1.02−0 ± 2.11−0 | 7.08−2 ± 1.40−1 | BR | 1.10−1 ± 2.11−0 | 7.85−2 ± 1.44−1 | 0.883 |
| CR | 1.380 ± 2.59−0 | 8.68−2 ± 1.54−1 | 0.841 | ||||
| OCR | 1.15−0 ± 2.19−0 | 6.19−1 ± 1.62−0 | 0.822 | ||||
| OSR | 2.78−6 ± 3.95−5 | 1.51−5 ± 2.15−5 | 0.230 | ||||
| Rivaroxaban | 4.95−2 ± 1.44−1 | 7.71−2 ± 3.74−1 | BR | 6.38−2 ± 1.70−1 | 6.46−3 ± 1.93−2 | 0.671 | |
| CR | 6.36−2 ± 1.57−1 | 6.20−3 ± 1.75−2 | 0.472 | ||||
| OCR | 7.05−2 ± 1.72−1 | 6.47−3 ± 1.73−2 | 0.600 | ||||
| OSR | 1.08−7 ± 6.51−7 | 2.89−1 ± 7.21−1 | 0.008 | ||||
| C | Atorvastatin | 3.54−2 ± 1.16−1 | 4.13−3 ± 1.48−2 | BR | 4.43−2 ± 1.24−1 | 5.36−3 ± 160−2 | <0.001 |
| CR | 5.55−2 ± 1.53−1 | 6.04−3 ± 1.87−2 | <0.001 | ||||
| OCR | 4.19−2 ± 1.19−1 | 5.10−3 ± 1.57−0 | <0.001 | ||||
| OSR | 9.38−7 ± 1.30−7 | 4.84−6 ± 2,37−6 | <0.001 | ||||
| Rosuvastatin | 7.02−1 ± 1.24−0 | 1.23−0 ± 3.94−0 | BR | 8.28−1 ± 1.22−0 | 9.13−2 ± 1.35−1 | 0.400 | |
| CR | 1.13−0 ± 1.63−0 | 1.08-−1 ± 1.56−1 | 0.373 | ||||
| OCR | 8.51−1 ± 1.27 0 | 1.00−1 ± 1.58−1 | 0.400 | ||||
| OSR | 7.89−6 ± 3.46−6 | 4.64−0 ± 6.98_0 | 0.001 | ||||
| D | Ciclopirox | 1.06−3 ± 6.85−3 | 2.35−3 ± 2.76−3 | BR | 2.63−3 ± 4.80−3 | 5.77−4 ± 8.86−4 | 0.035 |
| CR | 6.77−4 ± 1.01−3 | 3.60−3 ± 6.34−3 | 0.044 | ||||
| OCR | 9.24−4 ± 1.82−3 | 5.25−3 ± 1.15−3 | 0.056 | ||||
| OSR | 2.22−6 ± 6.29−7 | 7.86−6 ± 2.49−6 | <0.001 | ||||
| Econazole | 2.30−4 ± 5.61−4 | 1.07−3 ± 2.69−3 | BR | 2.80−4 ± 5.75−4 | 1.40−3 ± 3.05−3 | 0.095 | |
| CR | 2.75−4 ± 5.37−4 | 1.21−3 ± 2.38−3 | 0.104 | ||||
| OCR | 3.63−4 ± 7.59−4 | 1.65−3 ± 3.57−3 | 0.028 | ||||
| OSR | 1.60−6 ± 9.31−7 | 7.44−6 ± 4.00−6 | <0.001 | ||||
| G | Serenoa repens | 1.03−2 ± 4.00−2 | 1.19−2 ± 9.93−1 | BR | 8.79−3 ± 2.89−24 | 4.13−3 ± 1.31−2 | 0.008 |
| CR | 1.45−2 ± 4.68−2 | 8,90−3 ± 3.04−2 | 0.407 | ||||
| OCR | 1.79−2 ± 5.74−2 | 1.20−2 ± 3.91−32 | 0.915 | ||||
| OSR | 2.60−6 ± 2.75−6 | 4,50−1 ± 1.96−0 | 0.002 | ||||
| Tamsulosine | 1.08−2 ± 2.19−2 | 3.47−2 ± 1.39−1 | BR | 1.12−2 ± 1.78−2 | 5.42−3 ± 8.97−3 | 0.513 | |
| CR | 1.68−2 ± 2.79−2 | 8.22−3 ± 1.62−2 | 0.216 | ||||
| OCR | 1.50−2 ± 2.60−2 | 8.34−3 ± 1.57−2 | 0.450 | ||||
| OSR | 9.78−6 ± 6.69−6 | 1.17−1 ± 2.62−1 | <0.001 | ||||
| H | Prednisolone | 2.06−2 ± 5.44−2 | 2.09−2 ± 5.54−2 | BR | 2.69−2 ± 6.12−2 | 3.07−3 ± 6.53−2 | 1.000 |
| CR | 2.22−2 ± 4.45−2 | 1.90−2 ± 3.78−2 | 1.000 | ||||
| OCR | 3.34−2 ± 7.48−2 | 3.40−3 ± 7.75−2 | 1.000 | ||||
| OSR | 1.04−6 ± 4.22−7 | 4.77−6 ± 1.96−6 | <0.001 | ||||
| J | Amoxicillin | 3.08−3 ± 1.01−2 | 4.72−2 ± 1.38−2 | BR | 4.27−2 ± 1.18−2 | 6.85−3 ± 1.62−2 | 0.593 |
| CR | 4.43−2 ± 1.22−2 | 6.53−2 ± 1.62−2 | 0.962 | ||||
| OCR | 3.60−2 ± 1.03−2 | 5.49−2 ± 1.42−2 | 0.719 | ||||
| OSR | 9.93−7 ± 5.20−7 | 4.61−6 ± 2.47−6 | <0.001 | ||||
| Pyostacine | 5.24−2 ± 1.00−2 | 2.11−1 ± 5.97−1 | BR | 6.45−2 ± 1.14−1 | 6.22−2 ± 1.08−1 | 1.000 | |
| CR | 7.30−2 ± 1.28−1 | 5.41−2 ± 9.39−2 | 1.000 | ||||
| OCR | 7.21−2 ± 1.26−1 | 5.78−2 ± 1.01−1 | 1.000 | ||||
| OSR | 9.36−7 ± 3.29−7 | 6.70−6 ± 1.17−0 | 0.273 | ||||
| M | Ibuprofen | 1.04−2 ± 3.60−2 | 1.52−2 ± 3.60−2 | BR | 1.03−2 ± 2.86−2 | 1.66−2 ± 4.50−2 | 1.000 |
| CR | 1.49−2 ± 4.20−2 | 1.90−2 ± 5.49−2 | 1.000 | ||||
| OCR | 1.64−2 ± 4.98−2 | 2.51−2 ± 8.03−2 | 1.000 | ||||
| OSR | 9.55−7 ± 5.24−7 | 4.43−6 ± 2.48−6 | <0.001 | ||||
| N | Paracetamol | 4.68−2 ± 2.23−1 | 3.50−2 ± 2.00−1 | BR | 5.98−2 ± 2.20−1 | 4.14−2 ± 1.69−1 | 0.893 |
| CR | 7.09−2 ± 2.79−1 | 5.13−2 ± 2.49−1 | 0.845 | ||||
| OCR | 5.66−2 ± 2.64−1 | 4.73−2 ± 2.62−1 | 0918 | ||||
| OSR | 1.01−6 ± 4.48−6 | 4.85−6 ± 2.21−6 | <0.001 | ||||
| P | Ivermectin | 3.48−3 ± 8.45−3 | 1.60−2 ± 3.11−2 | BR | 6.95−3 ± 1.21−2 | 2.54−2 ± 4.46−2 | 1.000 |
| CR | 6.98−3 ± 1.21−2 | 2.19−2 ± 3.85−2 | 1.000 | ||||
| OCR | 1.26−6 ± 1.36−8 | 1.67−2 ± 2.91−2 | 1.000 | ||||
| OSR | 6.34−6 ± 7.29−7 | 1.26−6 ± 1.36−8 | 0.281 | ||||
| R | Salbutamol | 1.55−1 ± 7.08−1 | 3.28−1 ± 1.20−0 | BR | 3.53−1 ± 1.43−0 | 2.09−1 ± 8.32−1 | 0.999 |
| CR | 3.03−1 ± 1.21−0 | 2.35−1 ± 9.27−1 | 0.910 | ||||
| OCR | 2.63−1 ± 1.00−0 | 1.76−1 ± 6.75−1 | 1.000 | ||||
| OSR | 3.92−1 ± 1.15−0 | 1.59−5 ± 1.94−5 | <0.001 | ||||
| Tiotropium | 2.39−1 ± 3.26−1 | 3.53−1 ± 7.00−1 | BR | 3.39−1 ± 4.08−1 | 1.32−1 ± 1.50−1 | 0.980 | |
| CR | 3.04−1 ± 3.43−1 | 9.32−1 ± 1.00−1 | 0.750 | ||||
| OCR | 3.14−1 ± 3.36−1 | 9.15−1 ± 8.77−2 | 0.536 | ||||
| OSR | 3.62−6 ± 5.09−9 | 1.09−0 ± 1.15−0 | 0.068 | ||||
| S | Cromolyn sodium | 1.11−3 ± 2.27−3 | 6.33−3 ± 1.37−2 | BR | 1.12−3 ± 2.05−2 | 7.25−3 ± 1.48−2 | 0.857 |
| CR | 1.68−2 ± 2.91−2 | 9.18−3 ± 1.76−2 | 0.687 | ||||
| OCR | 1.61−3 ± 2.56−2 | 7.74−3 ± 1.32−2 | 0.848 | ||||
| OSR | 2.10−6 ± 8.98−7 | 6.94−6 ± 3.43−6 | 0.001 | ||||
| Timolol | 1.68−3 ± 5.57−3 | 8.90−2 ± 3.80−1 | BR | 1.82−2 ± 5.53−2 | 4.50−2 ± 1.47−2 | <0.001 | |
| CR | 2.29−2 ± 6.19−2 | 7.87−3 ± 2.23−2 | 0.004 | ||||
| OCR | 2.60−2 ± 7.25−2 | 7.94−3 ± 2.27−2 | 0.009 | ||||
| OSR | 1.54−5 ± 1.11−5 | 3.36−1 ± 7.07−1 | <0.001 |
| Medications | Sample Size | N | Achieved Power | |||
|---|---|---|---|---|---|---|
| Non-Centrality Parameters δ | Critical t | Df | Min. Sample Size | |||
| Metformin | 2.5084447 | 1.6568452 | 128 | 132 | 2760 | 1.0000000 |
| Insulin glargine | 2.5426355 | 1.6802300 | 44 | 48 | 80 | 0.8998699 |
| Acetysalicylic acid | 2.5530778 | 1.6895725 | 35 | 39 | 112 | 0.9960272 |
| Rivaroxaban | 2.54000013 | 1.6735649 | 54 | 58 | 144 | 0.9903044 |
| Atorvastatin | 2.5081065 | 1.6623540 | 88 | 92 | 3128 | 1.0000000 |
| Rosuvastatin | 5.0575634 | 2.9199856 | 2 | 6 | 120 | 1.0000000 |
| Econazole | 2.5281521 | 1.6706489 | 60 | 64 | 624 | 1.0000000 |
| Ciclopirox | 2.50300004 | 1.6536580 | 174 | 178 | 240 | 0.8250026 |
| Serenoa repens | 2.9247437 | 2.0150484 | 5 | 9 | 404 | 1.0000000 |
| Tamsulosine | 2.5345654 | 1.6838510 | 40 | 44 | 512 | 1.0000000 |
| Prednisolone | 2.5034009 | 1.6567516 | 129 | 133 | 520 | 0.9983722 |
| Amoxicillin | 3.4858480 | 2.1318468 | 4 | 8 | 2136 | 1.0000000 |
| Pyostacine | 2.6321916 | 1.7291328 | 19 | 23 | 32 | 0.8500695 |
| Ibuprofen | 2.4983873 | 1.6515642 | 228 | 232 | 976 | 0.9992094 |
| Paracetamol | 2.4913307 | 1.6481729 | 460 | 464 | 2120 | 0.9996143 |
| Ivermectin | 2.5241591 | 1.6665997 | 71 | 75 | 32 | 0.3567551 |
| Salbutamol | 2.4919857 | 1.6479629 | 491 | 495 | 352 | 0.5539792 |
| Tiotropium | 2.6147066 | 1.7396067 | 17 | 21 | 48 | 0.9716385 |
| Cromolyn Sodium | 3.4211741 | 2.1318468 | 4 | 8 | 216 | 1.0000000 |
| Timolol | 2.5712845 | 1.6923603 | 33 | 37 | 560 | 1.0000000 |
| Class | Medication | Predictor | Estimate | SE | p-Value | Pattern |
|---|---|---|---|---|---|---|
| A | Metformin | intercept | 0.1057 | 0.00730 | <0.001 | |
| prevalence | −0.3198 | 0.04229 | <0.001 * | |||
| population P–NP | −0.0957 | 0.01033 | <0.001 * | |||
| prevalence * population | 0.2896 | 0.05981 | <0.001 * | pattern-3 | ||
| Insulin glargine | intercept | 0.0441 | 0.0128 | <0.001 | ||
| prevalence | −0.1324 | 0.0740 | 0.077 | |||
| population P–NP | −0.0413 | 0.0181 | 0.025* | |||
| prevalence * population | 0.1240 | 0.1046 | 0.240 | |||
| B | Acetylsalicylic acid | intercept | 1.51 | 0.286 | <0.001 | |
| prevalence | −4.59 | 1.654 | 0.006 * | |||
| population P–NP | −1.41 | 0.404 | <0.001 * | |||
| prevalence * population | 4.28 | 2.339 | 0.070 | |||
| Rivaroxaban | intercept | 0.0820 | 0.0490 | 0.096 | ||
| prevalence | −0.2485 | 0.2839 | 0.0383 * | |||
| population P–NP | −0.1462 | 0.0693 | 0.037 * | |||
| prevalence * population | 1.3269 | 04014 | 0.001 * | pattern-1 | ||
| C | Atorvastatin | intercept | 0.0591 | 0.00317 | <0.001 | |
| prevalence | −0.1811 | 0.01835 | <0.001 * | |||
| population P–NP | −0.0522 | 0.00448 | <0.001 * | |||
| prevalence * population | 0.1600 | 0.02597 | <0.001 * | pattern-3 | ||
| Rosuvastatin | intercept | 1.17 | 0.510 | 0.024 | ||
| prevalence | −3.56 | 2.953 | 0.230 | |||
| population P–NP | −2.20 | 0.721 | 0.003 * | |||
| prevalence * population | 20.86 | 4.177 | <0.001 * | pattern-1 | ||
| D | Ciclopirox | intercept | 0.00186 | 0.000723 | 0.011 | |
| prevalence | −0.00610 | 0.00419 | 0.146 | |||
| population P–NP | 0.00179 | 0.00102 | 0.081 | |||
| prevalence * population | −0.00380 | 0.00592 | 0.522 | |||
| Econazole | intercept | 0.000723 | 0.000165 | 0.023 | ||
| prevalence | −0.00112 | 0.000956 | 0.241 | |||
| population P–NP | 0.00138 | 0.000234 | <0.001 * | |||
| prevalence * population | −0.00414 | 0.00135 | 0.002 * | pattern-2 | ||
| G | Serenoa repens | intercept | 0.0066 | 0.0754 | 0.826 | |
| prevalence | −0.0483 | 0.4366 | 0.912 | |||
| population P–NP | −0.1188 | 0.1061 | 0.263 | |||
| prevalence * population | 1.7352 | 0.6145 | 0.005 * | pattern-1 | ||
| Tamsulosin | intercept | 0.0177 | 0.00900 | 0.050 | ||
| prevalence | −0.0530 | 0.05210 | 0.309 | |||
| population P–NP | −0.0379 | 0.01272 | 0.003 * | |||
| prevalence * population | 0.4724 | 0.07368 | <0.001 * | pattern-1 | ||
| H | Prednisolone | intercept | 0.03395 | 0.00512 | <0.001 | |
| prevalence | −0.10179 | 0.02962 | <0.001 * | |||
| population P–NP | 0.000729 | 0.00723 | 0.920 | |||
| prevalence * population | −0.00303 | 0.04190 | 0.942 | |||
| J | Amoxicillin | intercept | 0.00516 | 0.000556 | <0.001 | |
| prevalence | −0.01592 | 0.00322 | <0.001 * | |||
| population P–NP | 0.00278 | 0.000786 | <0.001 * | |||
| prevalence * population | −0.00863 | 0.00455 | 0.058 | |||
| Pyostacine | intercept | 0.0869 | 0.152 | 0.571 | ||
| prevalence | −0.2631 | 0.878 | 0.767 | |||
| population P–NP | −0.1813 | 0.214 | 0.405 | |||
| prevalence * population | 2.5956 | 1.242 | 0.046 * | pattern-1 | ||
| M | Ibuprofen | intercept | 0.01701 | 0.00316 | <0.001 | |
| prevalence | −0.05031 | 0.01832 | 0.006 * | |||
| population P–NP | 0.00774 | 0.00447 | 0.084 | |||
| prevalence * population | −0.02294 | 0.02591 | 0.376 | |||
| N | Paracetamol | intercept | 0.0782 | 0.00993 | <0.001 | |
| prevalence | −0.2395 | 0.05749 | <0.001 * | |||
| population P–NP | −0.0202 | 0.01404 | 0.150 | |||
| prevalence * population | 0.0641 | 0.08130 | 0.430 | |||
| R | Tiotropium | intercept | 0.400 | 0.142 | 0.007 | |
| prevalence | −1.226 | 0.820 | 0.142 | |||
| population P–NP | −0.538 | 0.200 | 0.010 * | |||
| prevalence * population | 4.977 | 1.160 | <0.001 * | pattern-1 | ||
| S | Cromolyn sodium | intercept | 0.00181 | 0.00142 | 0.203 | |
| prevalence | −0.00541 | 0.00822 | 0.512 | |||
| population P–NP | 0.00824 | 0.00202 | <0.001 * | |||
| prevalence * population | −0.02503 | 0.01198 | 0.038 * | pattern-2 | ||
| Timolol | intercept | 0.0275 | 0.0232 | 0.235 | ||
| prevalence | −0.0819 | 0.01342 | 0.541 | |||
| population P–NP | −0.1030 | 0.0328 | 0.002 * | |||
| prevalence * population | 1.3374 | 0.1898 | <0.001 * | pattern-1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birault, F.; Le Bonheur, L.; Langbour, N.; Clodion, S.; Jaafari, N.; Perault-Pochat, M.-C.; Thirioux, B. Exposure to High Precariousness Prevalence Negatively Impacts Drug Prescriptions of General Practitioners to Precarious and Non-Precarious Populations: A Retrospective Pharmaco-Epidemiological Study. Int. J. Environ. Res. Public Health 2022, 19, 2962. https://doi.org/10.3390/ijerph19052962
Birault F, Le Bonheur L, Langbour N, Clodion S, Jaafari N, Perault-Pochat M-C, Thirioux B. Exposure to High Precariousness Prevalence Negatively Impacts Drug Prescriptions of General Practitioners to Precarious and Non-Precarious Populations: A Retrospective Pharmaco-Epidemiological Study. International Journal of Environmental Research and Public Health. 2022; 19(5):2962. https://doi.org/10.3390/ijerph19052962
Chicago/Turabian StyleBirault, François, Lakshmipriva Le Bonheur, Nicolas Langbour, Sandivanie Clodion, Nematollah Jaafari, Marie-Christine Perault-Pochat, and Bérangère Thirioux. 2022. "Exposure to High Precariousness Prevalence Negatively Impacts Drug Prescriptions of General Practitioners to Precarious and Non-Precarious Populations: A Retrospective Pharmaco-Epidemiological Study" International Journal of Environmental Research and Public Health 19, no. 5: 2962. https://doi.org/10.3390/ijerph19052962
APA StyleBirault, F., Le Bonheur, L., Langbour, N., Clodion, S., Jaafari, N., Perault-Pochat, M.-C., & Thirioux, B. (2022). Exposure to High Precariousness Prevalence Negatively Impacts Drug Prescriptions of General Practitioners to Precarious and Non-Precarious Populations: A Retrospective Pharmaco-Epidemiological Study. International Journal of Environmental Research and Public Health, 19(5), 2962. https://doi.org/10.3390/ijerph19052962






